Abstract
Biodegradable and ultrahigh content grafted chitosan-g-poly(acrylic acid) powder was successfully synthesized in a homogeneous system and used as adsorbents for the removal of Cu(II) in aqueous solution. The copolymer was characterized by various techniques. The fundamental adsorption behaviors of the material were studied. The adsorption isotherm was well fitted with Langmuir equation, while the adsorption kinetics preferred to be described the pseudo-second order equation. The maximum adsorption capacity obtained from the Langmuir model was 210.13 mg/g, indicating that the adsorption capacity of chitosan was enhanced remarkably after grafting poly(acrylic acid). Moreover, Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) have been used to investigate the adsorption mechanisms at molecular levels, which revealed that carboxyl groups are facile to form bidentate carboxylates with metal ions. Thus, the environment-friendly copolymer will be a promising candidate for application in removal of heavy metal ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With rapid development of modern industries in recent years, heavy metal commination in water resources has been a public concern due to the toxic effect on the human beings, animals, and plants in the environment. Among the metal pollutions, copper may cause temporary stomach and intestinal disorders, kidney or liver damage, and even cancer in human beings as a result of its cumulative effect [1]. The copper compounds are widely used in many industrial processes, though they are still hard to separate from wastewater [2]. Up to now, many techniques have been applied to remove heavy metal, including coagulation/coprecipitation, ion exchange, adsorption, membrane filtration, and solvent extraction [3]. Among these methods, adsorption has been recognized as one of the most popular techniques because of its cost-effectiveness, simplicity of operation, high efficiency, easy recovery, and sludge-free operation.
Recently, biopolymers have attracted much attention as the adsorbent for removing heavy metals from aqueous solution [4, 5]. In particular, chitosan is considered as outstanding candidates, since it is environment-friendly, low cost, and wide source [6]. However, the disadvantages of poor chemical resistance and high crystallinity limit its use as an effective adsorbent [7]. In order to improve the adsorption capacity of chitosan, the exploration of novel chitosan materials is still challenging and of great interest.
The number of chemical modifications devoted to the application of chitosan materials using cross-linking, grafting of functional groups, and blending polymer or inorganics for the removal of metals from wastewater has grown rapidly [8–10]. Owing to great chelating effects of carboxyl groups, various carboxylated chitosan products have been reported. Therein, poly(acrylic acid) (PAA) has attracted increasing attention as modifier material, which contains a carboxyl group in each repeated unit and favor adsorption of metal ions [8]. Most efforts have been made to blending or in situ grafting PAA on chitosan for enhance metal ions adsorption capacity [8, 10]. But the polymers obtained in these ways often contain ultrashort chains and low levels of PAA. Therefore, a higher grafting content should be obtained to enhance adsorption capacity, which can be accomplished from copolymerization of chitosan containing double bonds with acrylic acid. Furthermore, such copolymers are biodegradable and thus can reduce secondary wastes.
In this article, the chitosan-g-poly(acrylic acid) (CS-g-PAA) was synthesized via graft copolymerization of maleoylchitosan with acrylic acid in a homogeneous system, and the copolymer was characterized by various techniques (NMR, FT-IR, and SEM). The adsorption capacity of the material for metal ions has been improved obviously after introducing carboxyl groups of long-chain PAA into the chitosan. Various effecting parameters on the removal of Cu(II) from aqueous solution were investigated to determine adsorption behavior and performance of CS-g-PAA, including contact time, initial concentration, and pH value. Furthermore, the isothermal adsorption equilibrium and kinetics were also studied. The possible adsorption mechanism was further identified based on Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy.
Materials and method
Materials
Chitosan with an average molecular weight (Mn) of 10,000 Da was purchased from Yuhuan Biomedical Company (Zhejiang, China) and used without further purification. Acrylic acid (AA), maleic anhydride, potassium persulfate (K2S2O8), and N,N-dimathyl formamide (DMF) were supplied by Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). K2S2O8 was recrystallized from distilled water. DMF was distilled under reduced pressure from calcium hydride and stored over molecular sieves (4 Ǻ). All other reagents were of analytical grade and used without further purification. Distilled water was used in all experiments.
Preparation of chitosan-g-poly(acrylic acid) (CS-g-PAA)
The CS-g-PAA powder was prepared by following previously reported method [11] with minor modification. Briefly, 2 g of chitosan and 15 g of maleic anhydride were added into 100 mL of dry N,N-dimathyl formamide in a 250-mL three-neck round-bottom flask equipped with a magnetic stirring, a nitrogen inlet, and a condenser. The mixture was heated to 115 °C and kept stirring for 8 h under nitrogen flow. Then, the resulting mixture was cooled to room temperature and filtered. The pale tan solution was poured into ice water producing flocculent precipitate. The product was collected by filtration, washed completely by Soxhlet’s extraction with ethanol, and dried to afford maleoychitosan as a pale tan powder.
Dried maleoylchitosan (0.5 g) was dissolved in 70 mL DMF with stirring under nitrogen atmosphere and then treated with potassium persulfate (0.1 g) for 10 min. Ten milliliter aqueous solution with 1.5 g acrylic acid was subsequently added to the reacting system. Polymerization was initiated by K2S2O8 at 70 °C and proceeded for 4 h under nitrogen atmosphere. The product was filtered and made free from homopolymer by exhaustive extraction with hot deionized water and ethanol for 24 h. Finally, the granular product was dried in vacuum at 60 °C up to constant weight and stored for further experiments. Additionally, the graft content (G) was calculated as follows:
where W g and W 0 are weights of copolymer and maleoylchitosan, respectively.
Characterization
Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 510p spectrometer. The samples were mixed with KBr and pressed to a thin plate for measurement. 1H NMR spectra were recorded on a Bruker Avance-400 NMR spectrometer in DMSO-d6 to elucidate the stoichiometry of the complex. Scanning electron microscopy (SEM) studies were carried out in an S-4800 SEM instrument (Hitachi) after coating the sample with gold film. Thermogravimetry (TG) curves were obtained on a Henven HTG-3 thermometer at a heating rate of 15 °C min−1 from 5 to 750 °C. X-ray photoelectron spectroscopy (XPS) measurements were carried out with Thermo Escalab 250XI electron spectrometer. A standard Al Kα excitation source was employed. Copper ions content was determined using the oxalic acid bis-cyclohexylidene hydrazide spectrophotometry at the wavelength of 600 nm.
Adsorption experiment
Adsorption experiments were conducted by varying initial copper ions concentration, contact time, temperature, and pH value under the aspects of adsorption isotherms and adsorption kinetics. Typical experiment was performed under 200 rpm with stirring for 2 h, which was carried out in a series of 250-mL round-bottom flasks containing 50 mg of adsorbent powder and 50 mL of Cu2+ solution. After adsorption, the adsorbents were separated by direct filtration, and the final Cu2+ concentrations in the solution were determined. The amount of metal ions adsorbed onto adsorbent was calculated according to the following equation:
where q is the amount of sorbate per adsorbent (mg/g); C 0 and C are the liquid phase concentrations of Cu(II) at initial and final time, respectively (mg/L); V is the volume of the solution (L); m is the mass of dry adsorbent (g).
The effect of initial pH on adsorption behavior of CS-g-PAA for Cu(II) was studied by varying pH value from 3.0 to 7.0 at 30 °C for 2 h. The initial concentration of Cu(II) was 400 mg/L, and pH value was adjusted by adding appropriate concentrations of HCl or NaOH solutions. For the adsorption kinetic studies, 400 mg/L of Cu(II) solutions was contacted with 50 mg of adsorbent with different contact time (10 min to 8 h). The experiments were carried at pH = 5.8 and 20, 30, and 40 °C, respectively.
Equilibrium isotherms are used to determine the adsorbent capacity for metallic ions. Herein, Langmuir and Freundlich models were selected to demonstrate the adsorption equilibrium between the adsorbent and metal ions. Adsorption isotherm was instigated by varying the initial Cu(II) concentration from 50 to 500 mg/L for 2 h at 30 °C.
Results and discussion
Characterization of CS-g-PAA
The procedure to prepare CS-g-PAA power consists of two steps, as shown in Scheme 1. The maleoylchitosan was prepared by reacting chitosan with maleic anhydride. Subsequently, the cross-linked chitosan-g-poly(acrylic acid) (CS-g-PAA) was synthesized by copolymerization of maleoylchitosan with acrylic acid in the presence of potassium persulfate. As shown in Fig. 1, the peaks at 5.8–7.1 ppm assigned to the protons 7 and 8 in the maleoyl group were observed in the 1H NMR spectrum of maleoylchitosan in DMSO-d 6 , confirming the maleoylation modification of chitosan. The broad peaks at 2.7–3.8 ppm could be attributed to the back bone protons of chitosan. From the calculation, the graft content of CS-g-PAA was beyond 250% and higher than that for most similar chitosan-based copolymers reported by others [8, 12].
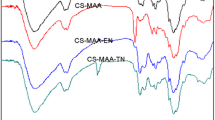
To further verify the construction of products, FT-IR spectra of chitosan, maleoylchitosan, and CS-g-PAA were initially measured (see Fig. 2). For maleoylchitosan, the intensity of amine band I at 1633 cm−1, which can be observed clearly in pure chitosan, decreased dramatically, and two new absorption bands at 1716 and 1637 cm−1 are observed, which can be assigned to the stretching vibration of carbonyl and the double bond adsorption of maleoyl group, respectively. The absorption peaks at 828 and 697 cm−1 also confirmed the presence of the syn-ring in maleoylchitosan. The FT-IR spectrum of the CS-g-PAA was shown in Fig. 2c. Comparing with the spectrum of maleoylchitosan, the peak at 1637 cm−1 (double bond) had almost disappeared, while the new peaks at 1577 and 1406 cm−1, which can be assigned to asymmetric and symmetric stretching vibrations of COO- groups, appeared. The results indicated that the poly(acrylic acid) chains were grafted on chitosan through polymerization to form graft copolymer.
Figure 3 shows the SEM photographs of chitosan and CS-g-PAA. It is clearly observed that chitosan presented a smooth and nonporous surface. In contrast, the surface of CS-g-PAA was obviously rough and porous, providing great surface area for practically useful adsorption purposes.
Adsorption properties
Cu(II) has been employed as the model ion to demonstrate the adsorption properties of CS-g-PAA. During the adsorption process, the pH value is an important parameter. The effect of initial solution pH on Cu(II) removal by CS-g-PAA is shown in Fig. 4. It is observed that the adsorption of Cu(II) increases gradually with increasing at pH < 5.0 and then increases abruptly at pH 5.0–7.0. At low pH, the adsorption efficacy of CS-g-PAA was low, because carboxylic groups in the polymer were protonized, and thus, electrostatic interaction was hindered. Therefore, nonionic bonding mechanism (for example hydrogen bonding) among functional groups occurs at low pH, which was responsible for lower metal ion adsorption efficacy. With an increase in pH values, the adsorption efficacy increased, since the deprotonating of functional groups is favored at higher pH values, by which electrostatic interaction between the polymer backbone and Cu(II) ions. Moreover, the precipitation constant of Cu(OH)2 is 5.6 × 10−20, corresponding to Cu(II) precipitation at pH = 7.4, which means that the precipitation might play an important role beyond 7.4 [13]. Based on these points, the most suitable pH value was selected as 6.0 for Cu(II) adsorption, at which there were no hydroxide precipitations. The maximum Cu(II) uptake was 197.2 mg/g at a pH 6.0. Compared with pure chitosan reported previously [8, 9], the CS-g-PAA copolymer exhibited higher adsorption capacity.
Thermal analyses curves were recorded in order to further confirm the adsorbing efficacy of adsorbent to Cu(II). As shown in Fig. 5, the polymer-Cu complex was degraded in four well-distinguished steps, which were more obvious in DTG curves. The first step ranges between 20 and 180 °C with 8.75% of the adsorbed and bound water weight loss. The second step was recorded in the temperature range of 181–267 °C and related to the chitosan degradation [14, 15]. The third step of weight loss starts at 268 °C and that continues to 338 °C, contributing to decomposition of the carboxyl groups of the PAA chain. The fourth step range of 339–467 °C was associated with breakage of PAA chain [16]. The final remnant weight of 20.6% attributed to copper oxide arised from high temperature oxidation. A similar decomposition mechanism also could be observed in the neat CS-g-PAA, and the remaining mass was 17.8% at about 666 °C resulted from sodium oxide. It has been calculated that Cu(II) uptakes of CS-g-PAA were 197.2 mg/g consistent with those mentioned above.
It is well known that poly(acrylic acid) has poor biodegradability and thus limits their practical application. Therefore, the strategy by grafting PAA on degradable chitosan cannot only enhance metal ions uptake but also improve biodegradability. Overall, CS-g-PAA could be considered as a promising adsorption adsorbent for the removal of copper ions due to high adsorption capacity and good biodegradability.
Adsorption isotherms
The adsorption isotherm can indicate the interactive behaviors between the solutes and adsorbents and illuminate the properties and affinity of the adsorbent. The adsorption isotherm was measured at 30 °C and pH = 5.8, which was presented in Fig. 6. It was found that the total amount of Cu(II) adsorbed increased sharply with the initial concentrations of metal ions increasing at the beginning, and then reached to surface saturation at high concentrations around 300 mg/L. This is attributed to an increase in Cu(II) concentration, which can enhance the permeation of metal ions into the polymer networks as a result of an increase in the driving force of concentration gradient. The correlation of equilibrium data was analyzed by the most commonly isotherm models, namely, Langmuir and Freundlich model, and the adsorption mechanism was further investigated.
Based on the assumption of a structurally homogeneous adsorbent, Langmuir isotherm is the commonly used model for monolayer adsorption process [10, 17]. It can be represented as follows:
where q e and C e are the amount adsorbed (mg/g) and the adsorbate concentration on solution (mg/L), both at equilibrium; b is the Langmuir adsorption constant related to the energy of adsorption; and q m is the maximum adsorption capacity of adsorbent (mg/g). The linearized form of Langmuir isotherm is represented by Eq. (4).
The Freundlich model is employed to describe heterogeneous system assumption of an exponentially decaying adsorption site energy distribution [7, 17]. The equation can be expressed as:
where K f is the Freundlich isotherm constant, and 1/n (dimensionless) is the heterogeneity factor.
The experimental data is fitted based on the Langmuir and Freundlich models. The model parameters obtained are all listed in Table 1, and adsorption isotherms are shown in Fig. 6. The residual sum of squares (RSSs) could be calculated by sum squared errors between data points and predicted values. The value of RSSs obtained for Langmuir (1832.1) model was not as high as that of Freundich (5354.3), which suggests that the data was better fitted to the Langmuir isotherm in this case. In addition, the high correlation coefficients (R 2) value for the Langmuir isotherm suggests that the Langmuir model fits fairly well with the experimental data points, as shown in Table 1. The maximal Cu(II) uptakes calculated from Langmuir model were quite close to corresponding experimental data. It can be concluded that the monolayer Langmuir adsorption isotherm is suitable to explain the adsorption of Cu(II) onto CS-g-PAA. In comparison to other reported chitosan-based materials as listed in Table 2, the CS-g-PAA exhibited significantly higher capacity to adsorb Cu(II) ions.
In order to determine the degree of suitability of adsorbent toward Cu(II) ions, a dimensionless constant (R L) called separation factor can be obtained by:
where b is the Langmuir constant and C 0 (mg/L) is the initial concentration of Cu(II) ions in solution. The value of R L indicates the tendency of the isotherm to be either unfavorable (R L > 1), linear (R L = 1), favorable (0 < R L < 1), or irreversible (R L = 0). The values of R L lie between 0.015 and 0.134, signifying that the adsorption of metal ions on CS-g-PAA is favorable [22].
Adsorption kinetics
The kinetic models have been used to examine the controlling mechanism for the adsorption process. The typical kinetic adsorption results of metal ions on adsorbent are shown in Fig. 7. It can be seen that Cu(II) adsorption on CS-g-PAA was a relatively fast process. The adsorption equilibrium time decreases as the temperature increases, indicating that the adsorption equilibrium could be achieved earlier at higher temperature. In addition, very similar equilibrium uptakes of Cu(II) at various temperatures confirm the temperature independence of isothermal adsorption behavior.
In order to interpret the experimental data further, the pseudo-first order, pseudo-second order, and intraparticle diffusion models were applied [23]. The pseudo-first order and pseudo-second order models are given as Eqs. (7) and (8), respectively:
These equations could be rearranged to linear form for the convenience of plotting and determining the constants (k) as below [20]:
where k 1 (min−1) and k 2 (g/mg min) are the rate constant of pseudo-first and pseudo-second order adsorption, respectively. q e and q t (mg/g) are the amounts of Cu(II) ions adsorbed onto adsorbent at equilibrium and at time t, respectively.
The intraparticle diffusion equation was employed to verify whether the intrapaticle diffusion behavior is the limiting step of the adsorption process [24, 25]. It is represented by:
where q t (mg/g) is adsorption capacity at time t (min), k p (mg/g min1/2) is the intraparticle diffusion rate constant, and C (mg/g) is related to the boundary layer thickness.
All of the experimental data have been linear fitted by the aforementioned kinetics models, and the obtained parameters are all listed in Table 3. From the given correlation coefficients (R 2), the pseudo-second order model was more appropriate to describe the adsorption kinetics behavior for Cu(II) onto the CS-g-PAA powder. Therefore, the graphical presentation for pseudo-second order model at 30 °C was selected and shown in the inset of Fig. 7. Theoretically simulated curve as solid lines fitted the experimental data quite well, showing that the rates of adsorption conformed to pseudo-second order kinetics, and the chemisorptions were the rate controlling mechanism. The results were consistent with those draw from adsorption isotherm analysis as mentioned above. Furthermore, the correlation coefficients of intraparticle diffusion model were quite low, revealing that the adsorption process was not limited by the intraparticle diffusion rate.
Adsorption mechanism
To illustrate the nature of adsorption and distinguish the possible adsorption sites of CS-g-PAA, the FT-IR spectra for adsorbent before and after adsorption of Cu(II) ions are obtained and shown in Fig. 2. After adsorption, the asymmetric vibration absorption of carboxyl group (COO-) has shifted from 1577 cm−1 to lower wave number of 1560 cm−1, while the symmetric vibration absorption band of carboxyl group remains almost the same (1406–1407 cm−1), implying that carboxyl groups are chelating functional groups for Cu(II). Additionally, the separation (Δ) between the symmetric and antisymmetric stretching modes of the coordinated carboxylic anions in CS-g-PAA was 153. According to previous study [26, 27], the Δ value for bidentate carboxylates was less than or equal to that from the free formate, for which the value of the sodium salt was 241 cm−1. Therefore, the FT-IR results from polymer-Cu complex have proved the existence of bidentate carboxylates, which were beneficial to be adsorption of Cu(II) ions.
To verify the results from the FT-IR spectra, XPS has been employed to analyze the surface structures of adsorbent before and after adsorption of Cu(II) ions. Figure 8 shows the XPS wide scan spectra of CS-g-PAA before (a) and after (b) Cu(II) adsorption. It is clear that a new peak at the binding energy (BE) of 934.43 eV appeared after Cu(II) adsorption, which represented the oxidation state +2 for the Cu 2p3/2 orbital. Thus, the new peak indicates that Cu(II) was adsorbed on the surface of CS-g-PAA.
The XPS data of O 1 s on CS-g-PAA before and after Cu(II) adsorption are shown in Fig. 8c, d, respectively. There were two BE peaks in O 1 s spectrum before adsorption of copper ions around 531.18 and 532.18 eV as seen in Fig. 8c, which were assigned to the oxygen atoms in the -C = O and -C–O–H groups, respectively [28]. However, the two peaks changed into one peak around 531.58 eV after adsorption, which suggested that the two oxygen atoms on the carboxyl group turned equivalent, and the carboxyl groups formed bidentate carboxylates with Cu(II). The N 1 s BEs did not show significant changes before and after Cu(II) adsorption, implying that the nitrogen atoms are not involved in the chemical adsorption of Cu(II).
Conclusions
A graft copolymer containing carboxyl and hydroxyl functional groups was synthesized from maleoylchitosan and acrylic acid and characterized by NMR, FT-IR, and SEM. The chitosan skeleton endows the biodegradability for materials; meanwhile, the poly(acrylic acid) provides abundant adsorption sites. Thus, the maximum adsorption capacity of CS-g-PAA to Cu(II) was 203.62 mg/g. Results from isotherms and kinetics study revealed that the adsorption isotherms obeyed the Langmuir equation, and the adsorption kinetics were well described by the pseudo-second order equation. It indicated that the Cu(II) adsorption behavior of adsorbent was monolayer chemisorption. XPS and FT-IR illustrated that the high adsorption capacity of CS-g-PAA was ascribed to formation of bidentate carboxylates with copper ions. The environment-friendly component of material is proposed to have promising potential for removal of heavy metals from aqueous solution.
References
Beppu MM, Arruda EJ, Vieira RS, Santos NN (2004) Adsorption of Cu(II) on porous chitosan membranes functionalized with histidine. J Member Sci 240(1–2):227–235
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83(4):1446–1456
Zhang L, Zeng Y, Cheng Z (2016) Removal of heavy metal ions using chitosan and modified chitosan: a review. J Mol Liq 214:175–191
Pal A, Das D, Sarkar AK, Ghorai S, Das R, Pal S (2015) Synthesis of glycogen and poly (acrylic acid)-based graft copolymers via ATRP and its application for selective removal of Pb2+ ions from aqueous solution. Eur Polym J 66:33–46
Ren H, Gao Z, Wu D, Jiang J, Sun Y, Luo C (2016) Efficient Pb(II) removal using sodium alginate–carboxymethylcellulose gel beads: preparation, characterization, and adsorption mechanism. Carbohydr Polym 137:402–409
Jiang TD (2007) Chitosan. Chemical Industry Press, Beijing
Peng S, Meng H, Ouyang Y, Chang J (2014) Nanoporous magnetic cellulose−chitosan composite microspheres: preparation, characterization, and application for Cu(II) adsorption. Ind Eng Chem Res 53(6):2106–2113
Dai J, Han Y, Yang H, Cheng R (2010) Simple method for preparation of chitosan/poly(acrylic acid) blending hydrogelbeads and adsorption of copper(II) from aqueous solutions. Chem Eng J 165(1):240–249
Song Q, Wang C, Zhang Z, Gao J (2014) Adsorption of Cu(II) and Ni(II) using a novel xanthated carboxymethyl chitosan. Separ Sci Technol 49(8):1235–1243
Wang X, Zheng Y, Wang A (2009) Fast removal of copper ions from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite composites. J Hazard Mater 168(2–3):970–977
Huang M, Jin X, Li Y, Fang Y (2006) Syntheses and characterization of novel pH-sensitive graft copolymers of maleoylchitosan and poly(acrylic acid). React Funct Polym 66(10):1041–1046
Zheng Y, Huang D, Wang A (2011) Chitosan-g-poly(acrylic acid) hydrogel with crosslinked polymeric networks for Ni2+ recovery. Anal Chim Acta 687:193–200
Ren Y, Li N, Feng J, Luan T, Wen Q, Li Z, Zhang M (2012) Adsorption of Pb(II) and Cu(II) from aqueous solution on magnetic porous ferrospinel MnFe2O4. J Colloid Interface Sci 367(1):415–421
Kyzass GZ, Siafaka PI, Lambropoulou DA, Lazaridis NK, Bikiaris DN (2014) Poly(itaconic acid)-grafted chitosan adsorbents with different cross-linking for Pb(II) and Cd(II) uptake. Langmuir 30(1):120–131
Al-Karawi AJM, Al-Qaisi ZHJ, Abdullah HI, Al-Heetimi DTA (2011) Synthesis, characterization of acrylamide grafted chitosan and its use in removal of copper(II) ions from water. Carbohydr Polym 83(2):495–500
Chen Y, Tan H (2006) Crosslinked carboxymethylchitosan-g-poly(acrylic acid) copolymer as a novel superabsorbent polymer. Carbohydr Res 341(7):887–896
El-Khaiary MI, Malash GF (2011) Common data analysis error in batch adsorption studies. Hydrometallurgy 105(3–4):314–320
Yan H, Dai J, Yang Z, Yang H, Cheng R (2011) Enhanced and selective adsorption of copper(II) ions on surface carboxymethylated chitosan hydrogel beads. Chem Eng J 174(2–3):586–594
Zhang S, Zhou Y, Nie W, Song L, Zhang T (2012) Preparation of uniform magnetic chitosan microcapsules and their application in adsorbing copper ion(II) and chromium ion(III). Ind Eng Chem Res 51(43):14099–14106
Yan H, Yang L, Yang Z, Yang H, Li A, Cheng R (2012) Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres and applications in the removal of copper(II) ions from aqueous solutions. J Hazard Mater 229-230:371–380
Xu J, Xu X, Zhao H, Luo G (2013) Microfluidic preparation of chitosan microspheres with enhanced adsorption performance of copper(II). Sensor Actuat B-Chem 183:201–210
Ong S-T, Tay EH, Ha S-T, Lee W-N, Keng P-S (2009) Equilibrium and continuous flow studies on the sorption of Congo Red using ethylenediamine modified rice hulls. Int J Phys Sci 4(11):683–690
Liu X, Zhang L (2015) Removal of phosphate anions using the modified chitosan bead: adsorption kinetic, isotherm and mechanism studies. Powder Technol 277:112–119
Malash GF, El-Khaiary MI (2010) Piecewise linear regression: a statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem Eng J 163(3):256–263
Rocha LS, Lopes CB, Borges JA, Duarte AC, Pereira E (2013) Valuation of unmodified rice husk waste as an eco-friendly sorbent to remove mercury: a study using environmental realistic concentrations. Water Air Soil Poll 224(7):1599
Deacon GB, Philips RJ (1980) Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord Chem Rev 33(3):227–250
Spinner E (1967) Vibration-spectral studies of carboxylate ions. Part III. Sodium formate, HCO2Na and DCO2Na; Raman-spectral depolarisation ratios in aqueous solution, and band splitting in the solid-state infrared spectrum. J Chem Soc B 9:879–885
Dietrich PM, Hennig A, Holzweber M, Lippitz A, Unger WES (2014) Surface analytical study of poly(acrylic acid)-grafted microparticles (beads): characterization, chemical derivatization, and quantification of surface carboxyl groups. J Phys Chem C 118(35):20393–20404
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51303003, 51303002) and Natural Science Foundation of Anhui (1408085QE76).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lin, Y., Hong, Y., Song, Q. et al. Highly efficient removal of copper ions from water using poly(acrylic acid)-grafted chitosan adsorbent. Colloid Polym Sci 295, 627–635 (2017). https://doi.org/10.1007/s00396-017-4042-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4042-8