Abstract
A sponge phase (L3 phase) was observed in aqueous solution of a nonionic surfactant polyethylene glycol ether of tridecyl alcohol with the average 3 of ethylene oxide (CH3(CH2)12(OCH2CH2)3OH, abbreviated as Trideceth-3) with tetradecyldimethylamino oxide (\( {\mathrm{CH}}_3{\left({\mathrm{CH}}_2\right)}_{13}\overset{\overset{\mathrm{O}}{\uparrow }}{\mathrm{N}}{\left({\mathrm{CH}}_3\right)}_2 \), abbreviated as C14DMAO). The L3 phase can be transferred to planar lamellar phase after the bilayer was protonated by the formic acid formed through the hydrolysis of methylformate. The addition of surface charge into the nonionic L3 phase through electrostatic repulsion on the ionic head groups will suppress the Helfrich undulation and induce the transition to planar lamellar phase. The planar lamellar phase can be transformed into multilamellar vesicles under shear. Rheological properties show that both of the storage modulus and the loss modulus of the lamellar phase were increased with the increment of surface charge density. The phase transition from L3 phase to vesicles was characterized by rheological measurements, 2H NMR spectra, and transmission electron microscope (TEM) observations. To our best knowledge, this is the first example of a controlled phase transition in nonionic surfactant mixtures through protonation and shear forces. The procedure provides a direction on how to achieve phase transition in surfactant solution by changing the conditions and an application of phase transition of controlled materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For mixed systems consisting of small particles and surfactants, it is a tremendous challenge to control the morphology of the colloid aggregates, especially for bilayer membranes. A multitude of self-assembled structures including micelles, nanodiscs, vesicles, liquid crystals, and fibers were produced in surfactant solutions [1,2,3,4,5]. L3 phase, i.e., the so-called sponge phase, is a low viscosity, slightly turbid, and optically isotropic phase which shows flow birefringence [6, 7]. From the structure point of view, L3 phase is a three-dimensional random multiply connected bilayer dividing the spaces into “inside” and “outside” regions [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. A symmetric sponge phase has equal amounts of inside and outside regions; however, in an asymmetric sponge phase, these regions are different in amounts. Freeze-fracture transition electron microscope (FF-TEM) micrographs clearly show that the bilayers are indeed multiply connected and have a saddle-shaped microstructures with a negative Gaussian curvature [16, 23].
The L3 phases in surfactant mixtures have been investigated widely. They consist mainly of ionic surfactant, alcohol (i.e., cosurfactants of alcohols having C4-C8 alkyl), and water (or oil) mixtures in dilute solution [24, 25]. However, Hoffmann et al. investigated nonionic surfactant C14DMAO mixed with hexanol as a cosurfactant to observe sponge phase with increasing hexanol in the spherical aqueous micellar solution of C14DMAO, and observed a phase sequence of vesicle phase (Lαl)-lamellar phase (Lαh) with flat bilayers-sponge phase (L3). This phase sequence is due to an increase in flexibility of the bilayers with increasing amount of the cosurfactant [26, 27]. In addition, other scholars observed a remarkable characteristic behavior of phase transition from L3 to Lα under shear forces in C14DMAO and hexanol mixtures in aqueous solution [17, 28, 29]. Basically, the appearance of a L3 phase is correlated with the presence of a lamellar phase, which strongly indicates that the structure of aggregate is locally formed bilayer membrane. On the basis of detailed diffusion measurements, it was suggested that L3 consists of disordered lamellar structures [30], which along with the bending energy of uncharged nonionic surfactant bilayers can be influenced by various additives. Under the controlled shear strain, orientated lamellar phase can transfer into densely packed onion, leading to a more negative Gaussian curvature modulus of membrane [31,32,33,34,35,36]. Porcar et al. studied the shear-induced transitions by the small angle neutron scattering studies, which complement the electron microscopy studies [37,38,39].
In the present report, L3 phase was observed in aqueous solution of nonionic surfactants Trideceth-3 mixed with nonionic surfactant C14DMAO. Trideceth-3 is a typical nonionic surfactant with a preference to form flat bilayers, which can close to vesicles by introducing charges [40]. Upon the addition of C14DMAO to the aqueous solution of Trideceth-3, the behaviors of phase transition among Lα, L3, and L1 were observed. Interestingly, the phase transition from L3 phase to planar lamellar was observed by introducing charges from formic acid formed by methylformate hydrolysis, but not shear forces in the system, i.e., HCOOCH3 + H2O → HCOOH + CH3OH and \( {\mathrm{CH}}_3{\left({\mathrm{CH}}_2\right)}_{13}\overset{\overset{\mathrm{O}}{\uparrow }}{\mathrm{N}}{\left({\mathrm{CH}}_3\right)}_2 \) + HCOOH → \( {\mathrm{CH}}_3{\left({\mathrm{CH}}_2\right)}_{13}\overset{\overset{{\mathrm{OH}}^{+}}{\uparrow }}{\mathrm{N}}{\left({\mathrm{CH}}_3\right)}_2 \) + HCOO−. Under shear forces, the phase transition from Lαl to vesicle phase was obtained. Our study shed light on our understanding of L3 phase and bilayer morphology.
Experimental
Materials
Trideceth-3 was purchased from SASOL Germany GmbH. It is a biphasic solution at room temperature due to the existence of trace amount of Trideceth-5 and Trideceth-8 and stirred before use. A 70% (w/v) C14DMAO aqueous solution was from Wacker Company, Germany, and purified at least three times using acetone. It was dissolved in deionized water that had been distilled for three times.
Phase diagram
Phase behavior of the nonionic surfactant mixture was studied by visual inspection using crossed polarizers of 400 solution samples. The samples were obtained by dissolving various amounts of C14DMAO and Trideceth-3 in aqueous solution. The solutions were stirred and kept at 25 °C for at least 4 weeks for equilibration.
Conductivity
The conductivity of samples was measured at 25 °C using a conductivity meter DDSJ-308A and a glass electrode DJS-10C (Shanghai Jingke, China).
Rheological measurement
The rheological measurements were performed using a Haake RS6000 rheometer with double-gap sensor (Z41° Ti for low viscosity samples) at temperature controlled within ±0.1 °C by a thermal controller (Haake TC81). The viscosity of samples was determined in amplitude sweep tests conducted in a range of frequency between 0.01 and 10 Hz. All samples for rheological measurements were prepared at least 4 weeks before.
2H NMR
2H NMR spectra were recorded on a Bruker AVANCE 400 spectrometer equipped with pulse field gradient module (Z axis). The samples in which the water was replaced to deuterium oxide were added into a 5-mm BBO probe and the temperature was controlled to ±0.1 °C by a thermal controller for 4 weeks for equilibration.
Transmission electron microscopy
The phase structural transitions from sponge phase, planar lamellar phase to vesicles were characterized by freeze-fracture TEM (FF-TEM) and cryo-TEM observation. For FF-TEM observations, a small part of sample was placed on a 0.1-mm-thick copper disk covered with a second copper disk. This copper sandwich was plunged into liquid propane, which had been cooled by liquid nitrogen, to freeze the sample. Fracturing and replication were carried out at −140 °C on a Balzers BAF-400D equipment. Pt/C was deposited at angle of 45°. The replicas were examined on a JEOL 100CX-II TEM operated at 100 kV. For cryo-TEM observations, specimens were prepared using carbon film grids with hole size between 1 and 12 mm. In brief, one drop of sample solution was placed on a coated TEM grid (copper grid, 3.02 mm, 200 meshes). Most of the liquid was removed with blotting papers to afford a thin film stretched over the holes. The resultant specimens were instantly snap frozen by plunging into liquid ethane in a temperature-controlled freezing unit (Zeiss, Oberkochen, Germany). After removing the remaining ethane using blotting papers, the specimens were inserted into a cryo-transfer holder (Zeiss, Oberkochen, Germany) and transferred to a Zeiss CEM 902 apparatus equipped with a cryo-stage. TEM analysis was conducted with an accelerating voltage of 80 kV at 90 K. Zero-loss filtered images (DE = 0 eV) were recorded under low-dose conditions. The mean lamellarity was determined based on the micrographs of three different areas of the specimen. All vesicles within several holes of the carbon film grid were counted and used to calculate the average number of small and large vesicles.
Results and discussion
Phase behavior and properties of L3 phase
Phase behavior was studied by visual inspection and with crossed polarizers. Figure 1 shows the section of the ternary phase diagram of mixture of C14DMAO, Trideceth-3, and H2O at low surfactant concentrations. One can see that the phase diagram exhibits a number of phases and transitions, including L1, vesicle, vesicle/L1, planar lamellar/L1, and L3 phases. The formation of a two-phase system can be ascribed to the coexistence of different aggregates and the different surfactant compositions, as well as the triggered difference of solution density between the upper and the lower phase. L3 phase was in a very narrow region and located in the Trideceth-3 rich region at Trideceth-3 concentration of 2.75–7%.
Phase diagram of C14DMAO/Trideceth-3/H2O system at 25.0 ± 0.1 °C. At low C14DMAO concentration, the solutions undergo transition from L3 phase to biphase planar lamellar (Lαl)/L3 and to planar lamellar phase with domain-like birefringence in a very narrow region, as shown as the inset on the right corner. With the increment of C14DMAO, the solutions undergo transition from birefringent vesicle phase (Lαv) to biphase vesicle (Lαv)/L1 and to L1 phase in a broad region
The samples at L3 phase can be prepared according to the phase diagram in Fig. 1. The sample is a little bluish, low viscosity, isotropic, and has flow birefringence. When samples at L3 phase were sheared violently, the instantaneous birefringence can be observed more than 1 h later. Figure 2 shows a typical diagram of the conductivity changing with time after samples at L3 phase were vigorously sheared. Without shear, the conductivity was about 67 μS cm−1. After shear, the conductivity decreased to about 10 μS cm−1. After stopping the shear process, the conductivity increased gradually to its original value. It is considered that upon shear, the bilayers can be oriented and fused into vesicles temporarily. The vesicles could lower ions mobility, decreasing the conductivity. After the shear was retreated, the bilayers came back to the original state, thus increasing the conductivity.
The shear forces to the L3 phase samples temporarily increased their viscosity. In order to demonstrate this phenomenon, we characterized the apparent viscosity of unsheared L3 phase samples as a function of shear rate from about 0.01 s−1 up to a few thousand s−1 over 1 h and then decreased again. The rheogram in Fig. 3 clearly shows that after being sheared at high shear rate, the returned apparent viscosity η is significantly increased, indicating that the L3 phase with bilayers has been transferred into other aggregates such as vesicles.
Transition and microstructures from L3 phase to planar lamellar phase
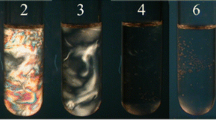
A 20 mmol L−1 methylformate was added into samples at L3 phase. After that, L3 phase became planar lamellar phase due to protonation of bilayer membranes by formic acid produced by methylformate hydrolysis. In this process, the shear forces were not introduced in the system. Phase transition from L3 phase to planar lamellar phase is shown in Fig. 4. It is clear from Fig. 4 that the domain-like birefringence gradually spread to the whole phase with methylformate hydrolysis. After complete protonation, the charges on the amphipathic bilayer membranes render the Gaussian modulus negative, thereby reducing the stability of L3 phase and increasing that of Lα phase [41, 42]. After the planar lamellar phase was sheared at 10 s−1 for 4 h, it is clear that the birefringent texture is different from the one without shearing, indicating that the planar lamellar phase was transformed into vesicle phase under shear.
FF-TEM and cryo-TEM are two powerful tools for unambiguously observing the structures of surfactant self-assembly at hydrate state [43, 44]. The bicontinuous bilayer structure of L3 phase was determined by FF-TEM, as shown in Fig. 5a. It is clear from the figure that the bicontinuous bilayer structure is as long as over several micrometers. The saddle point structures can clearly be identified from the image at enlarged scale. At such a point, the bilayers have both negative and positive curvatures. Without the shear forces, the L3 phase can be protonated by the formic acid formed through methylformate hydrolysis, and transformed into planar lamellar phase, as shown in Fig. 5b. After introducing shear force into planar lamellar phase, a new phase consisting of vesicles as shown in Fig. 5c can be formed.
FF-TEM images of aqueous samples containing 1.25% C14DMAO and 3.75% Trideceth-3 at the L3 phase (a), at the planar lamellar phase (b) formed without shear forces through protonation by the formic acid formed through methylformate hydrolysis, and at vesicle phase (c) formed through shaking the planar lamellar phase
Deuterium NMR is a well-established method to study the microstructures of surfactant aggregates in solution without destroying the essential properties of the sample [45, 46]. For surfactant self-assembly, some characteristic peaks have been summarized [31]. In the planar lamellar phase solution, a doublet quadrupole splitting is usually seen in the spectrum due to the long-range ordered anisotropic bilayer structure. In the vesicle phase, the water molecules bounded at the interface of curved bilayers exhibit local anisotropy but a long-range isotropy; therefore, only a single peak is seen in the spectrum. In Fig. 6, the phase transition from L3 phase to unsheared planar lamellar phase to vesicle phase is characterized by 2H NMR, showing no doublet splitting of L3 phase. This can be explained by that after charging the bilayer membranes of the isotropic solution at L3 phase, the newly formed planar lamellar phase with shaking shows obvious doublet splitting, indicating the formation of high anisotropic solution at the flat lamellar phase. The doublet splitting was not observed at vesicle phase after shaking planar lamellar phase to transform into vesicles, as only one 2H NMR signal was observed. It is mainly due to the closely packed spherical-like vesicles at the vesicle phase, which should be a less anisotropic environment of the individual water molecules without quadrupole coupling [47].
Rheological properties of the phase transition process
Figure 7 shows the time-dependent apparent viscosity variation of planar lamellar phase exposed to a constant shear rate of 10 s−1. Three regions could be determined. In the first region (I region) shortly after shearing, a shear thinning property is shown, indicating that the sample behaves like a plastic fluid, such as the flat lamellar phase. It is considered that this thinning is principally because of the formation of ordered bilayer structures being aligned in the flow direction. In the second region (II region), a shear thickening property is shown, suggesting that some bilayers start to close at this point, resulting in the formation of vesicles, which are more viscous than the extended bilayers. In the region III, the viscosity reaches a plateau, with viscosity further slightly increasing over time.
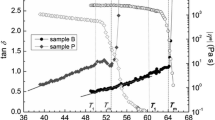
Figure 8 shows the rheograms of the L3 phase with bicontinuous bilayers, unsheared low viscosity planar lamellar phase with stacked lamellae, and the vesicle phase with vesicles obtained from the planar lamellar phase by applying a constant shear rate at 10 s−1 for 4 h. As shown in Fig. 8a, the L3 phase almost keeps its complex viscosity constant at stress around 0.002 Pa and behaves as Newtonian fluid. The loss modulus G″ is higher than the storage modulus G′, indicating that the L3 phase has low elasticity. As shown in Fig. 8b, even the storage modulus G′ of the unsheared planar lamellar phase is higher than the loss modulus G″, their values are very low, suggesting that the unsheared planar lamellar phase is a weakly viscoelastic fluid. The complex viscosity decreases with increase of the investigated frequency, indicating the fluid has a shear thinning property. After introducing shear forces into the planar lamellar phase, the newly formed vesicle phase consists of vesicles and is strongly viscoelastic, as shown in Fig. 8c. The storage modulus G′ and the loss modulus G″ are much higher than that of L3 phase and planar lamellar phase. The complex viscosities decrease with a slope of −1 over the whole frequency ranging from 0.01 to 10 Hz, suggesting the presence of uni- and multibilayer vesicles [48]. Thus, the rheological properties could indicate that vesicle phase behaves like a Bingham fluid with a yield stress value and has viscoelastic properties.
Conclusions
A L3 phase of bicontinuous bilayers in an isotropic, low viscosity aqueous solution of nonionic surfactant mixtures of C14DMAO and Trideceth-3 can be transformed to planar lamellar phase by protonation then to vesicle phase under shear. This study builds on previously investigation on sponge phase and phase transition from L3 to Lα under shear forces of nonionic surfactant C14DMAO mixed with cosurfactant [17, 26,27,28,29], by expanding the phase transition from L3 to vesicle phase. The phase behavior and rheology properties of L3 phase formed by nonionic surfactants Trideceth-3 mixed with nonionic surfactant C14DMAO aqueous solutions reported in this paper. When the L3 phase was vigorously sheared, the bilayers can be oriented and fused into vesicles temporarily. After the shear was retreated, the bilayers came back to the original state. We have systematically studied the phase transition and rheology properties of nonionic surfactants Trideceth-3 mixed with nonionic surfactant C14DMAO aqueous solutions. Due to hydrolysis of methylformate in the L3 phase solution, the charged lamellar structures can be obtained from L3 phase with a negative curvature and extended to flat planar lamellar phase without shear forces. The flat planar lamellar phase sample is weakly viscoelastic and highly anisotropic as indicated by the obvious double splitting in 2H NMR spectra. Under shear forces, the flat planar lamellar phase can close to vesicles with high viscoelasticity. An increase of ionic strength results in decreases in elasticity and viscosity of the solution.
We hope that the report could provide a guide for us on how to achieve controlled phase transition in nonionic surfactant mixtures and an application of phase transition of controlled materials.
References
Porte G, Appel J, Bassereau P, Marignan J (1989) Lα to L3: a topology driven transition in phases of infinite fluid membranes J Phys Fr 50:1335–1347
Kaler EW, Murthy AK, Rodriguez BE, Zasadzinski J (1989) Spontaneous vesicle formation in aqueous mixtures of single-tailed surfactants Science 245:1371–1374
Zemb T, Dubois M, Deme B, Gulik-Krzywicki T (1999) Self-assembly of flat nanodiscs in salt-free catanionic surfactant solutions Science 283:816–819
Kato T (2002) Self-assembly of phase-segregated liquid crystal structures Science 295:2414–2418
Yan D, Zhou Y, Hou J (2004) Supramolecular self-assembly of macroscopic tubes Science 303:65–67
O’Leary LER, Fallas JA, Bakota EL, Kang MK, Hartgerink JD (2011) Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel Nat Chem 3:821–828
Gazeau D, Bellocq AM, Row D, Zemb T (1989) Experimental evidence for random surface structures in dilute surfactant solutions Europhys Lett 9:447–452
Mitchell DJ, Tiddy GJT, Waring L, Bostock T, McDonald MP (1983) Phase behaviour of polyoxyethylene surfactants with water J Chem Soc Faraday Trans 79(4):975–1000
Gomati R, Appell J, Bassereau P, Marignan J, Porte G (1987) Influence of the nature of the counterion and of hexanol on the phase behavior of the dilute ternary systems: cetylpyridinium bromide or chloride-hexanol-brine J Phys Chem 91:6203–6210
Porte G, Marignan J, Bassereau P, May R (1988) Shape transformations of the aggregates in dilute surfactant solutions: a small-angle neutron scattering study J Physiol Paris 49:511–519
Anderson D, Wennerström H, Olsson U (1989) Isotropic bicontinuous solutions in surfactant-solvent systems: the L3 phase J Phys Chem 93:4243–4253
Penfold J, Chen M, Thomas RK, Dong C, Smyth TJP (2011) Solution self-assembly of the sophorolipid biosurfactant and its mixture with anionic surfactant sodium dodecyl benzene sulfonate Langmuir 27:8867–8877
Roux D, Cates ME, Olsson U, Ball RC, Nallet F, Bellocq AM (1990) Light scattering from a surfactant sponge phase: evidence for a hidden symmetry Europhys Lett 11:229–234
Cates ME (1992) Sponge phases, vol 66. Springer, Berlin Heidelberg, pp. 275–280
Strey R, Schomäcker R, Roux D, Nallet F, Olsson U (1990) Dilute lamellar and L3 phases in the binary water–C12E5 system J Chem Soc Faraday Trans 86:2253–2261
Strey R, Jahn W, Porte G, Bassereau P (1990) Freeze fracture electron microscopy of dilute lamellar and anomalous isotropic (L3) phases Langmuir 6:1635–1639
Miller CA, Gradzeilski M, Hoffmann H, Kramer U, Thanic C (1991) L3 phases: their structure and dynamic properties Progr Colloid Polym Sci 84:243–249
Skouri M, Marignan J, Appell J, Porte G (1991) Fluid membranes in the “semi-rigid regime”: scale invariance J Phys B Atomic Mol Phys 1(9):1121–1132
Roux D, Coulon C, Cates ME (1992) Sponge phases in surfactant solutions J Phys Chem 96:4174–4187
Vinches C, Coulon C, Roux D (1992) Viscosity of sponge phase in porous medium J Phys B Atomic Mol Phys 2(3):453–469
Waton G, Porte G (1993) Transient behavior and relaxations of the L3 (sponge) phase: T-jumb experiments J Phys B Atomic Mol Phys 3(4):515–530
Filali M, Porte G, Appell J, Pfeuty P (1994) L3 (sponge) phase in the very dilute regime: spontaneous tearing of the membrane J Phys B Atomic Mol Phys 4(2):349–365
Porte G, Appell J, Bassereau P, Marignan M, Skouri M, Billard I (1991) Scaling laws for some physical properties of the L3 phase Progr Colloid Polym Sci 84:264–265
Granek R, Cates ME (1992) Sponge phase of surfactant solutions: an unusual dynamic structure factor Phys Rev A 46:3319–3334
Watanabe K, Nakama Y, Tanaki T, Hoffmann H (2001) Novel vesicle and sponge phase prepared in amphoteric surfactant/anionic surfactant/oleic acid/water system Langmuir 17:7219–7224
Munkert U, Hoffmann H, Thunig C, Meyer HW, Richter W (1992) From vesicles to the L3 (sponge) phase in alkyldimethylamine oxide/heptanol systems Langmuir 8:2629–2638
Thunig C, Platz G, Hoffmann H (1992) Phase behavior and light scattering of the system dodecyldimethylaminoxide, n-hexanol and water in the very dilute region Ber Bunsenges Phys Chem 96:667–667
Mahjoub HF, McGrath KM, Kléman M (1996) Phase transition induced by shearing of a sponge phase Langmuir 12:3131–3138
Yamamoto J, Tanaka H (1996) Shear-induced sponge-to-lamellar transition in a hyperswollen lyotropic system Phys Rev Lett 77:4390–4393
Nilsson PG, Lindman B (1984) Nuclear magnetic resonance self-diffusion and proton relaxation studies of nonionic surfactant solutions. Aggregate shape in isotropic solutions above the clouding temperature J Phys Chem 88:4764–4769
Dong R, Zhong Z, Hao J (2012) Self-assembly of onion-like vesicles induced by charge and rheological properties in anionic-nonionic surfactant solutions Soft Matter 8:7812–7821
Medronho B, Shafaei S, Szopko R, Miguel MG, Olsson U, Schmidt C (2008) Shear-induced transitions between a planar lamellar phase and multilamellar vesicles: continuous versus discontinuous transformation Langmuir 24:6480–6486
Nettesheim F, Zipfel J, Olsson U, Renth F, Lindner P, Richtering W (2003) Shear-induced transitions between a planar lamellar phase and multilamellar vesicles: continuous versus discontinuous transformation Langmuir 19:3603–3618
Song S, Song A (2014) Self-assembled structures of amphiphiles regulated via implanting external stimuli RSC Adv 4:41864–41875
Song S, Wang H, Song A, Dong S, Hao J (2014) Sponge phase producing porous CeO2 for catalytic oxidation of CO Chem Eur J 20:9063–9072
Wang A, Shi W, Huang J (2016) Adaptive soft molecular self-assemblies Soft Matter 12:337–357
Gentile L, Behrens MA, Porcar L, Butler P, Wagner NJ, Olsson U (2014) Multilamellar vesicle formation from a planar lamellar phase under shear flow Langmuir 30(28):8316–8325
Porcar L, Hamilton WA, Butler PD, Warr GG (2003) Scaling of structural and rheological response of L3 sponge phases in the “sweetened” cetylpyridinium/hexanol/dextrose/brine system Langmuir 19(26):10779–10794
Porcar L, Hamilton WA, Butler PD, Warr GG (2004) Topological relaxation of a shear-induced lamellar phase to sponge equilibrium and the energetics of membrane fusion Phys Rev Lett 93(19):198301
Bergmeier M, Gradzielski M, Hoffmann H, Mortensen K (1999) Behavior of ionically charged lamellar systems under the influence of a shear field J Phys Chem B 103:1605–1617
Winterhalter M, Helfrich W (1992) Bending elasticity of electrically charged bilayers: coupled monolayers, neutral surfaces, and balancing stresses J Phys Chem 96:327–330
Helfrich W (1994) Lyotropic lamellar phases J Phys Condens Matter 6:A79–A92
Friedrich H, Frederik PM, de With G, Sommerdijk NAJM (2010) Imaging of self-assembled structures: interpretation of TEM and cryo-TEM images Angew Chem Int Ed 49:7850–7858
Song A, Dong S, Jia X, Hao J, Liu W, Liu T (2005) An onion phase in salt-free zero-charged catanionic surfactant solutions Angew Chem Int Ed 44:4018–4021
Lukaschek M, Miiller S, Hansenhindl A, Grabowski DA, Schmidt C (1996) Lamellar lyomesophases under shear as studied by deuterium nuclear magnetic resonance Colloid Polym Sci 274:1–7
Zana R (1987) Surfactant solutions.New methods of investigation. Marcel Dekker, New York,
Yuan Z, Dong S, Liu W, Hao J (2009) Transition from vesicle phase to lamellar phase in salt-free catanionic surfactant solution Langmuir 25:8974–8981
Li X, Dong S, Jia X, Song A, Hao J (2007) Vesicles of a new salt-free catanionic fluoro-hydrocarbon surfactant system Chem Eur J 13:9495–9502
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2016BQ30), the Young Talents Training Program of Shandong Academy of Agricultural Sciences, and Special Agricultural Research Program (201303103).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shen, Y., Hoffmann, H., Lin, H. et al. The phase transition from L3 phase to vesicles and rheological properties of a nonionic surfactant mixture system. Colloid Polym Sci 295, 1663–1670 (2017). https://doi.org/10.1007/s00396-017-4144-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4144-3