Abstract
The purpose of this work was to prepare a non-fluorinated superhydrophobic composite coating with heat/durability resistance and excellent adhesion properties. This procedure was based on organic–inorganic molecular hybrid Zinc oxide (ZnO)/Carbon Nanotubes (CNTs)-Poly(dimethylsiloxane) (PDMS) and Stearic acid compound. The coating produced was characterized in terms of their superhydrophobic properties and morphology. In optimized conditions, the contact angle (CA) for water and oil deposited on the coating were as high as 172, and 154°, respectively. In addition, the composite coating could maintain superhydrophobic even soaked in liquid with pH from 1 to 14. The resulting superhydrophobic composite coating showed good thermal stability, which could maintain hydrophobic even under a harsh environment with the high temperature over 150 °C. The contact angle of the prepared coating could still keep superhydrophobic even after being exposed in air about 90 days, showing a good performance in durability. The enhancement in these preliminary results will guide the design and fabrication of the non-fluorinated commercial superhydrophobic coatings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, superhydrophobic coatings, which display static water contact angles (WCAs) of water greater than 150° and slide angle (SA) of water less than 10°, have attracted tremendous attention in both scientific and industrial areas owing to their wide practical applications in various fields, including water–oil repellent fabrics, anti-adhesion films, stain resistant coatings, and anti-fogging films [1–4].
Typically, the surface morphology and chemical composition of the superhydrophobic coatings endowed them with high water−/oil-repellent [5, 6]. In addition, superhydrophobic coatings with high water adhesion are promising attributed to their representative superhydrophobic property and relative research value in various realms, such as barrier materials, micro fluidic systems, liquid transportation, etc. [7–9]. Therefore, generating a surface with high degree of roughness and lowering down the surface free energy of materials are the two main strategies for the preparation of superhydrophobic coatings [10–14]. Techniques for fabricating superhydrophobic surfaces are using large quantity of fluoride materials to provide low surface energy [15–19]. Naddaf et al. fabricated a superhydrophobic coating with FEP by oxygen glow discharge treatment and argon–ion etching; this combined treatment yield an increase in CA for water from 109 ± 2.2° to 152 ± 2.1° [20]. The drawback with this fluorine-functionalized coating is its high-cost, environmental hazards, and potential risks for human health during the lifetime [11–23].
Zinc oxide (ZnO) is an appropriate candidate for fabricating coatings with hydrophobic properties due to the special structure of nano-tetrapod-like [24]. Poly(dimethylsiloxane) (PDMS) has excellent properties such as low surface energy, low friction, chemical inertness, and weatherability [25–27]. For instance, Cho et al. fabricated a superhydrophobic film using chemical vapor deposition approach depositing uniformly ZnO hydrophobic nanoparticles (HNPs) on the Poly (dimethylsiloxane) (PDMS) film substrate [28]. Chakradhar et al. prepared ZnO–PDMS nanocomposite coatings by wet chemical route [29]. The engineering value of this surface is limited by the fragility and poor durability resistance. However, Poly(phenylene sulfide) (PPS) is an appropriate matrix for fabricating coatings with hydrophobic properties due to the outstanding adhesion, thermally stabilization, and thermo-plasticity [30]. However, the surface energy of PPS is high and unable to provide the function of low surface energy. Hence, preparing a superhydrophobic coating with greater heat/durability resistance via a non-fluorinated procedure will be challenging and significant in industry.
The present work, the non-fluorinated superhydrophobic PPS-based composite coatings were the first time to be prepared by the simple spraying method via designing reticular (network) structure and introducing the low surface energy material ZnO-PDMS and CNTs-PDMS by organic–inorganic molecular hybrid. The heat/durability resistance of the superhydrophobic coatings are investigated and discussed. It is believed that the work is of great interest to researchers, particularly in the fields of multi-functional nanocomposites and non-fluorinated superhydrophobic polymer coatings.
Experimental details
Materials
Aluminum plates (80 × 80 × 1 mm3, purity ≥ 99.9 wt%) are supplied by Jiayun aluminum product Co. Ltd., China. Polyphenylene sulfide (PPS, average diameter of 30 μm) is supplied by Yuyao Degao Plastic Technology Co. Ltd., China. Polydimethylsiloxane (PDMS, purity ≥ 98 wt%, reagent grade) is obtained from Jinan Hailan Reagent Factory of China. Stearic acid is purchased from Shenyang Huadong Reagent Factory. Zinc oxide (ZnO, average diameter of 50 nm) is obtained from DuPont, China. Carbon Nanotubes (CNTs) is purchased from Guangdong Tianxin Reagent Factory, China, and Ethanol (ET, reagent grade, purity ≥ 98 wt%) is supplied by Shenyang Huadong Reagent Factory, China.
Sample preparation
Fabrication of silylated CNTs/ZnO
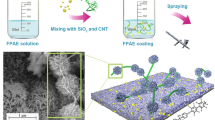
One milliliter H2O and 1 mL KH550 were dissolved in 18 mL anhydrous alcohol ultrasonically for 30 min. 0.1 mL PDMS was added with ultrasonically for another 30 min to allow the reaction as shown in Fig. 1. Then added 0.5 g oxidized CNTs into the resultant solution with 400 rpm stirring for 1–2 h to yield a final solution. Finally, products were filtered, washed by anhydrous alcohol (50 mL), and dried at 80 °C for 4 h. ZnO powders were silylated either in the same way. The dried CNTs-PDMS and ZnO-PDMS powders were obtained after drying at 80 °C for 24 h.
Fabrication of superhydrophobic coating on aluminum plate
Aluminum plates, as the substrate, with the size of 80 × 80 × 1 mm3 were first polished with 1000-grit waterproof abrasive paper to obtain a relatively rough surface, then ultrasonically cleaned in anhydrous alcohol for 10 min and dried in air at room temperature. The coating was obtained through spraying polymer solution, which was composed of PPS, CNTs-PDMS, and ZnO-PDMS nano-particles, on the aluminum plates. The samples were then sintered at 300–350 °C in a muffle furnace for 90 min. Finally, the sintered coating was soaking in the 0.03 mol/l stearic acid ethanol solution at 70 °C for 120 min; after that, the coating was heated at 120 °C for 30 min, and then the PPS/ZnO-PDMS/CNTs-PDMS composite coating was synthesized.
Characterization
Contact angles (CAs) of liquid droplets on the fabricated coatings were estimated by using JC2000A apparatus at ambient temperature via a sessile drop method. The water droplet was controlled at around 5 μL. All CA and SA measurements obtained were averages of five values made on different areas of coatings. The morphology of the coatings was observed by scanning electron microscope (SEM, Quanta 200). The chemical composition was examined by Fourier transform infrared spectrometer (FT-IR, Tensor27). Thermal stability characterization was calcining the fabricated coatings in a muffle furnace for 90 min. The wear resistance tests were carried out on a pin-on-disk friction and wear tester (MPX-2000, Xuanhua Testing Factory, China). The corrosion resistance was evaluated by using the electrochemical workstation (LK 2010) in 3.5 wt.%, NaCl solution. The adhesive ability of the prepared composite coating was evaluated according to GB/T9286. The painted aluminum plate was scribbled into 2 mm × 2 mm gridding with a razor to expose the base metal, and then the adhesive tapes were pressed and pulled to remove from on the scored surface in 5 min.
Results and discussion
Surface wettability
The effect of different nanoparticles content on the WCAs of PPS-based composite coatings is shown in Fig. 2 a. It can be seen that WCAs of PPS/ZnO/CNTs or PPS/ZnO-PDMS/CNTs-PDMS coatings increase gradually along with the promotion of CNTs or CNTs-PDMS content. When the CNTs-PDMS content increase to 9 %, the PPS-based composite coating shows the most excellent superhydrophobic property, whose contact angle can hold up to 171° and the sliding angle is as low as 3° (Fig. 2 c.. The obvious contrast is that the As of the coatings with CNTs under the same proportion is only 93°. Result has shown that the CNTs can improve the WCAs of the coatings by enlarging the micro/nano-structure on the surface. Nevertheless, the unmodified CNTs lead to the small increase of the WCAs due to the result from the FT-IR that there exist large amount of hydrophilic hydroxyl group on the surface.
a Effects of different CNTs content on the hydrophobic property of superhydrophobic PPS-based composite coatings, b Effects of different CNTs-PDMS content on the oleophobic property of superhydrophobic PPS-based composite coatings, c Continuous shooting of water drop sliding on the PPS/3 %ZnO-PDMS/9 %CNTs-PDMS composite coatings with the slant angle of 3°
The effects of different CNTs-PDMS content on the oleophobic property of superhydrophobic PPS-based composite coatings are shown in Fig. 2 b. When CNTs-PDMS content increases to 3 %, the contact angle for glycerine and ethylene glycol reaches to 113° and 95°, respectively. Moreover, the glycerine and ethylene glycol angle increases up to 126° and 121° when CNTs-PDMS content reaches 9 %. So far, the silicon-oxygen bond (−Si-O-) terminated surface has been reported to possess the lowest surface free energy [9, 31]. This illustrates that the existence of silicon-oxygen bond (−Si-O-) is a key factor to achieve the superhydrophobic and oleophobic properties.
The photos of water droplets of 5 μL on the un-stearic acid treated coating with different tilt angles (A: 0°, B: 90°, C: 180°) are shown in Fig. 3. It can be observed that the WCA of the un-stearic acid treated coating is 145°. In addition, the water can still maintain a spherical on the coating surface after incline 90° and 180°, showing high bond strength. This performance is mainly caused by a network-like rough grading structure. Network-like structure allows droplets immersed in a large gully among micron structure and ensure the droplets adhere to the surface of the coating even if the coating rollover 90° and 180°. This phenomenon contributes to the high contact angle hysteresis of coating. As to the network-like composite coating, the sizes of hierarchical micro and nanostructures are both larger than those of low contact angle hysteresis coatings [32, 33]. Moreover, take the FT-IR result into consideration, the surface of network-like composite coating exists not only the hydrophobic group, but also exist a certain amount of hydroxyl group. Therefore, the coexisting of hydrophobic group and hydroxyl group on superhydrophobic coating should be one of the main factors that cause the appearance of high water adhesion. At the same time, the volume of the droplet is a key factor to ensure this performance. The droplets, whose weight is lighter than the surface tension of composite coating, can adhere to the coating and the huge droplet may be dropped from the fabricated surface.
FT-IR analysis
As shown in Fig. 4, the ZnO and CNTs with PDMS modification are further characterized by FT-IR. It can be seen that the peaks at 1550 ~ 1650 and 3450 cm−1 are corresponding to the hydroxyl group on ZnO. The peaks of 784, 1022, and 1256 cm−1 represent the Si-C, C-C, and Si-O-C bonds of PDMS, respectively [34]. And the new peaks appear at 796, 1017, and 1261 cm−1 belong to the asymmetric bond types of Si-C, C-C, and Si-O-C, which indicates the ZnO and CNTs are modified with PDMS successfully [35]. Results show that the unmodified ZnO and CNTs have high level of hydroxyl on the surface, which makes them hydrophilic. In addition, hydroxyl group exists on the surface of ZnO-PDMS, CNTs-PDMS particles. Therefore, it can be demonstrated that the existence of silicon-oxygen bond (−Si-O-), which terminated on ZnO and CNTs, is providing the lowest surface free energy which is the key to superhydrophobic and oleophobic performance.
Surface morphology
Figure 5 clearly shows the SEM images and WCA of the PPS/ZnO-PDMS/CNTs-PDMS composite coating with different CNTs-PDMS content at low- and high-magnification, respectively. In this work, we designed network-like structures to exhibit superhydrophobicity and oleophobicity on PPS composite coatings. It can be observed that the surface morphologies of the prepared coating exhibit both internal and external nano/μ-structure. The surface with 1 % CNTs-PDMS content has more rough structure on external surface, but it has less network-like nano-structure under the high-magnification. As a result, it obviously shows the smaller WCAs than the coating with 9 % CNTs-PDMS. In addition, it could be clearly seen that there is much more nano-structure on the surface Fig. 5 d, while the surface morphology in Fig. 5 c is almost micro-structure. What is more, the low-magnification Fig. 5 a, b shows that surface of 9 % CNTs-PDMS coating is smoother and more uniform than that of 1 % CNTs-PDMS coating. Therefore, the nano-network structure is generated in the composite coating during the heat treatment; thus,CNTs and ZnO particles play an important role in fabricating superhydrophobic surfaces.
Mechanical property
The wear test of the PPS/3 %ZnO-PDMS/9 %CNTs-PDMS composites coating was conducted as reported in the literature [36]. Results shown in Fig. 6 depict that the WCA of the friction surface on the coating is a little higher than that of the fresh one after 1-hour wear test. While, after long times of wear test, the WCA of the coating is showing a declining trend. The reason of the above phenomenon can be explained from two aspects. First, the density of ZnO (5.6 g/cm3) is higher than CNTs (1.8 g/cm3), so the location of ZnO in the coating can be a little lower than that of CNTs after the coating calcinating for 2 h. Therefore, the appearance of the little higher of CA at 1 h, is attributed to the exposure of more rough structure on the surface such as the micro-ZnO after short time friction. Second, the stearic acid on the surface of coating is reducing with the increase of friction time. More and more residual hydroxyl and carboxyl groups are exposed. That is the point of the decreasing of WCAs of the friction surface on the coating. However, the whole special construction distributed uniformly in the coating; the WCA is still higher than 150°after wear test for 8 h, indicating that the coating has good wear resistance.
Figure 7 shows the bending resistance property and the adhesive ability of the prepared coatings. From Fig. 7a,b , we can observe that the surface of the prepared coating exhibit slight cracks when the coating is bended at 90 angle. It is well known that the PPS base coatings have the weakness of high brittleness [37]. We tested that the pure PPS can only be subjected to 35°bending. This result can be explained by the cross-linking effect of CNTs and it could obviously improve the bending resistance of the PPS based coating. In addition, the adhesive ability of the coating was qualitatively determined. According to GB/T92, grade 0 indicates the best adhesion property of the coating, and grade 5 corresponds to the worst. As shown in Fig. 7 c , the prepared composite coating has little peeling at the intersection of the scratches, and the impacted area in the cross incision is less than 5 %. This coating can be classified as grade 1.
Environmental tolerance
Thermal stability and acid-base resistance property of WCAs for the PPS/ZnO-PDMS/CNTs-PDMS composite coating are also tested (Fig. 8). The variations of WCAs under the temperatures of 50–200 °C on the PPS/ZnO-PDMS/CNTs-PDMS composite coating are shown in Fig. 8 a . There is no significant change of the WCAs (168 ± 2.0°) when the temperature increased from 50 to 100, which shows outstanding heat resistance. Moreover, there is only a little decrease of the WCAs although the temperature increased around 150–200 °C. All the WCAs are in the range of 167.2 to 169.5°, within experimental errors, indicating that the PPS/ZnO-PDMS/CNTs-PDMS composite coating can maintain the characteristic of superhydrophobic at both room temperature and the elevated temperature around 150 to 200 °C. The results of the decrease of WCAs under the temperature of 200 °C are attributed to the shape change of the network-like structure. It can be noticed that the surface roughness of the prepared coating decreased after 200 °C calcination. This should be related to the melting and softening phenomenon of the polymer (Fig. 9a, c .
The WCAs results of PPS/3 %ZnO-PDMS/9 %CNTs-PDMS composite coatings soaking in different pH conditions are shown in Fig. 8 b. The insets in Fig. 3 show the WCAs of the coating under strongly acidic (pH = 1) and alkaline conditions (pH = 14). Five drops are placed at five different locations on the coating surface, and then the averaged values are obtained. The tested coatings were immersed in the corrosion conditions for 12 h, then it was cleaned with distilled water and dried in 80 °C. For the corrosion conditions tested composite coating, there is no observable change of the CAs (168 ± 2.0°) when the pH increased from 1 to 14. All the WCAs are in the range of 167.2 to 169.5°, within experimental error, indicating that the composite coatings have a shield property not only for water but also corrosive liquids with pH range from 1 to 14. To probe the mechanisms of the corrosion surface, the morphologies of the acid-base treated coatings were explored (Fig. 9). Obviously, surface of base-treated coating have little change, while surface of acid-treated coating became more porous. Results indicate that the surface structure and the covered chemicals could not be damaged or changed by base liquid. In addition, results further indicate that PDMS covered on the surface of ZnO could partly protect it from acid corrosion. Therefore, the above results will have great significance in extending the application scope of PPS/3 %ZnO-PDMS/9 %CNTs-PDMS composite coating.
Durable stability
WCA on the as-prepared composite coatings after outdoors air exposure for different times are shown in Fig. 10. We can see that the WCA of the PPS/ZnO-PDMS/CNTs-PDMS composite coating appears only a little slight decrease after placed for 20, 60, and 90 days, showing high durability. This is mainly due to these: (1) Constructed of network-like structure with high stability within the coating provides a load supporting role and hierarchical structures for long time. (2) Chemically modified ZnO-PDMS CNTs-PDMS particles have a high chemical stability, which ensure that the coating always has a low surface energy.
Corrosion resistance of the prepared coating
The Tafel plots for (a) the pure aluminum plate, (b) PPS/ZnO/CNTs composite coating, (c) PPS/ZnO-PDMS/CNTs-PDMS composite coating are shown in Fig. 11. Compared with the aluminum plate, we can observe that the corrosion current (Icorr) decrease from 10–1.75 to 10–2.75 mA/cm2 after coated the PPS/ZnO-PDMS/CNTs-PDMS composite coating and the corrosion potential also revealed a slight decrease. While the corrosion current (Icorr) of PPS/ZnO/CNTs composite coating has no change and the corrosion potential decreased little. The reason for the improved corrosion resistance of the aluminum plate can be attributed to the enhanced shielding effect of highly hydrophobic PPS coating by introducing the ZnO-PDMS/CNTs-PDMS composite, which can keep oxygen gas and water from the aluminum plate. Both the modified PPS/ZnO/CNTs composite coating and the unmodified PPS/ZnO/CNTs composite coating are micro and nanostructures, the only difference is that the unmodified PPS/ZnO/CNTs composite coating has a larger amount of hydrophobic group on the surface. Accordingly, these results reveal that the nature of hydrophobic group, which can keep oxygen gas and water from inside the coating, is an important factor for anticorrosion.
Conclusions
In conclusion, a simple spraying approach is applied to obtain a non-fluorinated superhydrophobic composite coating with heat/durability resistance properties. The CA for water, glycerine, and ethylene glycol of PPS/ZnO-PDMS/CNTs-PDMS coatings reaches a maximum value of about 171 ,131, and 128°, respectively. The coating retain good superhydrophobic stability in a wide range of pH values (from 1 to 14). Moreover, its superhydrophobicity can be maintained even after the temperature increased from room temperature to the elevated temperature around 150 °C, which shows outstanding heat resistance. Besides, the un-stearic treated PPS/ZnO-PDMS/CNTs-PDMS composite coating shows a high bond strength even if the coating revolve 90 and 180°. In addition, there is no significantly decrease on the PPS/ZnO-PDMS/CNTs-PDMS composite coating after storing in air for as long as 90 days, showing a long-term weather ability. More importantly, results have indicated that coatings with hydrophobic group on the surface is showing a higher anticorrosion property than the coatings with the same surface morphology, which means the shielding effect is an important function for anticorrosion coating. Such superhydrophobic surfaces would have significant potentials for many applications, such as anti-drag transport of liquids, self-cleaning coating for buildings, and automobile decoration.
References
Ge J, Si Y, Fu F, Wang J, Yang J, Cui L, Ding B, Yu J, Sun G (2013) Amphiphobic fluorinated polyurethane composite microfibrous membranes with robust waterproof and breathable performances. RSC Adv 3(7):2248–2255
Rao AV, Gurav AB, Latthe SS, Vhatkar RS, Imai H, Kappenstein C, Wagh P, Gupta SC (2010) Water-repellent-porous silica films by sol–gel dip coating method. J Colloid Interface Sci 352(1):30–35
Xiong D, Liu G, Duncan ES (2013) Robust amphiphobic coatings from bi-functional silica particles on flat substrates. Polymer 54(12):3008–3016
Wang H, Sun F, Wang C, Zhu Y, Wang H (2015) ) A simple drop-casting approach to fabricate the super-hydrophobic PMMA-PSF-CNFs composite coating with heat-, wear-and corrosion-resistant properties. Colloid Polym Sci:1–7
G-Y W, Wang S-S, J-C W, Hsu M-Y, Chen H (2011) Preparation of highly adhesive and superhydrophobic epoxy-based thin film by sol–gel process. J Adhes Sci Technol 25(10):1095–1106
Motlagh NV, Birjandi FC, Sargolzaei J, Shahtahmassebi N (2013) Durable, superhydrophobic, superoleophobic, and corrosion resistant coating on the stainless steel surface using a scalable method. Appl Surf Sci 283:636–647
Hozumi A, Cheng DF, Yagihashi M (2011) Hydrophobic/superhydrophobic oxidized metal surfaces showing negligible contact angle hysteresis. J Colloid Interface Sci 353(2):582–587
Song J-1, W-j X, Liu X, Lu Y, Sun J (2012) Electrochemical machining of super-hydrophobic Al surfaces and effect of processing parameters on wettability. Applied Physics A 108(3):559–568
Tuteja A, Choi W, Ma M, Mabry JM, Mazzella SA, Rutledge GC, McKinley GH, Cohen RE (2007) Designing superoleophobic surfaces. Science 318(5856):1618–1622
Ganesh VA, Dinachali SS, Nair AS, Ramakrishna S (2013) Robust superamphiphobic film from electrospun TiO2 nanostructures. ACS Appl Mater Interfaces 5(5):1527–1532
Cui Z, Ding J, Scoles L, Wang Q, Chen Q (2013) Superhydrophobic surfaces fabricated by spray-coating micelle solutions of comb copolymers. Colloid Polym Sci 291(6):1409–1418
Dong F, Ha C-S (2012) Multifunctional materials based on polysilsesquioxanes. Macromol Res 20(4):335–343
Xue C-H, Jia S-T, Zhang J, Tian L-Q (2009) Superhydrophobic surfaces on cotton textiles by complex coating of silica nanoparticles and hydrophobization. Thin Solid Films 517(16):4593–4598
Zhou H, Wang H, Niu H, Gestos A, Wang X, Lin T (2012) Fluoroalkyl silane modified silicone rubber/nanoparticle composite: a super durable, robust superhydrophobic fabric coating. Adv Mater 24(18):2409–2412
Andrews H, Eccles E, Schofield W, Badyal J (2011) Three-dimensional hierarchical structures for fog harvesting. Langmuir 27(7):3798–3802
Chhatre SS, Choi W, Tuteja A, Park K-C, Mabry JM, McKinley GH, Cohen RE (2009) Scale dependence of omniphobic mesh surfaces. Langmuir 26(6):4027–4035
Mazrouei-Sebdani Z, Khoddami A (2011) Alkaline hydrolysis: a facile method to manufacture superhydrophobic polyester fabric by fluorocarbon coating. Prog Org Coat 72(4):638–646
Kang SM, Kim SM, Kim HN, Kwak MK, Tahk DH, Suh KY (2012) Robust superomniphobic surfaces with mushroom-like micropillar arrays. Soft Matter 8(33):8563–8568
Grigoryev A, Tokarev I, Kornev KG, Luzinov I, Minko S (2012) Superomniphobic magnetic microtextures with remote wetting control. J Am Chem Soc 134(31):12916–12919
Pan S, Kota AK, Mabry JM, Tuteja A (2012) Superomniphobic surfaces for effective chemical shielding. J Am Chem Soc 135(2):578–581
Zhou S, Ding X, Wu L (2013) Fabrication of ambient-curable superhydrophobic fluoropolysiloxane/TiO 2 nanocomposite coatings with good mechanical properties and durability. Prog Org Coat 76(4):563–570
Lee J-P, Choi S, Park S (2010) Extremely superhydrophobic surfaces with micro-and nanostructures fabricated by copper catalytic etching. Langmuir 27(2):809–814
Jafari R, Menini R, Farzaneh M (2010) Superhydrophobic and icephobic surfaces prepared by RF-sputtered polytetrafluoroethylene coatings. Appl Surf Sci 257(5):1540–1543
Feng X, Jiang L (2006) Design and creation of superwetting/antiwetting surfaces. Adv Mater 18(23):3063–3078
Ge B, Zhang Z, Zhu X, Ren G, Men X, Zhou X (2013) A magnetically superhydrophobic bulk material for oil removal. Colloids Surf A Physicochem Eng Asp 429:129–133
Gnanappa AK, Papageorgiou DP, Gogolides E, Tserepi A, Papathanasiou AG, Boudouvis AG (2012) Hierarchical, plasma nanotextured, robust superamphiphobic polymeric surfaces structurally stabilized through a wetting–drying cycle. Plasma Process Polym 9(3):304–315
Zhou H, Wang H, Niu H, Gestos A, Lin T (2013) Robust, self-healing superamphiphobic fabrics prepared by two-step coating of fluoro-containing polymer, fluoroalkyl silane, and modified silica nanoparticles. Adv Funct Mater 23(13):1664–1670
Tsai H-J, Lee Y-L (2007) Facile method to fabricate raspberry-like particulate films for superhydrophobic surfaces. Langmuir 23(25):12687–12692
Chakradhar R, Kumar VD, Rao J, Basu BJ (2011) Fabrication of superhydrophobic surfaces based on ZnO–PDMS nanocomposite coatings and study of its wetting behavior. Appl Surf Sci 257(20):8569–8575
Wang H, Zhao J, Zhu Y, Meng Y, Zhu Y (2013) The fabrication, nano/micro-structure, heat-and wear-resistance of the superhydrophobic PPS/PTFE composite coatings. J Colloid Interface Sci 402:253–258
Ghosh N, Bajoria A, Vaidya AA (2009) Surface chemical modification of poly (dimethylsiloxane)-based biomimetic materials: oil-repellent surfaces. ACS Appl Mater Interfaces 1(11):2636–2644
Feng L, Zhang Y, Xi J, Zhu Y, Wang N, Xia F, Jiang L (2008) Petal effect: a superhydrophobic state with high adhesive force. Langmuir 24(8):4114–4119
Chang Y, Liu X, Yang H, Zhang L, Cui Z, Niu M, Liu H, Chen J (2016) Nonsolvent-assisted fabrication of multi-scaled polylactide as superhydrophobic surfaces. Soft Matter 12(10):2766–2772
Almutairi Z, Ren CL, Simon L (2012) ) Evaluation of polydimethylsiloxane (PDMS) surface modification approaches for microfluidic applications. Colloids Surf A Physicochem Eng Asp 415:406–412
dos Santos MP, Magosso HA, Yoshida IV, Gushikem Y (2012) Poly (dimethylsiloxane) (PDMS)-Schiff base, a new polymeric network and its adsorbent capability for copper ions from ethanol. Colloids Surf A Physicochem Eng Asp 398:1–8
Wang H, Liu Z, Wang E, Zhang X, Yuan R, Wu S, Zhu Y (2015) Facile preparation of superamphiphobic epoxy resin/modified poly (vinylidene fluoride)/fluorinated ethylene propylene composite coating with corrosion/wear-resistance. Appl Surf Sci 357:229–235
Wang H, Gao D, Meng Y, Wang H, Wang E, Zhu Y (2015) Corrosion-resistance, robust and wear-durable highly amphiphobic polymer based composite coating via a simple spraying approach. Prog Org Coat 82:74–80
Acknowledgments
The research is financially supported by the National Young Top Talents Plan of China (2013042), National Science Foundation of China (51175066), FANEDD (201164), New Century Excellent Talents in University (NCET-12-0704), the Science Foundation for Distinguished Young Scholars of Heilongjiang Province (JC201403), and the Northeast Petroleum University Innovation Foundation For Postgraduate (YJSCX2016-016NEPU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this article.
Rights and permissions
About this article
Cite this article
Wang, H., Wang, C., Gao, D. et al. Heat/durability resistance of the superhydrophobic PPS-based coatings prepared by spraying non-fluorinated polymer solution. Colloid Polym Sci 294, 1519–1527 (2016). https://doi.org/10.1007/s00396-016-3914-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3914-7