Abstract
The thermotropic phase behavior of the cholesterol (Chol)-containing binary membrane of dihexadecylphosphatidylcholine (DHPC) was investigated as a function of the mole fraction of Chol Xch by DSC and fluorescence spectroscopy using 6-propionyl-2-(N,N-dimethylamino)naphthalene (Prodan). The main-transition enthalpy ΔHm was almost constant at Xch < ca. 0.05, but it decreased almost linearly with increasing Xch above 0.05 until it reached 0 at Xch = ca. 0.50, suggesting that the interaction between DHPC and Chol is relatively weaker than that between dipalmitoylphosphatidylcholine (DPPC) and Chol. The Prodan fluorescence spectra changed depending on the phase state, which was characterized by the wavelength at the emission maximum. Particularly, the two-phase state of the interdigitated (LβI) and non-interdigitated (Lβ) gel was sensitively detected as the superposition of the two elementary spectra corresponding to each phase state, allowing us to explain the Xch dependent non-linear behavior of the Chol-induced Lβ phase by introducing a proportionality factor ξ(Xch) and by assuming a simple probabilistic process. By comparing the phase diagram constructed for the DHPC–Chol binary membrane with that for the DPPC–Chol binary bilayer, a difference was observed in the minimum Xch value for the entire formation of the liquid ordered (Lo) phase. This difference could be explained in terms of the hydrophobic mismatch at the bilayer midplane by taking into account the difference in the capability of hydrogen bonding with the 3β-OH group of Chol due to the presence or absence of the carbonyl oxygens at the glycerol backbone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
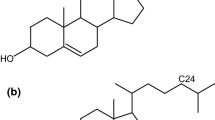
One of the phospholipid species that have been most often used in model membrane studies is dipalmitoylphosphatidylcholine (DPPC), which consists of a polar headgroup of phosphocholine (PC) and two palmitoyl chains attached to the glycerol backbone by ester linkages. The DPPC bilayer membrane exhibits several types of phase transitions with variations in the surroundings: it undergoes the pretransition at ca. 34 °C and the main transition at ca. 42 °C under the atmospheric pressure [1, 2]. The former and latter transitions correspond to the conversion from a lamellar gel (Lβ′) phase to a ripple gel (Pβ′) phase and the conversion from the Pβ′ phase to a liquid crystalline (Lα) phase, respectively. On the other hand, some kinds of phospholipids are known to be capable of forming molecular aggregates with non-bilayer structures. Dihexadecylphosphatidylcholine (DHPC) is one of those phospholipids. DHPC has the same chemical structure as DPPC except that the two hydrocarbon chains of the DHPC molecule are attached to the glycerol backbone by ether linkages (Fig. 1). Hence, DHPC is often categorized as an ether-linked PC lipid while DPPC is an ester-linked PC lipid. Seemingly, the DHPC membrane shows similar thermotropic phase behavior to that of the DPPC bilayer membrane: it undergoes the pretransition at ca. 35 °C and the main transition at ca. 44 °C under atmospheric pressure [3,4,5,6]. However, the pretransition of the DHPC membrane is not the Lβ′/Pβ′ transition but the transition from a so-called interdigitated gel (LβI) phase to the Pβ′ phase. In the LβI phase, the hydrocarbon chains of the DHPC molecules in the two opposing monolayers of a bilayer alternately interpenetrate beyond the bilayer midplane [4, 7]. Several previous studies [8, 9] have shown that a notable difference between the DPPC and the DHPC bilayer membrane can be seen in the structure of water in hydration shells and that the interaction between adjacent polar headgroups is weaker in the DHPC membrane than in the DPPC bilayer. In general, therefore, it is thought that weaker interaction in the hydrophilic region of the bilayer membrane is related to the propensity of DHPC to form the interdigitated structure.
In recent model-membrane studies, binary and ternary phospholipid bilayer membranes containing cholesterol (Chol) have often been used. Chol is a major component of the plasma membrane and is also thought to be essentially relevant to the formation of functional microdomains called lipid rafts [10]. Ipsen et al. [11] predicted the phase behavior of the DPPC–Chol binary bilayer membrane by constructing the compositional phase diagram on the basis of theoretical calculations. Their phase diagram suggested several significant features: a third phase that is distinguished from a solid ordered (So) phase like Lβ′ and Pβ′ phase and a liquid disordered (Ld) phase like Lα phase occurs in a higher Chol composition region (> ca. 30 mol%) and two-phase regions of the Ld (or So) and the third phase appear in an intermediate Chol composition region. The third phase was termed liquid ordered (Lo) phase, where constituent lipids have higher conformational order of the acyl chains than in the Ld phase and relatively faster lateral diffusion than in the So phase. These theoretical predictions were experimentally confirmed by Vist and Davis [12]. We also have recently reported the temperature (T)–Chol mole fraction (Xch) phase diagrams for the Chol-containing binary bilayer membranes of a series of linear saturated diacylphosphatidylcholines with different acyl chain length n (i.e., CnPC, n = 14–18) [13,14,15]. Our phase diagrams suggested several interesting features about the phase behavior of these binary bilayer membranes. One is that the phase behavior of those binary bilayer membranes was quite similar to typical peritectic melting behavior, suggesting that Chol tends to form molecular complexes with its surrounding phospholipids within the binary bilayer membranes. Another is that biphasic n-dependence was observed for the Xch values at which the pretransition is abolished. These features can be reasonably explained by applying the so-called superlattice view (i.e., regular distribution of Chol within the binary bilayer membrane) [16].
On the other hand, very few studies have been conducted on the phase behavior of Chol-containing binary membrane of ether-linked PC lipids like DHPC [17] despite the fact that they are peculiar in their capability of forming the interdigitated structure without any inducer. In this study, we investigated the phase transitions of the DHPC–Chol binary membrane by means of differential scanning calorimetry (DSC) and fluorescence spectroscopy using 6-propionyl-2-(N,N-dimethylamino)naphthalene (Prodan) as a membrane probe [18, 19] to construct the T–Xch phase diagram for the DHPC–Chol binary membrane. Since different structures of lipid membranes are generally dealt with as different phases, constructing a compositional phase diagram is a suitable way to examine the overall effect of Chol on the lateral organization of lipid membranes. The first aim of this study is to clarify similarities and differences in the phase behavior of the cholesterol-containing binary membranes of DHPC and DPPC and to discuss how those similarities and differences are associated with the difference in the chemical structure between DHPC and DPPC. As described above, the difference in the chemical structure between DHPC and DPPC is very small, but it can be a non-trivial factor when considering the interaction with Chol, because the carbonyl group in DPPC can function as a hydrogen bond acceptor. In fact, an MD simulation study [20] has shown that the formation of hydrogen bonding networks in the headgroup region via the 3β-OH groups of Chol plays a crucial role in the formation of 1:1 or 1:2 complexes of Chol and DPPC, which leads to the occurrence of membrane domains.
The second aim of this study is to examine the effect of Chol incorporation on the formation of the interdigitated structure in the DHPC membrane. A previous X-ray scattering and calorimetric study [21] has revealed that Chol incorporated into the DHPC membrane has a perturbing effect on the chain interdigitation and induces non-interdigitated (i.e., bilayer) gel structure, and also that the interdigitated and non-interdigitated gel state can coexist until the chain interdigitation is completely abolished at the Chol concentration of 5 mol% (Xch = 0.05). However, this Chol concentration is not consistent with their own DSC result, which shows that the pretransition persists up to 10 mol% (Xch = 0.10). Then we re-examined the Chol effect on the chain interdigitation by applying Prodan fluorescence technique to consider the process of the conversion from the interdigitated gel to the non-interdigitated gel induced by the incorporation of increasing amount of Chol. The Prodan fluorescence technique can sensitively detect the conversion from the interdigitated gel to the non-interdigitated gel phase as a clear shift of the wavelength at the emission maximum (λmax) [22].
Experimental
A synthetic phosphatidylcholine, DHPC (1,2-di-O-hexadecanoyl-sn-glycero-3-phosphocholine, purity > 99%), was purchased from Avanti Polar Lipids (Alabaster, AL) and cholesterol (5-cholesten-3β-ol, purity ≥ 99%) was from Sigma-Aldrich Co. (St. Louis, MO). The chemical structures of DHPC and Chol, along with that of DPPC, are shown in Fig. 1. The fluorescent probe of Prodan (6-propionyl-2-(N,N-dimethylamino)naphthalene) was purchased from Molecular Probes Inc. (Eugene, OR). All the materials were used without further purification. Water was distilled twice from dilute alkaline permanganate solution to prepare all sample solutions. Aqueous dispersions of multilamellar vesicles containing Chol were prepared by the following procedure. First, appropriate amounts of stock solutions of Chol/chloroform and Prodan/ethanol were added to a weighed amount of phospholipid powder. Second, the mixed solution was dried in vacuum to remove all residual solvents. Finally, a given amount of distilled water was added to the resulting dry film, and subsequently, the heterogeneous aqueous mixture was vortexed and sonicated for a few minutes at about 50 °C to obtain a homogeneously translucent aqueous dispersion. The concentrations of the phospholipid and Prodan were fixed at 1 mmol kg−1 and 2 μmol kg−1 (i.e., the molar ratio of Prodan to phospholipids is 1:500), respectively [19]. We have confirmed that such a slight amount of Prodan has no significant effect on the thermotropic phase behavior of phospholipid membranes [19]. The mole fraction of Chol Xch in a binary membrane defined as
was varied in the range from 0 to 0.50. Here, mch, mDHPC, nch, and nDHPC denote the molalities of Chol and DHPC and the number of moles of Chol and DHPC, respectively.
Fluorescence spectroscopic measurements were performed using an F-3010 fluorescence spectrophotometer (Hitachi High-Technology Corp., Tokyo) at constant temperatures ranging from 5 to 80 °C. The temperature was controlled within an accuracy of 0.1 °C by circulating thermostated water from a temperature-regulated water bath. A light with the wavelength of 361 nm was used for the excitation of the membrane probe Prodan and the emission spectra were acquired in the range of 400–600 nm. Data analyses were carried out by using Excel 2013 (Microsoft) and Kaleida graph ver. 4.0 (Synergy software).
DSC measurements were carried out using a high-sensitivity differential scanning calorimeter MCS (MicroCal, Northampton, MA). DSC thermograms were recorded in the temperature range of 10–80 °C at the heating rate of 0.33 °C min−1 by using water as a reference solution. Each DSC measurement was composed of two heating scans, namely a first heating scan and a second heating scan performed immediately after the first scan was completed, and at least two independent DSC measurements were applied to each sample solution. A transition temperature was determined as the temperature at the top of an endothermic peak and an enthalpy change with a transition ΔH was estimated by integrating an endothermic peak using Excel 2013 as well as data-analyzing software ORIGIN ver. 7.0. In the analysis of DSC data using Excel 2013, baseline subtraction was done to obtain a ΔH value by producing a baseline by least-squares regression with a polynomial function.
Results
DSC measurements
Figure 2(a) shows the overall DSC thermograms for the DHPC–Chol binary membranes with different Chol concentrations (Xch = 0–0.45) to see the rough tendency of how the endothermic peak of the main transition changed with increasing Xch. For the pure DHPC membrane (i.e., Xch = 0, curve a-1), a small endothermic peak, arising from the pretransition, and a large sharp endothermic peak, from the main transition, were clearly observed at 34 and at 44 °C, respectively, which is consistent with previous studies [3,4,5,6,7]. As Xch increased, the main-transition peak gradually increased in width but markedly decreased in height, and as a result, it became smaller as a whole. At Xch = 0.20, only a very broad peak was observed (curves a-5 and a-6). This broad peak also became broader and smaller with further increases in Xch and eventually became completely undetectable at Xch = 0.50. The peak-top temperature was also changed slightly with Xch: it shifted slightly to lower temperatures with increasing Xch up to ca 0.15, whereas it shifted gradually to higher temperatures with increasing Xch above 0.15. Figure 2(b) shows the magnified view of the DSC thermograms in the vicinity of the pretransitions at every 0.02 up to Xch = 0.10. The endothermic peak of the pretransition also became smaller and broader with increasing Xch and finally it became undetectable at Xch > 0.10. This Xch value is slightly higher than that for the DPPC–Chol binary bilayer (i.e., 0.07–0.08 [15]) and much higher than that for the DSPC–Chol binary bilayer (i.e., 0.035–0.04 [13]). Parallel to this change in the peak shape, the peak top progressively shifted to lower temperatures with increasing Xch.
Overall (a, c) and magnified (b, d) view of DSC thermograms for a series of DHPC–Chol binary membranes with different Chol concentrations (Xch). Schematic illustration represents the molecular arrangements in interdigitated (LβI) phase, ripple gel (Pβ′) phase, and liquid crystalline (Lα) phase of pure DHPC membrane. (a) Overall behavior of the Chol effect on the main transition. Curves 6–11 have been 20-fold magnified in the y-axis direction to improve visibility. Note that curves 5 and 6 are the same DSC thermogram. (b) Chol effect on the pretransition. (c, d) Change in the shape of main-transition peak with increasing Xch. Curves 4–6 have been 5-fold magnified in the y-axis direction relative to curves 1–3. Note that curves 3 and 4 are the same DSC thermogram
In Fig. 2(c, d), the magnified view of the main-transition peaks at several specific Xch in the intermediate Xch region (0.05 ≤ Xch ≤ 0.20) is shown to see in more detail how the line shape of the main-transition peak changes with the variation in Xch. An obviously asymmetric peak was observed at Xch = 0.06 (curve c-2), though the main-transition peak observed at Xch = 0.05 (curve c-1) was almost symmetric. This peak asymmetry is apparently attributable to the presence of another non-negligible peak at a slightly higher temperature than that of the original main-transition peak. The heights of both original and second peak decreased with an increase in Xch. The second peak could be comparatively more clearly observed in the DSC thermogram at Xch = 0.12 (curve c-3). From its line shape, the second peak seems almost as sharp as the original main-transition peak. In addition, this thermogram also shows that there is a slight mismatch between the heights of the baseline before and after the peaks, which suggests that there is a very broad peak as a third component that has a maximum at a temperature slightly higher than that of the second peak. This mismatch can be more clearly seen in the thermogram further magnified in the vertical direction (curve d-4 in Fig. 2(d)). This broad peak was relatively less affected by the increase in the Xch, whereas the other two sharp peaks became noticeably smaller with an increase in Xch (curve d-5). The two sharp peaks eventually became almost undetectable at Xch = 0.20, and as a result, only the third broad peak remained as a detectable component (curve d-6). The DPPC–Chol binary bilayer membrane also exhibited similar behavior in almost the same Xch range except that only two component peaks (i.e., a sharp and a broad component peak) were observed [15]. As will be described later, this Xch-dependent complicated change in the DSC endothermic peaks is probably related to the heterogeneous distribution of Chol within the binary membrane due to two-phase coexistence.
From the DSC results, we were able to determine several typical thermodynamic properties that characterize the main transition of a series of DHPC–Chol binary membranes with different Chol concentrations. Figure 3(a–c) shows the Xch dependence of the main-transition enthalpy (ΔHm), the main-transition temperature (Tm), and the peak width at half height of the main-transition peak (ΔT1/2), respectively. For comparison, the same kinds of data obtained in our previous studies for the binary bilayers of DPPC–Chol [15] and DSPC–Chol [13] are also plotted together in each of Fig. 3(a–c). As seen from Fig. 3a, the ΔHm value of the DHPC–Chol binary membrane was almost constant at Xch ≤ 0.05, which is evidently different from the behavior for the binary bilayers of the other two ester-linked PCs in the same Xch range. It is well known that the main transition is associated with the trans–gauche conformational change of the hydrocarbon chains of the constituent lipid molecule [23] and also that Chol incorporated into a phospholipid membrane has an inhibitory effect on this conformational change of its surrounding lipid molecules [24]. Taking this into account, this invariance of the ΔHm value observed at Xch ≤ 0.05 can be interpreted as indicating that virtually no DHPC molecules are restricted from the trans–gauche conformational change at the main transition in the presence of such a small amount of Chol. The difference in the behavior of ΔHm in this Xch region between DHPC and the other ester-linked PCs may suggest that the phospholipid–Chol interaction is weaker within the Chol-containing binary membrane of DHPC than within those of ester-linked PCs.
Chol concentration (Xch) dependence of (a) enthalpy change with main transition (ΔHm), (b) main-transition temperature (Tm), and (c) peak width at half height (ΔT1/2) for DHPC–Chol binary membrane (square). The same results for the Chol-containing binary bilayer of DPPC (circle) and DSPC (triangle) obtained in the previous studies [13, 15] are also plotted for comparison. In (b), solid lines represent the calculated curve based on the general thermodynamic relation (Eq. (2))
With a further increase in Xch, the ΔHm value decreased almost linearly and became 0 finally at an Xch between 0.45 and 0.50. Taking the above inhibitory effect of Chol into account, this almost linear decrease in ΔHm with Xch can be considered to imply that the population of the DHPC molecules capable of causing the thermotropic trans–gauche conformational change decreases nearly in proportion to the number of the Chol molecules incorporated into the binary membrane. In addition, this linearity may suggest that Chol tends to be scattered uniformly over the entire membrane rather than to be localized to form a distinct region with extremely high concentration of Chol. Finally, the main transition is completely abolished when the trans–gauche conformational change of all the DHPC molecules within the binary membrane is completely suppressed by Chol incorporated into the membrane. The Xch value of the abolition of the main transition (Xch*main) can be estimated from Fig. 3(a) at ca. 0.50 for the DHPC. This value is obviously higher than that for the DPPC–Chol binary bilayer (i.e., Xch*main~0.33) but is close to that for the DSPC–Chol binary bilayer (i.e., Xch*main~0.50). This difference may also be due to the fact that the DHPC–Chol interaction is weaker than the DPPC–Chol interaction.
Tm of the DHPC–Chol binary membrane, as shown in Fig. 3(b), decreased slightly with increasing Xch in the low Xch region. This seems to be similar to the Xch dependence of Tm for the DPPC– and the DSPC–Chol binary bilayers. In this figure, the theoretical curve based on the following thermodynamic relation is also shown as a solid line for each binary membrane:
Here, R is the gas constant, and ΔHm° and Tm° represent the enthalpy change and temperature of the main transition for the pure membrane (i.e., the values of ΔHm and Tm at Xch = 0), respectively. This equation predicts the extent of the depression of the main-transition temperature when the binary membrane can be regarded as an ideal mixture, where phospholipid and Chol molecules are completely miscible in a liquid crystalline state but are perfectly immiscible in a gel state. For the DHPC–Chol binary membrane, agreement between the experimental results and the theoretical prediction can be seen in the very dilute region (i.e., Xch < ca. 0.02). The DPPC–Chol binary bilayer also exhibited similar behavior (i.e., Xch < ca. 0.02), whereas the agreement was observed in a comparatively wider Xch region (i.e., Xch < ca. 0.04) for the DSPC–Chol binary bilayer. This indicates that these binary membranes can be regarded as ideal binary mixtures in these very limited Xch regions; that is, these kinds of phospholipids and Chol are almost completely immiscible in the gel state but ideally miscible in the liquid crystalline state. In the higher Xch region, on the other hand, the experimental values of Tm became gradually higher than the theoretical prediction for each binary membrane with increasing Xch. In general, the positive deviation from the theoretical prediction can be caused by either non-ideal mixing in the liquid crystalline state or imperfect immiscibility in the gel state, or both. Although it is quite difficult to accurately identify which effect is dominant, the deviation observed here seems to be attributable mainly to the latter reason, because, as will be described later, the distribution of Chol within the binary membrane in the gel state is expected to be rather heterogeneous, suggesting that the Chol and the DHPC molecules are partially miscible even in the gel state.
A notable feature of the Xch dependence of ΔT1/2 (Fig. 3(c)) is that the dependence drastically changes in the vicinity of Xch = 0.15: the ΔT1/2 value varied very slightly with Xch below that Chol concentration, while it increased steeply with increasing Xch above that concentration. As described above, the drastic change in the Xch dependence of ΔT1/2 is due to the fact that the broad component peak becomes relatively dominant as compared to the sharp component peaks at Xch ≥ ca. 0.15. Since ΔT1/2 is inversely related to the cooperativity of the transition in general [23, 25], the steep increase in ΔT1/2 at Xch ≥ ca. 0.15 indicates that the intermolecular cooperativity at the chain-melting transition is markedly reduced with increasing Xch in the higher Xch region. As seen from Fig. 3(c), this is a common feature to the binary membranes of DHPC–, DPPC–, and DSPC–Chol. In our previous studies [14, 15], it has been demonstrated that similar behavior is also observed for the other Chol-containing binary bilayers of CnPCs (n = 14, 15, and 17) in addition to DPPC (i.e., C16PC) and DSPC (i.e., C18PC), and we found that the Lo phase begins to be formed within the binary bilayer as one of coexisting two phases at this common Xch value of ca. 0.15 on the T–Xch phase diagrams. This is also the case for the DHPC–Chol binary membrane, as will be confirmed later on the phase diagram.
In contrast to the qualitative similarity, an obvious difference in the values of ΔT1/2 can be seen in the higher Xch region: the ΔT1/2 value increased to more than 25 °C for the DHPC–Chol binary membrane, while the main transition was abolished in the DPPC– and the DSPC–Chol binary bilayer before it reached 15 °C. This indicates that the incorporation of Chol produces much greater degree of reduction in the cooperativity of the main transition in the DHPC membrane than in the DPPC and the DSPC bilayers. The cooperativity of the main transition is a measure of how fast or how far the effect of the trans–gauche conformational change will propagate within the membrane when one constituent phospholipid molecule underwent the conformational change. In the case of binary membranes in a two-phase state, therefore, it would depend on long-range order of the lateral organization of the binary membrane rather than short-range molecular interactions including interactions between the same species. Therefore, the difference in the ΔT1/2 value may suggest that there is some difference in the size or morphology of the individual phase regions within the binary membrane in the two-phase state.
Prodan fluorescence
Figure 4(a) shows the Prodan fluorescence spectra F(λ) (λ: wavelength) obtained for the DHPC membrane at different temperatures ranging from 5 to 50 °C. Each spectrum had a unique local maximum in the λ-range of 400–600 nm and the wavelength at the emission maximum (λmax) slightly varied depending on the temperature. In our previous studies [19, 26,27,28,29,30], it has been shown for bilayer membranes of several ester-linked saturated PCs that there is a good correlation between the λmax value and the membrane phase state: λmax = 480–490 nm for the Lα phase, 430–440 nm for the Lβ′ (or Lβ), and the Pβ′ phase and 500–510 nm for the LβI phase. As seen from Fig. 4(a), Prodan in the DHPC membrane exhibited similar fluorescence behavior: the emission maximum can be seen at 500 nm (i.e., LβI phase) below the pretransition temperature (ca. 34 °C) while that can be seen at 490 nm (i.e., Lα phase) above the main-transition temperature (ca. 44 °C). On the other hand, the λmax value for the Pβ′ phase (488 nm) was much higher compared to the case for the ester-linked saturated PC bilayers. This is presumably due to the difference in the location of the Prodan molecule along the depth of the bilayer membrane: it is located around the phosphate group in the DHPC membrane, while it exists around the glycerol backbone in the bilayer membranes of ester-linked PCs [22].
To clarify the relationship between the variation in λmax and the phase state of the DHPC membrane, the temperature dependence of λmax is shown in Fig. 4(b). The λmax value steeply decreased from ~500 to ~490 nm around the pretransition temperature and very slightly increased at the main-transition temperature, which means that these transition temperatures can be determined from the variation in λmax for the DHPC membrane. In our previous studies, the intensity ratio of F(430)/F(490) was also used for the observation of the bilayer phase transitions of ester-linked saturated PCs [15]. Similarly, the temperature dependence of this ratio for the DHPC membrane is shown in Fig. 4(c). The ratio increased at the pretransition temperature and decreased at the main-transition temperature, indicating that this ratio is also applicable to the detection of the phase transitions of the DHPC membrane. Although the characteristic λmax value of the LβI phase is ca. 500 nm, we used the ratio F(430)/F(490), and not F(500)/F(490), as an index for the detection of the bilayer transition, because the two wavelengths of 490 nm and 500 nm are too close as compared to the width of each fluorescence peak.
Figure 5 shows the Prodan fluorescence spectra obtained at different temperatures for the DHPC–Chol binary membranes with various Chol concentrations. At Xch = 0.028, the binary membrane exhibited relatively complicated spectral change with increasing temperature (Fig. 5(a)). The spectrum obtained at 5 °C showed two local maxima at ca. 430 and ca. 500 nm, suggesting that this spectrum consists of two elementary spectra superposed, each of which has a maximum in the vicinity of 430 or 500 nm (curve 1 in Fig. 5(a)). It is known that Chol tends to induce an Lβ-like phase, in which the hydrocarbon chains of the constituent phospholipids are oriented almost parallel to the bilayer normal [31]. Considering that the characteristic λmax value of the Lβ phase is 430–440 nm, the appearance of the local maximum at ca. 430 nm indicates that the Lβ phase was partially formed in the binary membrane. The intensities at 430 and 500 nm increased and decreased with increasing temperature, respectively (curves 2–5), and eventually, at 30 °C the component peak at ca. 500 nm was detected as a shoulder peak (curve 6). At 31 °C, the shape of the spectrum changed very slightly (curve 7). This spectral change appears to be brought about by the shift of the component peak at ca. 500 nm to slightly lower wavelengths. Taking into account the fact that the λmax shift from 500 to 488 nm occurs at the pretransition in the pure DHPC membrane, this slight spectral change is presumably due to the pretransition of the binary membrane. The subsequent increase in the temperature up to 35 °C produced almost no change in the shape of the spectra, but above that temperature the intensities at 430 nm and 490 nm gradually decreased and increased with increasing temperature, respectively. The λmax value abruptly shifted from ca. 430 to ca. 490 nm into the temperature range of 39–43 °C, and finally, the spectra with a single peak at ca. 490 nm were observed above 43 °C. Since the λmax value of 490 nm is characteristic of a disordered phase like the Lα phase, this spectral change is attributable to the fact that the binary membrane underwent the main transition in this temperature range. On the other hand, there was no obvious variation in λmax during the spectral change due to the pretransition, and so, the pretransition temperature could not be determined from the temperature dependence of λmax. However, it can be estimated from the temperature dependence of the ratio of F(430)/F(490), where the ratio reaches maximum in the vicinity of the temperature at which the spectral change due to the pretransition was observed.
Prodan emission spectra F(λ) obtained at different temperatures for DHPC–Chol binary membranes at various Chol concentrations (Xch): (a) Xch = 0.028, (b) 0.077, (c) 0.15, (d) 0.24, (e) 0.40, (f) 0.54. Note that curves 6 and 7 in (a) are shown twice to clarify the spectral change accompanying the pretransition. The temperatures for curves in (c)–(f) are as follows: (c): (1) 5.0 °C, (2) 20.0, (3) 30.1, (4) 41.0, (5) 42.0, (6) 43.1, (7) 44.1, (8) 45.0, (9) 46.0, (10) 48.0, (11) 50.0, (12) 55.1; (d): (1) 30.0 °C, (2) 35.1, (3) 40.0, (4) 43.0, (5) 45.1, (6) 47.0, (7) 50.0, (8) 52.0, (9) 55.0, (10) 60.0, (11) 65.0, (12) 70.0; (e) and (f): each spectrum was obtained at every 5 ± 0.1 °C from (1) 30.0 °C to (11) 80.0 °C . In (c)–(f), only the first and the last number are given to designate the respective spectra because of the limited space, but the spectral change with temperature is monotonic (i.e., from top (1) to bottom (11 or 12))
At Xch = 0.077, as shown in Fig. 5(b), the component peak at ca. 500 nm observed at lower temperatures was detected only as a small shoulder peak even at 5 °C and became further smaller with increasing temperature. However, the overall spectral change with increasing temperature observed for this binary membrane was qualitatively similar to that for the binary membrane of Xch = 0.028, though the spectral change by the pretransition could not be recognized. At Xch = 0.15, the component peak at ca. 500 nm was no longer observed at any temperature (Fig. 5(c)), suggesting that the pretransition was completely abolished at this Chol concentration. In addition, the spectral change with increasing temperature became relatively simple: a spectrum with a single peak at ca. 430 nm was observed at 5 °C, the intensities at 430 and 490 nm decreased and increased gradually with increasing temperature, respectively, λmax shifted to higher wavelengths in the temperature range of ca. 41–48 °C, and finally spectra with a single peak at ca. 490 nm were observed at temperatures above that temperature range. Similar spectral changes were observed for the binary membranes with higher Chol concentrations, as shown in Fig. 5(d, e), though the temperature range in which λmax shifts from ca. 430 to ca. 490 nm moved to higher temperatures as Xch increased. Finally, the λmax shift to 490 nm was not observed at Xch = 0.54 in the temperature range up to 80 °C (Fig. 5(f)), suggesting that the binary membrane no longer exhibits a distinct phase transition from an ordered state to a disordered state at that higher Chol concentration.
Figure 6 shows the temperature dependence of λmax and F(430)/F(490) in the temperature range of 20–80 °C for the DHPC–Chol binary membranes with various Chol concentrations. Although two local maxima were observed at ca. 430 and ca. 500 nm in the Prodan fluorescence spectra below 30 °C at Xch = 0.028, we determined the λmax value as a unique index according to the definition of λmax. Therefore, the λmax values for Xch = 0.028 are approximately 430 nm in the temperature range between 20 and 30 °C in Fig. 6(a). After the λmax value increased very slightly at 31 °C, it remained almost constant up to 37 °C, and then, it started to increase and reached almost 490 nm at 43 °C. The slight increase in λmax at 31 °C corresponds to the slight spectral change due to the pretransition (see Fig. 5(a), curves 6 and 7), but this λmax change is not a typical behavior of the pretransition of this binary membranes. In fact, no characteristic behavior of λmax indicative of the pretransition was found in the temperature dependence of λmax at any Xch above 0.028, although it was indicated by the DSC results that the pretransition persists as high as Xch = 0.10. This means that it is impossible to determine the pretransition temperature for the binary membranes at Xch > 0.028 on the basis of the temperature dependence curve of λmax. On the other hand, the steep increase in λmax to ca. 490 nm is a characteristic change observed commonly for many lipid bilayers when the main transition occurs. Therefore, this result indicates that the binary membrane of Xch = 0.028 exists in a disordered state (i.e., Ld phase) above 43 °C.
Temperature dependence of (a) the wavelength at the maximum emission (λmax) and (b) the intensity ratio F(430)/F(490) for DHPC–Chol binary membranes at various Chol concentrations (Xch). In the inset in (b), the temperature dependence curves of d(F(430)/F(490))/dT, i.e., the derivative of F(430)/F(490) with respect to T, are given for (4) Xch = 0.15, (5) 0.19, (6) 0.24, and (10) 0.54. Curves 6 and 10 have been magnified in the y-axis direction to improve visibility. The magnification factors are given on the right hand side
At Xch = 0.077, the abrupt λmax shift was also clearly observed at 41 °C; subsequently, the λmax value increased gradually with the temperature rise and finally reached the plateau at 45 °C. Almost similar behavior was observed at Xch = 0.11, but the gradual increase in the λmax value subsequent to the abrupt λmax shift became more obvious, which seems to indicate that the conversion from an ordered state to a disordered state is no longer a discontinuous change like a phase transition but a more continuous process taking place in a finite temperature range. At Xch = 0.15, this process-like λmax behavior can be seen more clearly: the λmax value started to increase comparatively gradually at 42 °C and reached the plateau at ca. 49 °C through the abrupt change at 45 °C and the subsequent gradual increase. As seen from Fig. 6(a), the initial increase in λmax prior to the abrupt λmax shift became markedly more gradual with increasing Xch, and as a result, the temperature at which the λmax value reaches the plateau shifted considerably to higher temperatures with increasing Xch. This means that the temperature range where the binary membrane can exist in a completely disordered state shifts to higher temperatures as Xch increases. Finally, at Xch = 0.54, the λmax shift to ca. 490 nm was not observed in the temperature range up to 80 °C, which suggests that the binary membrane no longer exhibits the conversion from the ordered state to the disordered state at that higher Chol concentration. This is consistent with the DSC results which indicates that the DHPC–Chol binary membrane no longer exhibits the main transition above Xch = 0.50.
The temperature dependence of the intensity ratio F(430)/F(490) and its derivative d(F(430)/F(490))/dT also provides the information about the Xch-dependent behavior of not only the main-transition temperature but also the pretransition temperature (Fig. 6(b)). At Xch = 0.028, initially, the F(430)/F(490) value increased gradually with increasing temperature and reached the maximum at 31 °C. This temperature is equal to the temperature at which the slight spectral change due to the pretransition was observed, and also comparable to the pretransition temperature determined from the DSC thermogram at Xch = 0.03. This suggests that the pretransition temperature can be estimated from the temperature dependence of F(430)/F(490) though the pretransition could not be clearly identified from the spectral change. As Xch increased, the temperature at the maximum intensity ratio decreased, and finally, no maximum was found at an intermediate temperature at Xch above ca. 0.12, which may suggest that the pretransition is abolished above that Chol concentration. This also agrees well with the DSC result which indicates that the pretransition is abolished above Xch = ca. 0.10.
On the other hand, the main transition was detected more clearly as a steep decrease in the intensity ratio. At Xch = 0.028, the intensity ratio started to decrease gradually with increasing temperature after it reached the maximum, and a relatively steep decrease in the ratio was observed at 42–43 °C, which signifies that the binary membrane underwent the main transition around this temperature. This temperature was in agreement with the temperature determined from the temperature dependence of λmax and that from the DSC thermogram. The further increase in temperature above the main-transition temperature produced only a very slight decrease in the intensity ratio. The temperature of the steep decrease in the intensity ratio shifted very slightly to lower temperatures (at most 2 °C) with increasing Xch up to ca. 0.06, but the further increase in Xch produced virtually no effect on the temperature until Xch exceeded 0.20. In addition, the decrease in the intensity ratio tended to be less steep progressively with increasing Xch, and as a result, the intensity ratio decreased more continuously over the whole temperature range at higher Xch, especially above ca. 0.20, which made it difficult or almost impossible to determine the transition temperature definitely. Hence, we computed the derivative of the intensity ratio with respect to the temperature, d(F(430)/F(490))/dT, to determine the transition temperature uniquely as a peak temperature, as shown in the inset of Fig. 6(b). The temperature dependence curve of the derivative exhibited a similar change in the line shape with increasing Xch to that observed for a series of DSC thermograms.
Discussion
Effect of Chol incorporation on the formation of interdigitated structure
On the basis of the above DSC and Prodan fluorescence results, we can discuss the Xch-dependent phase behavior of the DHPC–Chol binary membrane. Figure 7(a) shows the Prodan fluorescence spectra obtained at 20 °C (± 0.1 °C) for the binary membranes with different Chol concentrations within the Xch range from 0 to 0.107. Each spectrum shown in this figure is the normalized spectrum F°(λ) with respect to the integrated intensity:
(a) Normalized spectra of Prodan fluorescence for a series of DHPC–Chol binary membranes varying in Chol concentration (Xch). (b) Chol concentration (Xch) dependence of proportionality factor ξ(Xch) (circles). Relative enthalpy change with the pretransition ΔHp/ΔHp,0, obtained from DSC measurements, is also plotted as a function of Xch (crosses). The solid line represents the theoretical curve of Eq. (7) with K1 = 19 and K2 = 2.2
It can be clearly seen that the peak intensity at 500 nm decreases and that at 430 nm increases with increasing Xch. Since these wavelengths correspond to the λmax value characteristic of the membrane phase states of LβI and Lβ, this Xch-dependent spectral change can be considered to indicate that the conversion from the LβI phase to the Lβ phase gradually proceeds with increasing Xch and is almost completed at Xch = 0.107 and that the LβI and the Lβ phase coexist within the binary membrane at intermediate Chol concentrations within this Xch range. The previous small-angle X-ray scattering study [21] has also demonstrated that two different regions with different thicknesses (i.e., interdigitated and non-interdigitated lamellar gel phase) coexist in the Chol-containing binary membrane of DHPC at low Chol concentrations. With respect to this two-phase coexistence, this figure shows another important feature: there is an isoemissive point at ca. 468 nm, namely the point at which all the spectra cross one another. This feature indicates the possibility that each spectrum can be represented as the superposition of the spectra at Xch = 0 and 0.107 with a proportionality factor ξ(Xch) (0 ≤ ξ(Xch) ≤ 1):
Considering this possibility, we compared the calculated spectra based on this equation with the experimental spectra and found that the relative difference between both spectra is less than ca. 5%. This agreement means that the observed Xch-dependent spectral change is due only to the variation in the proportionality factor, which allows us to discuss the Xch-dependent conversion from the LβI phase to the Lβ phase from a quantitative aspect.
A similar Xch-dependent spectral change was observed at any temperature measured in the range of 5–30 °C, and thus we determined the ξ(Xch) value at each Xch as the average value of all the ξ(Xch) values obtained in this temperature range. In Fig. 7(b), the ξ(Xch) value thus determined are plotted against Xch. In this figure, the error bars represent the full range of the variation in the ξ(Xch) values within that temperature range. The ξ(Xch) value decreased monotonically with increasing Xch in such a manner that the slope of the decrease in ξ(Xch) becomes smaller with increasing Xch. Assuming that the distribution of the Prodan molecules within the binary membrane is uniform regardless of the membrane phase state [32], the ξ(Xch) value can be regarded as equivalent to the ratio occupied by the LβI phase in the whole binary membrane. Therefore, this behavior of ξ(Xch) indicates that the LβI/Lβ conversion induced by the incorporation of Chol gradually diminishes as Xch increases. In Fig. 7(b), ΔHp/ΔHp,0, namely enthalpy change of the pretransition (i.e., ΔHp) normalized by that at Xch = 0 (i.e., ΔHp,0), is also plotted against Xch. Similar Xch dependence was seen for ΔHp/ΔHp,0, though the plots were somewhat scattered. Since ΔHp is the enthalpy change accompanying the transformation of the membrane state from the LβI phase to the Pβ′ phase, this Xch dependence of ΔHp/ΔHp,0 also supports that the region occupied by the LβI phase decreases with increasing Xch in a similar non-linear manner.
This non-linear behavior of the Chol-induced LβI/Lβ conversion can be roughly explained by a simple process of the Chol incorporation based on the following three assumptions. First, when a small amount of Chol is incorporated into the binary membrane in a two-phase state at a certain Xch, the additional Chol molecules are partitioned into either LβI or Lβ region without any phase preference, and the amounts of the Chol incorporated into the LβI and the Lβ region are proportional to ξ(Xch) and (1 − ξ(Xch)), respectively. Second, the Chol molecules partitioned into the LβI region induce the conversion from the LβI state to the Lβ phase and the extent of the LβI/Lβ conversion is in proportion to the amount of Chol partitioned at any Xch. Third, the Chol molecules partitioned into the Lβ region expand the Lβ region in proportion to the amount of Chol partitioned at any Xch. According to this simple probabilistic process for the Chol incorporation, the variation in ξ(Xch) with a small increment in Chol molecules (dnch) can be described by the following equation:
Here, k1 and k2 denote the proportional constants in the second and the third assumption, respectively. Since the following relation is obtained by differentiating Eq. (1)
ξ(Xch) can be expressed as a function of Xch as follows:
Here, K1 = nDHPCk1 and K2 = nDHPCk2. Note that in this process, nDHPC is constant and that the constants K1 and K2 are dimensionless. The calculated curve of ξ(Xch) based on this equation with K1 = 19 and K2 = 2.2 is shown by a solid line in Fig. 7(b). The calculated curve agrees well with the experimental data, which provides us with quantitative information about the Chol-induced LβI/Lβ conversion. First, the tangent line at Xch = 0 intersects with the Xch-axis at Xch = 0.0526 (= 1/K1). This means that the first Chol molecule incorporated into the DHPC membrane, which is entirely in the LβI state, has the LβI/Lβ conversion effect on 18 DHPC molecules around the Chol molecule. Second, the calculated curve of ξ(Xch) becomes 0 at Xch = 0.114. This Chol concentration approximately corresponds to the average molar ratio of 1:8 (Chol/DHPC), and thus, the average molar ratio of Chol in the overall Lβ region increases continuously from 1:18 at Xch~0 to ca. 1:8 at Xch = 0.114 as Xch increases. If the process of Chol incorporation we assumed is true, the distribution of the Chol molecules in the overall Lβ region will be heterogeneous at intermediate Xch. As expected from the K1 and the K2 value, the Chol molecules incorporated into the Lβ region and those into the LβI region make different contributions to the expansion of the overall Lβ region, and thus, there can exist microregions or microdomains of the Lβ phase with different molar ratios of Chol within the whole binary membrane. Finally, when the entire binary membrane is occupied by the Lβ region at Xch = 0.114, the binary membrane totally becomes a bilayer and no longer undergoes the pretransition.
T–Xch phase diagram of DHPC–Chol binary membrane
We now discuss the overall phase behavior of the DHPC–Chol binary membrane. Figure 8(a) shows the T–Xch phase diagram of the DHPC–Chol binary membrane constructed on the basis of the DSC data (i.e., peak temperature) and the Prodan fluorescence data. For comparison, the T–Xch phase diagram of DPPC–Chol binary bilayer reported in the previous study [15] is also shown in Fig. 8(b). In the previous study [15], we interpreted the phase diagram of the DPPC–Chol binary bilayer on the basis of the general thermodynamic knowledge about the solid/liquid phase equilibria of peritectic binary mixtures. We applied the same thermodynamic concept to the phase diagram of the DHPC–Chol binary membrane to perform the phase assignment: A, Ld phase; B, Ld + Pβ′; C, Pβ′ + Lβ (1:8); D, LβI + Lβ (1:8); E, Ld + Lβ (1:6); F, Lβ (1:8) + Lβ (1:6); G, Ld + Lo; H, Lβ (1:6) + Lo; I, Lo. Here, the plus sign means two-phase coexistence. The ratio in parentheses denotes the average molar ratio of Chol to DHPC, and the Lβ (1:8) phase and the Lβ (1:6) phase are considered to be thermodynamically different phases.
(a) Temperature (T)–Chol concentration (Xch) phase diagram of DHPC–Chol binary membrane. Phase assignment is as follows: (A) Ld phase, (B) Ld + Pβ′, (C) Pβ′ + Lβ (1:8), (D) LβI + Lβ (1:8), (E) Ld + Lβ (1:6), (F) Lβ (1:8) + Lβ (1:6), (G) Ld + Lo, (H) Lβ (1:6) + Lo, (I) Lo. Here, the plus sign means the two-phase coexistence. (b) T–Xch phase diagram of DPPC–Chol binary bilayer constructed in our previous study [15] is also shown for comparison. Phase assignment is the same as above except the followings: (C) Pβ′ + Lβ (1:12), (D) Lβ′ + Lβ (1:12), (F) Lβ (1:12) + Lβ (1:6). Circle and square symbols represent the data from the DSC and the Prodan fluorescence measurements, respectively
As a common feature, the phase diagrams of these binary membranes suggest the presence of a characteristic point like a peritectic point at Xch = ca. 0.15. In general, a peritectic point occurs as a result of incongruent melting, and this type of melting behavior is usually observed for binary systems where the two components react to produce a solid compound with a specific stoichiometry that is stable in a solid state but unstable in a liquid state. It is therefore suggested that the Chol molecules tend to form complexes or molecular clusters with surrounding DHPC molecules within the binary membrane. In addition, the stoichiometry of the complex is estimated at 1:6 (Chol/DHPC) from the fact that the peculiar point like a peritectic point was observed at Xch = ca. 0.15 (the ratio of 1:6 is equivalent to Xch = 0.143). The same result has been also found for the other Chol-containing binary bilayers of CnPC (n = 14, 15, 17, 18) [13,14,15]. This is very interesting because it is expected that the strength or mode of the interaction between the Chol and constituent phospholipid molecules should more or less change when the molecular structure of the constituent phospholipid varies. It has been reported that Chol molecules tend to be distributed regularly within the binary bilayer of CnPCs [33,34,35] and the distribution is well represented with the hexagonal lattice (i.e., superlattice view [16]). Accordingly, the 1:6 complexes of Chol and phospholipids would be a weak unit occurring as a result of the regular distribution of Chol within the binary membrane rather than a rigid complex resulting from some specific or more direct Chol–phospholipid interaction.
On the other hand, the phase behavior of the DHPC– and the DPPC–Chol binary membrane is different in respect of the minimum Chol concentration at which the Lo phase is formed as a single phase throughout the binary membrane: Xch = ca. 0.5 for DHPC and Xch = ca. 0.33 for DPPC. The Lo phase is often characterized as the membrane state where the chain-melting transition no longer occurs and this is thought to be due to the suppression of the trans–gauche conformational change in the hydrocarbon chains of the constituent phospholipids by the rigid Chol molecules existing adjacent to them. Accordingly, the minimum Xch value of the entire formation of the Lo phase is equivalent to Xch*main. In the previous study [15], the same difference in the Xch*main value was observed between the binary bilayer of DSPC (i.e., C18PC) and those of the other CnPCs (n = 14–17). We speculated that this difference is attributable to the mismatch in the effective length of the hydrophobic region between Chol and the constituent phospholipid in the binary membrane on the basis of the fact that the hydrophobic mismatch is minimized at the chain length of 17 [31, 36].
The difference in the Xch*main value between the binary membranes of DHPC and DPPC could also be explained in terms of the hydrophobic mismatch at the bilayer midplane, although the hydrocarbon-chain length of DHPC is equal to that of DPPC. It has been reported that the Chol molecules reside within the bilayer such that their hydrophobic part (i.e., the four sterol rings and the side chain) is buried into the hydrophobic core of the bilayer with its 3β-OH group protruding into the hydrophilic region of the bilayer [37,38,39]. In the case that the constituent phospholipid is DPPC, this position of the Chol molecule in the depth direction within the bilayer is expected to be stabilized by hydrogen bonding between the 3β-OH group and the carbonyl oxygen at the glycerol backbone of an adjacent DPPC molecule. Meanwhile, in the case of DHPC, it is expected that the Chol molecule could alter its vertical position within the membrane relatively less restrictedly as compared to the case of the DPPC bilayer, because DHPC is not capable of hydrogen bonding with the 3β-OH group of the neighboring Chol molecule at the glycerol backbone owing to the absence of carbonyl oxygens. Consequently, the effect of the hydrophobic mismatch at the bilayer midplane is expected to be more or less reduced in the DHPC membrane, which would result in the higher Xch*main value. This would be supported by the DSC data suggesting that the Chol–phospholipid interaction is weaker within the binary membrane of DHPC than within that of DPPC (or DSPC).
In the last place, we discuss the comparison with a phase diagram of the same system given in the previous study by Laggner et al. [21]. Their phase diagram was also constructed on the basis of DSC data, but the phase boundary was determined as the onset and the completion temperatures of the main-transition endothermic peaks. The loop-like boundary thus obtained was explained from the viewpoint of a typical liquid–liquid phase separation due to a miscibility gap. This explanation logically implies that DHPC and Chol are miscible within the binary membrane to form a homogeneous phase even in the gel state (i.e., below the main-transition temperature). In fact, there is no indication about the phase states in their phase diagram, although the phase boundaries of the pretransition and even the subtransition (i.e., transition from the lamellar crystal (Lc) phase to the LβI) are provided. Therefore, our phase diagram, suggesting solid–liquid phase equilibria, is utterly different from their phase diagram. In other words, the major difference between their and our phase diagram would arise from the difference in the thermodynamic interpretation of the phase behavior of the binary membrane rather than from the experimental results themselves.
This is evident from the fact that our phase diagram looks more similar to the phase diagram re-constructed by Marsh [17] in his comprehensive work, which is based on the same data reported in the original study by Laggner et al. [21]. His re-constructed phase diagram suggests that the DHPC–Chol binary membrane basically exhibits eutectic melting behavior, and then, there are several two-phase states in the gel state depending on the Chol concentration, which is similar to our phase diagram. The essential difference between his and our phase diagram lies in the difference of whether the melting behavior that the binary membrane exhibits is eutectic or peritectic, as long as some quantitative differences are ignored. In this regard, we need to perform further investigation to obtain more direct or strong evidence for the presence of the 1:6 complex of Chol and DHPC within the binary membrane that we propose on the basis of the present and our previous studies on the Chol-containing binary membranes [13,14,15].
Conclusions
In this study, we performed DSC and Prodan fluorescence spectroscopic measurements to investigate the thermotropic phase behavior of the DHPC–Chol binary membrane as a function of Xch. The DSC measurements demonstrated that the thermal behavior of the DHPC–Chol binary membrane is different in several respects compared to that of the DPPC–Chol binary bilayer. First, the main-transition enthalpy decreased to 0 at Xch = ca. 0.50 for the DHPC–Chol binary membrane, while it decreased almost linearly with increasing Xch and reached 0 at Xch = ca. 0.33 for the DPPC–Chol binary bilayer. This Xch dependence of ΔHm for the DHPC–Chol binary membrane suggests weaker interaction between DHPC and Chol as compared to that between DPPC and Chol. Second, the increase in ΔT1/2 with increasing Xch above ca. 0.15 was strikingly larger than that observed for the DPPC–Chol binary bilayer. This difference would reflect the difference in the long-range order of the membrane lateral organization between the DHPC– and the DPPC–Chol binary membrane. Prodan fluorescence spectroscopy was successfully applied to the observation of the phase transitions of the DHPC–Chol binary membranes. The Prodan fluorescence spectra changed depending on the membrane phase states, and the spectral change was basically characterized by the λmax value and the intensity ratio of F(430)/F(490). Particularly, the two-phase state of the LβI and the Chol-induced Lβ phase could be sensitively detected as the superposition of the two elementary spectra corresponding to the respective phase states. By introducing a proportionality factor ξ(Xch), we could analyze the Xch-dependent spectral change due to the LβI/Lβ conversion induced by the Chol incorporation, and we found that the incorporation of Chol into the binary membrane in the two-phase state can be explained as a simple probabilistic process. Our calculation revealed that in this process, one Chol molecule incorporated into the LβI region has the effect of inducing the Lβ region on its surrounding 18 DHPC molecules.
On the basis of the DSC and Prodan fluorescence data, we constructed the T–Xch phase diagram for the DHPC–Chol binary membrane to compare it with that of the DPPC–Chol binary membrane. A difference was observed in the minimum Xch value for the entire formation of the Lo phase. This difference could be explained in terms of the hydrophobic mismatch at the bilayer midplane. We can finally conclude that the degree or extent of the Chol effect on the membrane phase behavior is pronouncedly affected by the difference in the chemical structure between DHPC and DPPC, namely the difference in the capability of hydrogen bonding with the 3β-OH group of Chol due to the presence or absence of the carbonyl oxygens at the glycerol backbone.
References
Lewis RNAH, Mak N, McElhaney RN (1987) A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry 26:6118–6126
Ichimori H, Hata T, Matsuki H, Kaneshina S (1998) Barotropic phase transitions and pressure-induced interdigitation on bilayer membranes of phospholipids with varying acyl chain-lengths. Biochim Biophys Acta 1414:165–174
Ruocco MJ, Siminovitch DJ, Griffin RG (1985) Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry 24:2406–2411
Laggner P, Lohner K, Degovics G, Müller K, Schuster A (1987) Structure and thermodynamics of the dihexadecylphosphatidylcholine–water system. Chem Phys Lipids 44:31–60
Koynova R, Koumanov A, Tenchov B (1996) Metastable ripple gel phase in saturated phosphatidylcholines: calorimetric and densitometric characterization. Biochim Biophys Acta 1285:101–108
Maruyama S, Matsuki H, Ichimori H, Kaneshina S (1996) Thermotropic and barotropic phase behavior of dihexadecylphosphatidylcholine bilayer membrane. Chem Phys Lipids 82:125–132
Kim JT, Mattai J, Shipley GG (1987) Gel phase polymorphism in ether-linked dihexadecylphosphatidylcholine bilayers. Biochemistry 26:6592–6598
Gawrisch K, Ruston D, Zimmerberg J, Parsegian A, Rand RP, Fuller N (1992) Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys J 61:1213–1223
Matsuki H, Miyazaki E, Sakano F, Tamai N, Kaneshina S (2007) Thermotropic and barotropic phase transitions in bilayer membranes of ether-linked phospholipids with varying alkyl chain lengths. Biochim Biophys Acta 1768:479–489
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Ipsen JH, Karlström G, Mouritsen OG, Wennerström H, Zuckermann MJ (1987) Phase equilibria in the phosphatidylcholine–cholesterol system. Biochim Biophys Acta 905:162–172
Vist MR, Davis JH (1990) Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry 29:451–464
Tamai N, Uemura M, Takeichi T, Goto M, Matsuki H, Kaneshina S (2008) A new interpretation of eutectic behavior for distearoylphosphatidylcholine–cholesterol binary bilayer membrane. Biophys Chem 135:95–101
Tamai N, Uemura M, Goto M, Matsuki H, Kaneshina S (2008) Lateral phase separation in cholesterol/diheptadecanoylphosphatidylcholine binary bilayer membrane. Colloids Surf B Biointerfaces 65:213–219
Tamai N, Izumikawa T, Fukui S, Uemura M, Goto M, Matsuki H, Kaneshina S (2013) How does acyl chain length affect thermotropic phase behavior of saturated diacylphosphatidylcholines–cholesterol binary bilayers? Biochim Biophys Acta 1828:2513–2523
Somerharju P, Virtanen JA, Cheng KH (1999) Lateral organisation of lipids: the superlattice view. Biochim Biophys Acta 1440:32–48
Marsh D (2010) Liquid-ordered phases induced by cholesterol: a compendium of binary phase diagrams. Biochim Biophys Acta 1798:688–699
Chong PLG (1988) Effects of hydrostatic pressure on the location of PRODAN in lipid bilayers and cellular membranes. Biochemistry 27:399–404
Kusube M, Matsuki H, Kaneshina S (2005) Effect of pressure on the Prodan fluorescence in bilayer membranes of phospholipids with varying acyl chain lengths. Colloids Surf B Biointerfaces 42:79–88
Hénin J, Chipot C (2006) Hydrogen-bonding patterns of cholesterol in lipid membranes. Chem Phys Lett 425:329–335
Laggner P, Lohner K, Koynova R, Tenchov B (1991) The influence of low amounts of cholesterol on the interdigitated gel phase of hydrated dihexadecylphosphatidylcholine. Chem Phys Lipids 60:153–161
Kusube M, Goto M, Tamai N, Matsuki H, Kaneshina S (2007) Effect of pressure on the Prodan fluorescence in bilayer membrane of ether-linked lipid, dihexadecylphosphatidylcholine. High Press. Biosci. Biotechnol.: Proc. 4th Int. Conf. High Press. Biosci. Biotechnol 1:61–67
Yeagle PL (2005) The roles of cholesterol in the biology of cells. In: Yeagle PL (ed) The structure of biological membrane 2nd edn. CRC Press, London Chapter 2
Dufourc EJ, Parish EJ, Chitrakorn S, Smith ICP (1984) Structural and dynamical details of cholesterol-lipid interaction as revealed by deuterium NMR. Biochemistry 23:6062–6071
Tsong TY (1974) Kinetics of the crystalline–liquid crystalline phase transition of dimyristoyl L-α-lecithin bilayers. Proc Natl Acad Sci U S A 71:2684–2688
Kusube M, Tamai N, Matsuki H, Kaneshina S (2005) Pressure-induced phase transitions of lipid bilayers observed by fluorescent probes Prodan and Laurdan. Biophys Chem 117:199–206
Goto M, Kusube M, Tamai N, Matsuki H, Kaneshina S (2008) Effect of hydrostatic pressure on the bilayer phase behavior of symmetric and asymmetric phospholipids with the same total chain length. Biochim Biophys Acta 1778:1067–1078
Goto M, Sawaguchi H, Tamai N, Matsuki H, Kaneshina S (2010) Effect of vesicle size on the prodan fluorescence in diheptadecanoylphosphatidylcholine bilayer membrane under atmospheric and high pressure. Langmuir 26:13377–13384
Goto M, Matsui T, Tamai N, Matsuki H, Kaneshina S (2011) Prodan fluorescence detects the bilayer packing of asymmetric phospholipids. Colloids Surf B Biointerfaces 84:55–62
Goto M, Wilk A, Kataoka K, Chodankar S, Tamai N, Fukui M, Kohlbrecher J, Ito H, Matsuki H (2012) Study on the subgel-phase formation using an asymmetric phospholipid bilayer membrane by high-pressure fluorometry. Langmuir 28:12191–12198
McMullen TPW, Lewis RNAH, McElhaney RN (1993) Differential calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholine. Biochemistry 32:516–522
Matsuki H, Kato K, Okamoto H, Yoshida S, Goto M, Tamai N, Kaneshina S (2017) Ligand partitioning into lipid bilayer membranes under high pressure: implication of variation in phase-transition temperatures. Chem Phys Lipids 209:9–18
Chong PLG (1994) Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc Natl Acad Sci U S A 91:10069–10073
Tang D, van der Meer BW, Chen SYS (1995) Evidence for a regular distribution of cholesterol in phospholipid bilayers from diphenylhexatriene fluorescence. Biophys J 68:1944–1951
Virtanen JA, Ruonala M, Vauhkonen M, Somerharju P (1995) Lateral organization of liquid-crystalline cholesterol–dimyristoylphosphatidylcholine bilayers. Evidence for domains with hexagonal and centered rectangular cholesterol superlattices. Biochemistry 34:11568–11581
McIntosh TJ (1978) The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta 513:43–58
Karolis C, Coster HGL, Chilcott TC, Barrow KD (1998) Differential effects of cholesterol and oxidised-cholesterol in egg lecithin bilayers. Biochim Biophys Acta 1368:247–255
Bhattacharya S, Haldar S (2000) Interactions between cholesterol and lipids in bilayer membranes. Role of lipid headgroup and hydrocarbon chain–backbone linkage. Biochim Biophys Acta 1467:39–53
Khelashvili G, Pabst G, Harries D (2010) Cholesterol orientation and tilt modulus in DMPC bilayers. J Phys Chem B 114:7524–7534
Funding
This work was supported in part by JSPS Grant-in-Aid for Scientific Research (C) (24550157) and also by JSPS Grant-in-Aid for Scientific Research (C) (26410016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tamai, N., Uemura, M., Izumikawa, T. et al. Phase behavior of cholesterol-containing binary membrane of an ether-linked phospholipid, dihexadecylphosphatidylcholine. Colloid Polym Sci 296, 697–711 (2018). https://doi.org/10.1007/s00396-018-4280-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4280-4