Abstract
Covalent immobilization of inulinase, produced by the marine-derived fungus Aspergillus terreus, on novel gel beads was made by the combination of alginate and carboxymethyl cellulose. Optimization of the loading time and loading units was done by response surface methodology. The bound enzyme displayed a change in optimum operating pH from 5.0 to 5.5 while the optimum operating temperature increased from 50 to 55 °C. K m value has been increased (from 3.6 to 7.1 mg/ml) in comparison with the free enzyme. However, the V max was lowered (from 145 to 77.5 U/g carrier) after immobilization. The immobilized inulinase showed enhancement in thermal stability against high temperature. There was an observed increase in half-lives and D values which revealed the improvement in the enzyme thermal stability. Thermodynamically, after immobilization, a remarkable increase in enthalpy and free energy was observed due to the enhancement of enzyme stability. Immobilized inulinase showed retention of 60% of its original activity after 10 successive cycles. Stability and reusability of immobilized inulinase on alginate–CMC enable the enzyme to be more convenient for industrial application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inulinases catalyze inulin hydrolysis to produce inulo-oligosaccharides, fructose, and glucose. These can be classified into two types: endoinulinase (E.C. 3.2.1.7) that produces inulo-oligosaccharides) IOS) and exoinulinase (E.C. 3.8.1.80) which splits the terminal units of fructose. Exoinulinase is used for the production of high-fructose syrup (HFS) from inulin, while endoinulinase can be used for production of inulo-oligosaccharides of different lengths [1]. Fructose has a higher sweetening power compared to sucrose (about 70% higher), and it is used as a sweetener in food and beverage industries. It is well tolerated by diabetics, improves iron absorption by children, and helps in the removal of ethanol from the blood of alcoholics [2]. Fructo-oligosaccharides (FOS) increase the population of the gut bifidobacteria and improve the absorption of minerals [3].
The use of immobilized enzymes in industrial processes is more advantageous than the free forms as they help in the separation of reactants and products, allow the recovery of the enzyme for reuse, increase the enzyme stability, and reduce the costs of the process. Additionally, immobilization increases the selectivity of enzymes and reduces its inhibition by the products [4–7].
Many immobilization techniques were used previously for enzyme immobilization, such as gel entrapment [8], cross-linking [9, 10], physical adsorption [11], and covalent binding. Some authors have worked for many years on enzyme covalent binding immobilization using natural hydrogels and performing different modifications on gel formation to increase the enzyme resistance at high temperature and also to improve the enzyme reusability and the shelf stability of the enzymes [12–16].
The response surface method (RSM) is a combination of mathematical and statistical techniques for experimental designing, model planning, and studying the effects of some factors to obtain the most favorable conditions. The RSM was established as an appropriate method to develop the optimal conditions or the region that fits the operation specification. The optimum conditions for enzyme immobilization have been reported frequently [17].
In the present work the marine-derived Aspergillus terreus inulinase was immobilized on modified grafted alginate beads. A 22 factorial design was employed for the planned statistical optimization of immobilization. The catalytic properties of both the free and immobilized enzymes were studied. Moreover, the shelf stability and reusability were studied for the development of continuous use in industrial processes.
Materials and methods
All experiments were carried out in triplicate and data are means ± SD (n = 3).
Chemicals
Sodium alginate (Alg) was obtained from Fluka. Carboxymethyl cellulose (CMC) was purchased from Sigma; polyethyleneimine (PEI) (MW: 423), cat no. 468533, was obtained from Aldrich; and inulin was obtained from Sigma. Crude inulinase was prepared in our laboratory. Other chemicals were of Analar or equivalent quality. Inotech Encapsulator, model IE-50, was purchased from Inotech Encapsulator in Switzerland.
Microorganism and its maintenance media:
The marine-derived A. terreus was used in this study as a source for inulinase enzyme. A. terreus was isolated from decayed wood samples collected from Ismailia, Egypt [18]. The fungal isolate was routinely grown on malt extract agar medium at 27 °C and preserved at −80 °C in 50% (v/v) glycerol.
Cultivation conditions and crude enzyme extraction
Three grams of artichoke leaves were taken into 250-ml Erlenmeyer flasks and moistened with 15 ml of Tris–maleate buffer, 0.1 M, pH 8.5 contained (g %) 0.8, KH2PO4; 1, NH4H2PO4; 1, glucose; 1, sucrose; 0.006, CaCl2; and 0.5 MgSO4 [18]. Flasks were autoclaved for 20 min at 121 °C and cooled to room temperature before inoculation. Sterilized solid substrate was inoculated with 1.0 ml inoculum containing (5 × 106 spores/ml) of 5 days old culture prepared by inoculating a slant of the fungus in 50 ml of malt extract broth medium. The contents of the inoculated flasks were well mixed and incubated at 28 °C for 10 days. At the end of incubation period, 50 ml of sodium acetate buffer 0.1 M, pH 4.0, was added to the cultures and placed on a rotary shaker for 30 min. The suspension was filtered through a nylon cloth, followed by centrifugation at 5000 rpm for 15 min at 4 °C. Inulinase was precipitated from the culture filtrate with 60% acetone and then used for immobilization during the study.
Determination of enzyme activity
Inulinase activity was assayed by measuring the amount of the reducing sugar fructose released from inulin by Nelson’s method [19]. The reaction mixture containing 0.5 ml of enzyme extract (0.5 g immobilized enzyme) and 0.5 ml of 1% (w/v) inulin in 0.2 M sodium acetate buffer (pH 5) was incubated at 50 °C for 15 min. The amount of reducing sugars released was measured using Somogyi’s copper reagent. Absorbance was read at 520 nm. One unit of inulinase (IU) was defined as the amount of enzyme which librated 1 μmol of fructose per min under the assay conditions.
Preparation and grafting of alginate beads (Alg + CMC/Ca2+/PEI/GA)
For gel beads formation, solutions of sodium alginate (Alg) and carboxymethyl cellulose (CMC) in a concentration of 2 and 3% (w/v), respectively, mixed together. The Alg–CMC solution was dropped through a nozzle of 300 μm using the Inotech Encapsulator in a hardening solution containing 2% (w/v) CaCl2 (Ca2+). The generated beads were soaked for 3 h in 1% PEI solution. The unreacted PEI was then removed from the beads by successive washing with distilled water. After washing, aminated gel beads were soaked in glutaraldehyde (GA) solution of 2.5% (v/v) for 3 h to incorporate the new functionality, aldehyde group, and then gel beads were washed with distilled water to remove unreacted GA [17]. After that, the activated gel beads were ready for immobilization step as shown in Scheme 1 for covalent immobilization of enzyme.
Enzyme immobilization
One milliliter of the partially purified inulinase was mixed with 1 g of the prepared grafted alginate carrier .The mixture was left for desired time at 4 °C. At the end of incubation period gel, beads were washed twice with distilled water and used for inulinase assay.
Elucidation of the modified gels using ATR–FTIR
The attenuated total reflectance-Fourier transform infrared (ATR–FTIR) has been used to identify the new functionalities on the grafted gels. IR transmission spectra were obtained using a FTIR spectrophotometer (FTIR-8300, Shimadzu, Japan). The test is aiming to prove the presence of the new functional group; carbonyl group formed at all the different formulas. A total of 2% (w/w) of the sample, with respect to the potassium bromide (KBr; S. D. Fine Chem) disk, was mixed with dry KBr. The mixture was ground into a fine powder using an agate mortar before it was compressed into a KBr disk under a hydraulic press at 10,000 psi. Each KBr disk was scanned 16 times at 4 mm/s at a resolution of 2/cm over a wave number range of 400–4000/cm, using Happ–Genzel apodization. The characteristic peaks were recorded.
Differential scanning calorimetry and thermal gravimetric analysis
Thermal gravimetric analysis (TGA) was performed to prove the formation of a strong polyelectrolyte complex between the Alg–CMC and the PEI followed by Alg–CMC + PEI and GA. The thermal behavior of the different gel formulations Alg–CMC, Alg–CMC + PEI, Alg–CMC + PEI + GA, and Alg–CMC + PEI + GA + enzyme was characterized by the DSC (SDT 600, TA Instruments, USA). Approximately 3–6 mg of the dried gels was weighed into an alumina pan. TGA was performed by using (Q500 TA instrument, New Castle, DE, USA). A typical sample was heated from 50 to 300 °C at a heating rate of 10 °C/min, under a constant nitrogen flow rate of 100 ml/min.
Optimization of the enzyme loading capacity and loading time using grafted Alg–CMC gel beads using 22 full-factorial central composite experimental designs
Optimization of loading capacity and loading time of inulinase on the Alg–CMC gel beads was carried out by using 22 full-factorial central composite design [17, 20] with four-star points (±∞) and three replicates at the center point. Design matrix of 11 trials experiment (Table 1) shows the coded and actual values. The independent variables, loading time (X 1), and enzyme unit solution (X 2) were fitted with a second-order polynomial function to correlate the relationship between independent variables and response of immobilized units per gram gel disk/bead as follows:
where Y Activity is the predicted amounts of inulinase per gram gel beads, i.e., U/g beads. β 0 is the intercept, β 1 and β 2 are linear coefficients, β 11 and β 22 are quadratic coefficients, and β 12 is cross-product coefficient. Statistical software SPSS (version 16.0) was used for the regression analysis of the experimental data obtained. Statistical analysis of the model was performed to evaluate the analysis of variance (ANOVA). Statistical significance of the model equation was determined by Fisher’s test value, and the proportion of variance explained by the model was given by the multiple coefficients to determine each variable; the quadratic models were represented as contour plots (3D), and response surface curves were generated by using STATISTICA (0.6). Experiments were performed in triplicate and mean values were given.
Optimization and stability of the free and immobilized inulinases
To validate the efficiency of the novel carriers for immobilization of inulinase to be used in industries, the following experiments were conducted.
Effect of pH on immobilized and free inulinases
To determine the optimum pH for the free and immobilized inulinases, the enzymes were incubated at 50 °C for 15 min into 1 ml of 1% (w/v) inulin dissolved in 0.2 M sodium acetate buffer (pH 4.0–7.0). The data were normalized to 100% activity. The highest enzyme activity is expressed as 100%, and each pH is expressed relatively as a percentage of the 100% activity.
Effect of temperature and thermodynamics of free and immobilized enzymes
The effect of temperature on the activity of soluble and immobilized inulinase was determined by carrying out the reaction at temperatures ranging from 40 to 65 °C for 15 min. The temperature showing maximum activity was taken as optimum for the enzyme. The residual activity (%) at each temperature was calculated by considering the enzyme activity at the optimum temperature as 100%. The activation energy (E a) of catalysis for both free and immobilized inulinase forms was determined from the slope of the Arrhenius plot [log V (logarithm of % residual activity) versus reciprocal of absolute temperature in Kelvin (1000/T)], which is given by the following expression.
To study the thermal stability, both free and immobilized enzyme forms were pre-incubated at different temperatures ranging from 50 to 65 °C up to 90 min. Samples were withdrawn at intervals of time and analyzed for activity in standard enzyme assays at the optimum temperature. The residual activity was calculated by taking the enzyme activity at 0 min incubation as 100%. Results were also expressed as first-order thermal deactivation rate constants (k d), half-lives (t 1/2), and D values (decimal reduction time or time required to pre-incubate the enzyme at a given temperature to maintain 10% residual activity) at each temperature. The k d was determined by regression plot of log relative activity (%) versus time (min). The t 1/2 and D value of immobilized inulinase were determined from the relationships.
The temperature rise necessary to reduce D value by one logarithmic cycle (z value) was calculated from the slope of the graph between log D versus T (°C) using the equation
The activation energy (E d) for inulinase denaturation was determined by a plot of log denaturation rate constants (ln k d) versus the reciprocal of the absolute temperature (K) using the equation
The change in enthalpy (ΔH°, kJ/mol), free energy (ΔG°, kJ/mol), and entropy (ΔS°, J/mol/K) for thermal denaturation of xylanase was determined using the following equation
where T is the corresponding absolute temperature (K), R is the gas constant (8.314 J/mol/K), h is the Planck constant (11.04 × 10−36 J/min), and k B is the Boltzmann constant (1.38 × 10−23 J/K).
Inulin hydrolysis
Ten units of free and immobilized enzymes were incubated at 55 °C for 1 h at pH 5.5 into the assay mixture. Samples were withdrawn at interval times from 15 min to 120 h and analyzed for inulin hydrolysis.
K m and V max of free and immobilized inulinases
The Michaelis–Menten constant (K m) and maximum velocity (V max) of free and immobilized enzymes were calculated using Lineweaver–Burk plot. Inulin was employed as a substrate (10–100 mg/ml) dissolved in sodium acetate (0.2 M, pH 5.0) at constant pH and temperature. The Lineweaver–Burk plot (double reciprocal) method was used to obtain the Michaelis–Menten kinetic models adequate for the description of the hydrolysis of inulin by the free and immobilized enzymes. Apparent K m and V max of free and immobilized inulinases were determined by plotting 1/[S] against 1/[V], respectively.
[S]/V o = 1/V max × [S] + K m/V max
where [S] is the substrate concentration (inulin), V o is the initial enzyme velocity, V max is the maximum enzyme velocity, and K m is the Michaelis–Menten constant and is defined only in experimental terms and equals the value of [S] at which V o equals one half V max.
Operational stability and shelf stability of immobilized inulinase
The reusability of inulinase covalently immobilized on grafted alginate–CMC gel beads was studied. One gram of gel beads was incubated into 1 ml of 1% (w/v) inulin at pH 5 and 50 °C for 15 min, and the substrate solution was assayed. The same gel beads were then washed with distilled water and reincubated with another substrate solution; this procedure was repeated 13 times, and the initial activity was considered as 100%. The relative activity was expressed as a percentage of the starting operational activity.
The shelf stability was studied for the free and the immobilized enzyme over a period of 12 weeks at 4 °C. Ten grams of the immobilized enzyme containing 100 U and their equivalent of the free enzyme (100 U/ml) were stored in 0.2 M sodium acetate buffer (pH 5.0) at 4 °C. The samples were covered to avoid dehydration and loss of the buffer. A sample of the free enzyme (1 ml) or the immobilized enzyme (1 g gel beads) has been withdrawn every week and assayed for enzyme activity. The starting operational activity was considered as 100% relative activity, and data were normalized to 100% activity.
Results and Discussion:
Elucidation structure of the grafted alginate
The FTIR spectroscopic analysis of (1) the generated (Alg–CMC) gel beads, (2) the activated beads, and (3) the immobilized beads were carried out in a range varied between 400 and 4000/cm (Fig. 1). The IR spectrum of Alg–CMC beads showed characteristic beaks (curve A). A new beak at 3429/cm was displayed which was corresponding to NH2 group, indicating the presence of the amino group on the beads surface (curve B); however, the activated beads showed two new beaks. The first one was at 1717/cm referred to the (C=O) group of glutaraldehyde-free aldehyde end, and the other beak is at 1660/cm referring to (C=N−) group which resulted from the reaction of NH2 end groups with glutaraldehyde (curve C). Moreover, immobilized beads give broader beak at 3457/cm, pointed to increasing NH2 group’s concentration. From those data, it was revealed that the processes of amination, activation, and immobilization were successful. These results were in agreement with the results of obtained by other published results [21].
TGA
The TGA thermogram of Alg–CMC, Alg–CMC/PEI, Alg–CMC/PEI/GA, and Alg–CMC/PEI/GA/enzyme gels are shown in Fig. 2, and data were indicated in (Table 2). A gradual improvement in TGA was observed with the different treatment steps of Alg–CMC with PEI and GA. The TGA of Alg–CMC was 168 °C while Alg–CMC/PEI was 198 °C, and TGA for Alg–CMC/PEI/GA was 220 °C. A remarkable increase in TGA (317 °C) was determined after enzyme immobilization [22]. It was referred that the thermal improvement of the gel might be due to the polyelectrolyte interaction formation between the –NH+3 of the PE and the –COO– of Alg–CMC. Moreover, the hardening of the beads by using GA showed an increase in the TGA of Alg–CMC/PEI/GA up to 230 °C.
The results in (Table 2) for TGA 50% at which 50% of the polymer (alginate–CMC) has been degraded showed that the improvements in the TG might be due to the formation of a stronger crosslink of the beads due to of Schiff’s base formation between the free PE’s amino groups and GA. Danial et al. [13] reported that when calcium alginate was treated with PEI and followed by GA, an obvious improvement in their TGA 50% was observed.
Optimization of loading time and loading capacity by grafted Alg–CMC gel beads using 22 full-factorial central composite experimental designs:
The design matrix of 11 trial experiments of 22 full-factorial central composite design and the results are shown in Table 1. Maximum activity 46 U/g was obtained in the trial no. 6 when 10 U of inulinase incubated with one gram of Alg–CMC gel beads for 24 h. This result was very similar to the predicted value obtained by the polynomial equation (45.60 U/g beads).
The results obtained by 22 full-factorial central composite design were analyzed by the standard analysis of variance (ANOVA) (Table 3). The second-order regression equation indicating the enzyme activity as a function of loading time (X 1) and amount of loading units (X 2) can be predicted by the following equation
Y Activity = 0.475 + 3.016X 1 + 3.372 X 2 − 0.066X 12 − 0.112X 22 − 0.04X 1 X 2
Table 3 shows the ANOVA results of inulinase immobilized onto gel beads where the amounts of enzymes loaded onto the gel beads (Y Activity) were calculated the results of ANOVA and the polynomial equation. The F value was 12.52 which made the model significant. Model terms having values of Prop > F (0.007) less than 0.05 were considered significant. ANOVA indicated that the R 2 value of 0.926 for response Y Activity and which indicated that 92.6% of data variability can be illustrated by the model. The observed R 2 was in agreement with the adjusted R 2 of 0.852 which assured a satisfactory adjustment of the quadratic model to the experimental data [23]. The correlation between observed and predicted results was 96.2%. All the above proved the applicability and accuracy of the central composite design for optimization of inulinase immobilization process. To explore the interaction between variables and determining the optimum value of 22 full-factorial central composite design, the three-dimensional response surfaces were plotted (Fig. 3). This was used by many authors for optimization of enzyme loading [17, 20].
Effect of reaction pH on free and immobilized enzyme activities
The activity of free and immobilized inulinases was tested at different pH values. The results showed in Fig. 4 showed that optimum pH values were 5 and 5.5 for free and immobilized inulinases, respectively. Immobilized inulinase had an activity at pH 5.5 which is slightly shifted in comparison with the free enzyme. Shifting to higher pH values is usually observed after inulinase immobilization [12, 13]. On the other hand, Ettalibi and Baratti, De Paula et al., and Risso et al. [24–26] observed that there is no change in the optimum pH after immobilization.
Effect of reaction temperature on free and immobilized enzyme activities
By investigating the effect of temperature, immobilized and free inulinases were assayed at different temperatures, the pH kept at the optimum. The results showed that the optimum temperature recorded for free and immobilized inulinases were 50 and 55 °C, respectively (Fig. 5a). The formation of covalent bonds between the enzyme molecules and the carrier gave rigidity to the enzyme molecule structure, so the enzyme was less affected by temperature denaturation [27, 28]. Covalent immobilization is a technique used to engineer the enzymes to use them at high temperatures [13, 29]. High temperature was considered more interesting and preferable for improvement of conversion rates; moreover, high temperature helped in decreasing microbial contamination and allowed inulinase solubility as substrate. Conversion improvements maximize for production of the HFS by inulinase [30]. Danial et al. [13] and Sankalia et al. [31] also reported an increase in the optimum temperature for immobilized inulinase. The increase in the energy barrier of the reaction resulted from the activation energy (E a) and Gibbs free energy (ΔG°) which were calculated from Arrhenius and Eyring–Polanyi equations, respectively [32].
By using Arrhenius plot, the activation energy (E a) of the free and immobilized inulinases was calculated (Fig. 5b). Arrhenius plot regression equations were as follows: y = −3.4176x + 12.596 and y = −1.9505x + 8.0546, respectively. The covalent immobilization of inulinase lowered the E a from 28.41 to 16.216 kJ/mol, consequently a higher catalytic efficiency of the immobilized inulinase.
Thermal stability of the immobilized and free inulinases was also studied. The results in Fig. 6a showed that the free enzyme lost about 30% of its activity after 15 min at 50 °C while the results showed that immobilized enzyme retained 86% of its original activity at the same conditions. Free enzyme activity decreased to half after 60 min while the immobilized enzyme retained 63% of its activity at the same temperature. The activity of free enzyme dropped to 9% after 15 min at 60 °C while the immobilized enzyme retained about 20% of its original activity. Increasing in enzyme thermal stability and optimum temperature after immobilization indicates that the immobilization process strengthened the enzyme structure [33]. The increase in thermal stability of many immobilized enzymes is in agreement with other authors [34, 35]. Generally, the immobilization of an enzyme protects it against heat inactivation [36, 37]. Retaining of enzyme activity at higher temperatures might be due to the restriction of interaction between the immobilized enzymes molecules [38]. The thermal stability of inulinase at high temperature increases its suitability for use in industrial applications [39]. The heat inactivation rate for the immobilized enzyme was investigated at temperatures 50, 55, and 60 °C, respectively.
The plots of log of residual activity versus time were linear and indicated a first-order kinetic reaction of the immobilized enzyme (Fig. 6b). The thermostability parameters of the free and immobilized inulinases are illustrated in Table 4. The half-lives and D values of inulinase after covalent immobilization indicated better thermal stability of the immobilized inulinase. Tyagi and Gupta [34] also reported an increase in the half-life after immobilization of Aspergillus xylanase at 60 °C. The activation energy for thermal denaturation (E d) was determined from the Arrhenius plot (Fig. 6c).
The E d for immobilized inulinase was 113.7 kJ/mol which was higher than that of the free enzyme (96.65025 kJ/mol) which indicated that the immobilized enzyme was more compact, stable, and resistant to denaturation by heat. The higher value of E d meant that we require more energy for denaturation of the immobilized enzyme [40]. A similar increase in the thermal stability after immobilization of many enzymes was observed by many researchers [34, 35].
At 50 °C, the ΔH° of free inulinase was 70.926 kJ/mol and that of the immobilized form was 87.976 kJ/mol which mean that more energy was required for the heat denaturation of the immobilized inulinase. The values of ΔH° were decreased when the temperature was increased in both cases. This indicates that at high temperatures, low energy was required for free enzyme denaturation but more in the case of the immobilized inulinase (Table 4). The change in ΔH° also indicated that at higher temperatures, the enzyme exhibited a conformational change in both states [41].
Gibbs free energy (ΔG°) of thermal unfolding was decreased with increasing temperature and revealed a slight difference between the free and immobilized enzymes. This means that immobilization of inulinase did not affect its thermal unfolding at high temperatures. The unfolding of enzyme structure was accompanied by increasing the disorder or entropy of deactivation. However, inulinase had negative entropy (ΔS°) which revealed that the native enzyme was in more ordered state (Table 4). The immobilized inulinase also had a negative ΔS° value but with a lower magnitude than in the case of the free form. The less negative value for ΔS° in the immobilized enzyme is normal due to binding with the substrate. The variation in entropy at different temperatures might be due to the changes in conformations and thermal agitation effect.
The z values of free and immobilized inulinases were calculated from the slope of the graph between log D and temperature as 18.14 and 21.27 °C, respectively. Tayefi-Nasrabadi and Asadpour [40] reported that the high z values revealed more sensitivity to heat treatment duration, and the lower z values mean more sensitivity to temperature increase. Therefore, the lesser z value of immobilized inulinase compared with the free form indicated that the immobilization process made the enzyme more sensitive to temperature increase rather than the duration of heat treatment.
Hydrolysis of inulin using the immobilized and free inulinases
The results for inulin hydrolysis by immobilized and free inulinases are illustrated in Fig. 7. At 45 min, the free inulinase reached a maximum conversion rate (100%) of inulin, while the immobilized inulinase reached 100% after 60 min and onward. High concentration of product formation by immobilized enzyme represents the stability as well as a positive conformational change of the enzyme within the gel. At the same time, such increment in the reaction time also supports the possibility of resistance toward accessibility of high-molecular weight substrate to diffuse into the matrix easily [42–44].
Effect of substrate concentration on the activity of free and immobilized inulinases
The kinetic parameters regarding the substrate concentration effect for both the free and immobilized inulinases were calculated using Lineweaver–Burk plot (Fig. 8). The K m values for free and immobilized inulinases were 3.6 and 7.1 mg/ml, respectively. An increase in K m after enzyme immobilization might be due to the low diffusion rate of the substrate to the enzyme active site [44]. The immobilized enzymes have high K m values especially with high-molecular weight substrates as inulin [37, 45]. The increase in K m value after immobilization has been reported earlier by many other investigators [46, 47]. The free and immobilized enzymes had V max values of 145 and 77.5 U/g, respectively. V max value of the immobilized inulinase was less than that of the free enzyme and this was reported by some other authors [25, 42, 48]. A variation in the kinetic constants values could be observed due to the types of the polymer used, the variation in assay method, and the type of the inulin used. As inulin is available from different sources and has high-molecular weight; therefore, it has low dispersion ability and may cause the reduction in the efficiency of the process [42].
Operational stability of immobilized inulinase
Immobilized enzymes are preferred because they can be easily separated and reused to reduce the production costs. The inulinase immobilized on alginate–CMC beads could be reused for 10 successive cycles with retaining 60% of its original activity (Fig. 9). The enzyme retained 100% of its activity for the first five cycles after that the enzyme loss only 10% reaching the 8 cycles. The loss in activity after that was due to inactivation of the enzyme due to continuous use [17].
Shelf stability of the free and immobilized inulinases
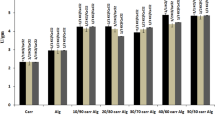
The enzyme stability is of great significance in the production of biocatalysts. Not only the easy separation of the enzyme from its products and enzyme reusability is the main advantage of enzyme immobilization but also the immobilized enzyme stabilization during storage considered as one of the most important advantages for enzyme immobilization. The data in Fig. 10 indicated that the inulinase immobilized on grafted alginate–CMC beads retained over 100% of its activity after 9 weeks at 4 °C, while the free enzyme completely lost its activity. This may be due to the formation enzyme-gel polyelectrolyte complexes with ionic interaction which increased the shelf stability of the immobilized inulinase [17].
Conclusion
This study justified the use of a grafted alginate–CMC as a suitable matrix for the covalent binding immobilization of inulinase. The kinetic parameters of the immobilized inulinase were evaluated with reference to free inulinase. Covalent immobilization increased inulinase optimum temperature and the reaction time. This matrix also increased the inulinase stability against higher temperatures compared to the free enzyme. Immobilized inulinase beads can be reused for many consecutive cycles retaining its activity.
References
Sumat CJ, Jain PC, Kango N (2012) Production of inulinase from Kluyveromyces marxianus using Dahlia tuber extract. Braz J Microbiol 43:1517
Turner P, Mamo G, Karlsson EN (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microbial Cell Factory 6:2859–2869
Ali S, Shahzadi H (2015) Nutritional optimizations for improved exo-inulinase production from Aspergillus oryzae for high fructose syrup preparations. Int J Curr Microbiol App Sci 4(5):618–631
Varavinit S, Chaokasem N, Shobsngob S (2002) Immobilization of a thermostable alpha-amylase. Sci Asia 28:247–251
Kahraman M, Bayramoglu G, Kayaman-Apohan N, Gungor A (2007) Alpha amylase immobilization on functionalized glass beads by covalent attachment. FoodChem 104:1385–1392
Riaz A, UlQader S, Anwar A, Iqbal S (2009) Immobilization of a thermostable alpha-amylase on calcium alginate beads from Bacillus subtilis. Aust J Basic Appl Sci 3:2883–2887
Mateo C, Palomo JM, Fernandez-lorente G, Guisan JM, Fernandez-Lorente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization. Enzym Microb Technol 40:1451–1463
Betancor L, Luckarift HR, Seo JH, Brand O, Spain JC (2008) Three-dimensional immobilization of 훽-galactosidase on a silicon surface. Biotechnol Bioeng 99(2):261–267
Wang Y, Wang X, Luo G, Dai Y (2008) Adsorption of bovine serum albumin (BSA) onto the magnetic chitosan nanoparticles prepared by a microemulsion system. Bioresour Technol 99(9):3881–3884
Synowiecki J, Wołosowska S (2006) Immobilization of thermostable 훽-glucosidase from Sulfolobus shibatae by crosslinking with transglutaminase. Enzym Microb Technol 39(7):1417–1422
Salman S, Soundararajan S, Safina G, Satoh I, Danielsson B (2008) Hydroxyapatite as a novel reversible in situ adsorption matrix for enzyme thermistor-based FIA. Talanta 77(2):490–493
Elnashar MM, Danial EN, Awad GE (2009) Novel carrier of grafted alginate for covalent immobilization of inulinase. Ind Eng Chem Res 48:9781–9785
Danial EN, Elnashar MM, Awad GE (2010) Immobilized inulinase on grafted alginate beads prepared by the one-step and the two-steps methods. Ind Eng Chem Res 49:3120–3125
Elnashar MM, Mohamed EH, Ghada EA (2013c) Grafted carrageenan gel disks and beads with polyethylenimine and glutaraldehyde for covalent immobilization of penicillin G acylase. Journal of Colloid Science and Biotechnology 2:1–7
Awad GE, Amer H, El-Gammal E, Hely W, Essawy M, Elnashar MM (2013) Production optimization of invertase by Lactobacillus brevis Mm-6 and its immobilization on alginate beads. Carbohydrates Polymers 93:740
Ghada E.A. Awad, Mona A. Esawy, Eman W. El-Gammal, Hanan Mostafa Ahmed, Magdy M. Elnashar, Nagwa A. Atwa. Comparative studies of free and immobilized phytase, produced by Penicillium purpurogenu GE1, using grafted alginate/carrageenan beads. Egyptian Pharmaceutical Journal 2015, IP: 197.35.212.170.
Elnashar MM, Awad GE, Hassan ME, Mohy Eldin MS, Haroun BM, El-Diwany AI (2014) Optimal immobilization of β-galactosidase onto κ-carrageenan gel beads using response surface methodology and its applications. Sci World J:1–7
Abd El Aty AA, Wehaidy HR, Mostafa FA (2014) Optimization of inulinase production from low cost substrates using Plackett-Burman and Taguchi methods. Carbohydr Polym 102:261–268
Nelson N (1944) A photometric adaptation of the Somogyi method for determination of glucose. J Biol Chem 153:375–380
Lee DH, Park CH, Yeo JM, Kim SW (2006) Lipase immobilization on silica gel using a cross-linking method. Journal of Industrial and Engineering Chemistry Research 12(5):777–782
Mohy Eldin SM, El Enshasy HA, El Sayed M, El Sayed S, Haroun B, Hassan EA (2012) Covalent immobilization of penicillin G acylase onto chemically activated surface of poly (vinyl chloride) membranes for 6-penicillic acid production from penicillin hydrolysis process I. Optimization of surface modification and its characterization. J Appl Polym Sci 124:E27–E36
Elnashar, M. M. and Hassan, M. E. Novel epoxy activated hydrogels for solving lactose intolerance. BioMed Research International, 2014, 2014, Article ID 817985, 9 pages
Munk V, Pásková J, Hanus J (1963) Glucose oxidase of Aspergillus niger. Folia Microbiol 8:203–214
Ettalibi M, Baratti JC (1992) Immobilization of Aspergillus ficuum inulinases on porous glass. Biocatalysis 5:175–182
De Paula FC, Cazetta ML, Monti R, Contiero J (2008) Sucrose hydrolysis by gelatin-immobilized inulinase from Kluyveromyces marxianus var. bulgaricus. Food Chem 111:691–695
Risso FVA, Mazutti MA, Costa F, Treichel H, Maugeri F, Rodrigues MI (2010) Comparative studies of the stability of free and immobilized inulinase from Kluyveromyces marxianus NRRL Y-7571 in aqueous-organic solutions. Braz J Chem Eng 27(4):507–516
Martin MT, Alcalde M, Plou FJ, Dijkhuizen L, Ballesteros A (2000) Synthesis of malto-oligosaccharides via the acceptor reaction catalyzed by cyclodextrin glycosyl transferases. Biocatal Biotrans 19:21–35
Ferrarotti SA, Bolivar JM, Mateo C, Wilson L, Guisan JM, Fernandez LR (2006) Immobilization and stabilization of a cyclodextrin glycosyltransferase by covalent attachment on highly activated glyoxyl-agarose supports. Biotechnol Prog 22:1140–1145
Nwagu TN, Aoyagi H, Okolo BN, Yoshida S (2012) Immobilization of a saccharifying raw starch hydrolyzing enzyme on functionalized and nonfunctionalized sepabeads. J Mol Catal B Enzym 78:1–8
Gill PK, Manhas RK, Singh P (2006) Hydrolysis of inulin by immobilized thermostable extracellular exoinulinase from Aspergillus fumigatus. J Food Eng 76:369–375
Sankalia MG, Mashru RC, Sankalia JM, Sutariya VB (2006) Stability improvement of alpha-amylase entrapped in kappa-carrageenan beads: physicochemical characterization and optimization using composite index. Int J Pharm 312:1–14
Arrhenius S (1889) Uber die reaktionsgeschwindigkeitbei der inversion von rohrzuckerdurch sauren. Z Phys Chem 4:226–248
Ortega N, Perez-Mateos M, Pilar MC, Busto MD (2009) Neutrase immobilization on alginate–glutaraldehyde beads by covalent attachment. J Agric Food Chem 57:109–115
Tyagi R, Gupta MN (1995) Immobilization of Aspergillus niger xylanase on magnetic latex beads. Biotechnol Appl Biochem 21:217–222
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microbial Technol 40:1451–1463
Sardara M, Roy I, Gupta MN (2000) Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer Eudragit TM L-100. Enzyme Microbial Technol 27:672–679
Gouda MK, Abdel-Naby MA (2002) Catalytic properties of the immobilized Aspergillus tamari xylanase. Microbiol Res 157:275–281
Ping W, Sheng D, Waezsada SD, Tsao AY, Davinson BH (2001) Enzyme stabilization by covalent binding in nanoporous sol-gel glass for nonaqueous biocatalysis. Biotechnol Bioeng 74:249–255
Maalej I, Belhaj I, Masmoudi NF, Belghith H (2009) Highly thermostable xylanase of the thermophilic fungus Talaromyces thermophilus: purification and characterization. Appl Biochem Biotechnol 158:200–212
Tayefi-Nasrabadi H, Asadpour R (2008) Effect of heat treatment on buffalo (Bubalus bubalis) lactoperoxidase activity in raw milk. J Biol Sci 8:1310–1315
Marin E, Sanchez L, Perez MD, Puyol P, Calvo M (2003) Effect of heat treatment on bovine lactoperoxidase activity in skim milk: kinetic and thermodynamic analysis. J Food Sci 68:89–93
Bibi Z, Shahid F, Ul Qader SA, Aman A (2015) Agar–agar entrapment increases the stability of endo-β-1,4-xylanase for repeated biodegradation of xylan. Int J Biol Macromol 75:121–127
Elnashar MMM, Mostafa H, Morsy NA, Awad GEA (2013) Biocatalysts: isolation, identification, and immobilization of thermally stable lipase onto three novel biopolymeric supports. Ind Eng Chem Res 52:14760–14767
Dalal S, Sharma A, Gupta MN (2007) A multipurpose immobilized biocatalyst with pectinase, xylanase and cellulase activities Chem. Cent J 1:16–20
Gupta MN, Guoquiang D, Kaul R, Mattiassion B (1994) Purification of xylanase from Trichoderma viride by precipitation with an anionic polymer Eudragit S 100. Biotechnol Tech 8:117–122
Abdel-Naby AM (1993) Immobilization of Aspergillus niger NRC 107 xylanase and β-xylosidase, and properties of the immobilized enzymes. Appl Biochem Biotechnol 38:69–81
A. L. Erarslan, T. F. Bozoglu and B. Ray, Current advances in metabolism, genetics and applications (Bozoglu ed.), Springer, Ber lin, Germany, 1996, 381–398.
Mahmoud DAR, Refaat HW, Abdel-Fattah AF, Mahdy EME, Shousha WGH (2011) Novel application of Luffa cylindrica in production of fructose. Aust J Basic & Appl Sci 5:2127–2137
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Awad, G.E.A., Wehaidy, H.R., Abd El Aty, A.A. et al. A novel alginate–CMC gel beads for efficient covalent inulinase immobilization. Colloid Polym Sci 295, 495–506 (2017). https://doi.org/10.1007/s00396-017-4024-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4024-x