Abstract
We report the thermotropic properties and measurements of the temperature and concentration dependences of the refractive index in isotropic micellar L 1 phase and nematic-calamitic N C mesophase of the hexadecyltrimethyl ammonium bromide/water (CTAB/H2O) and hexadecyltrimethyl ammonium bromide/water/1-decanol (CTAB/H2O/DeOH) lyotropic liquid crystalline systems. The effect of amphiphile and aliphatic alcohol concentrations on the refractive properties of L 1 phase and N C mesophase has been studied. Jump-like changes of the refractive properties in regions of the L 1 phase–N C mesophase lyotropic and thermotropic phase transitions have been found. The sufficient effect of CTAB addition on the refractive index value has been found for the CTAB/H2O and CTAB/H2O/DeOH lyotropic systems. In this work, the shape of micelles in L 1 phase and N C mesophase has been also estimated. Estimation showed that structural units of L 1 phase are the isometric spherical micelles, and structural units of N C mesophase are the anisometric rod-like micelles. Typical textures of N C mesophase in the CTAB/H2O and CTAB/H2O/DeOH lyotropic systems and the heterophase regions of the N C mesophase–isotropic liquid thermotropic phase transitions are presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lyotropic systems exhibit various types of optically isotropic phases and optically anisotropic mesophases. These phases and mesophases take place in the strongly definite temperature and concentration intervals [1–6]. Between these phases and mesophases, the biphasic and multiphasic regions and also the thermotropic and lyotropic phase transitions arise. Phase states, phase and mesophase boundaries, and the mesomorphic properties of lyotropic systems are determined by the phase diagrams [7–12].
Phases and mesophases of lyotropic systems display various physical properties (optical, dielectric, diamagnetic, viscous-elastic, acoustic, etc. properties). Because of the importance of the thermo-optical, magneto-optical, electro-optical, and acoustical-optical applications of lyotropic and thermotropic liquid crystalline systems, the optical properties of lyotropic phases and mesophases have special interest from both fundamental and application points of view. General optical parameter for isotropic phases and anisotropic mesophases in liquid crystals is the refractive index. This parameter determines the optical refractive properties of media and can change with concentration, temperature, number, and types of components in lyotropic liquid crystalline systems [13–18]. As it is noted in [19–21], study of the refractive indexes is a key for fundamental studies and practical applications of liquid crystals. Therefore, detailed investigations of the optical refractive properties and effect of components on these properties in lyotropic liquid crystalline systems have special interest.
In this work, we are interested in the thermo-morphologic and optical refractive properties of optical isotropic micellar L 1 phase and optical anisotropic nematic-calamitic N C mesophase in the binary and ternary lyotropic systems. The lyotropic systems under investigation are based on amphiphile as hexadecyltrimethyl (cetyltrimethyl) ammonium bromide (CTAB). The effect of amphiphile, water, and aliphatic alcohol on the thermo-morphologic and optical refractive properties of the abovementioned lyotropic systems have been investigated. We are also interested in the shape of micelles in the abovementioned lyotropic systems in L1 phase and ND mesophase for corresponding concentration intervals of lyotroopic systems under investigations. Results of such investigations are presented in this work.
Experimental
In this work, isotropic micellar L 1 phase and nematic-calamitic N C mesophase of the binary CTAB/H2O and ternary CTAB/H2O/DeOH lyotropic systems have been used. CTAB (cat. No.814119) and DeOH (cat. No.803463) were purchased from Merck. These materials have the high degree of purity and therefore were used without further purification. Water, which was used as the general solvent, was triple distilled and deionized.
The samples as the microslides were used in this work. The thickness of liquid crystalline layer in the microslides was 120 ± 1.0 μm. The samples were hermetically closed at once after filling by liquid crystalline system.
Investigations of the mesomorphic and thermo-morphologic properties of the CTAB/H20 and CTAB/H20/DeOH lyotropic liquid crystalline systems have been carried out using the polarizing optical microscopy (POM) method. Our setup consists of a trinocular polarizing microscope with orthoscopic/conoscopic observations, microphotographic system, Berek compensator and quartz plate from Olympus Optical Co., λ-plates (λ = 137 μm and λ = 530 μm), optical filters, special heater thermostat with digital temperature control system, differential Cu–Co thermocouples, power supply, and multimeters.
The refractive properties of the CTAB/H20 and CTAB/H20/DeOH lyotropic liquid crystalline systems have been investigated by using the polythermic refractometry setup (PR). The PR setup consists of Abbe’s Precision Refractometer with Digital Thermometer from Atago Co. Ltd and recirculation immersion thermostat Ultraterm 200 Selecta. Accuracy of the refractive indices measurements was as 0.1 %. Temperature of liquid crystal under investigation was also controlled by Co–Co thermocouple with accuracy as ± 0.1 K. Thermocouple was placed in close vicinity of the lyotropic system.
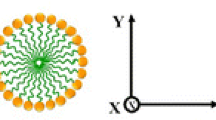
For estimation of the shape of micelles in lyotropic phases and mesophases of lyotropic liquid crystalline systems under investigations, the method of the electrical conductivity anisotropy in the orientational shear flow has been used. Principles of this classic method were described in detail in [22–24]. In accordance with this method, the sum of changes of the electrical conductivity values for both the rod-like and disc-like (plate-like) micelles in the three mutually perpendicular directions must be equal to zero \( \left({\displaystyle \sum \frac{\sigma_i-{\sigma}_0}{\sigma_0}=0}\right) \) [22, 23, 25, 26] (here i = x, y, z.). However, in the case of the rod-like micelles, a decrease of the electrical conductivity anisotropy in the X- and Z-directions and an increase of the electrical conductivity anisotropy in the Y-direction must take place. In the case of the disc-like (plate-like) micelles, a decrease of the electrical conductivity anisotropy in the X-direction and an increase of the electrical conductivity anisotropy in the Y- and Z-directions must take place [22, 23, 25, 26]. Therefore, by determination of the values of the electrical conductivity anisotropy in the three mutually perpendicular directions, the shape of micelles can be identified from the sign of these values [22–28]. In Fig. 1, the scheme of coaxial couette, which consists of the rotor, stator, and electrodes, is presented. The electrodes allow to determine the electrical conductivity anisotropy in the direction of the velocity gradient (X-direction), in the direction of the shift flow (Y-direction), and in the direction, which simultaneously is perpendicularly to the shift flow and to the velocity gradient (Z-direction).
We would like to note that the abovementioned method is similar to method of determination of the micelle shape by the optical birefringence of anisometric micelles in the shear flow [29].
Results and discussion
In this work, we are interested in the refractive properties of micellar L 1 phase and nematic-calamitic N C mesophase. For L 1 phase in binary lyotropic system, samples S1a–S5a with variable ratio of CTAB/H20 and in ternary lyotropic system, samples S6a–S10a with constant ratio of CTAB/H20, and variable concentration of DeOH have been investigated (Table 1). For N C mesophase in binary lyotropic system, samples S1b–S5b with variable ration of CTAB/H20 and also in ternary lyotropic system, samples S6b–S10b with constant ratio of CTAB/H20, and variable concentration of DeOH have been investigated (Table 2). L 1 phase in the CTAB/H20 and the CTAB/H20/DeOH lyotropic systems exhibits optical isotropic texture with black background. N C mesophase in the CTAB/H20 and the CTAB/H20/DeOH lyotropic systems displays by textures, which are presented in Fig. 4a, b. As seen in Fig. 2a, b, these textures are the same type and are typical schlieren textures for NC mesophase. Textures consist of the thread-like formations, singular points, and small uniform regions and are well known for N C mesophase of lyotropic liquid crystalline systems [9, 30–35].
The homeotropic uniform alignment of N C mesophase has been obtained by application of magnetic field of 7.0 kG, which was applied parallel to the reference surfaces of the microslide. In the uniform-aligned regions, the rod-like micelles are oriented perpendicularly to the reference surfaces of the microslide. In this case, the director of N C mesophase is oriented perpendicularly to the reference surfaces and molecules of amphiphile in the rod-like micelles are oriented parallel to these surfaces. Such arrangement of the optical axis and amphiphile molecules is cause of the negative optical birefringence (Δn = n || – n ⊥ < 0) for N C mesophase.
By the heating, The N C–isotropic liquid (NC–I) thermotropic phase transition has been observed in the CTAB/H2O and CTAB/H2O/DeOH lyotropic systems (Fig. 3). The thermo-morphologic investigations showed that schlieren texture of N C mesophase exists without any change up to the thermotropic transition to an isotropic liquid state. This fact indicates on thermal stability of this mesophase in the abovementioned lyotropic systems.
For control and confirmation of presence of the spherical and rod-like micelles in the corresponding regions (regions of L 1 phase and N C mesophase), the method of the electrical conductivity anisotropy in the orientational shear flow has been used. Investigations showed that for the samples in concentration region L 1 phase did not have any electrical conductivity anisotropy in the X-, Y-, and Z-directions. As an example, in Fig. 4a, the dependence of the electrical conductivity anisotropy vs. rotational frequency for L 1 phase is presented. As known, such behavior of the electrical conductivity anisotropy indicates on the spherical shape of micelles in lyotropic systems [19, 20, 23, 36, 37] (Fig. 5a). Investigations showed that for the samples in concentration region of N C mesophase, the electrical conductivity anisotropy in the X- and Z-directions was negative and in the Y-direction was positive. As an example, in Fig. 4b, the dependences of the electrical conductivity anisotropy vs. rotational frequency for N C mesophase are presented. As is known, such behavior of the electrical conductivity anisotropy indicates on the rod-like micelles in lyotropic systems [19, 20, 23, 36, 37] (Fig. 5b).
The electrical conductivity anisotropy vs. rotational frequency for L 1 phase in 20 wt%CTAB/80 wt%H20 and (20 wt%CTAB/80 wt%H20)+0.5 wt%DeOH lyotropic systems (a) and the electrical conductivity anisotropy vs. rotational frequency for N C mesophase in 24 wt%CTAB/76 wt%H20 (in red) and (24 wt%CTAB/76 wt%H20)+0.5 wt%DeOH (in blue) (b) lyotropic systems
Investigations of the temperature and concentration dependences of the refractive index for L 1 phase (samples S1a–S5a) showed that this index linearly decreases with an increase of temperature (Fig. 6). As is seen in Fig. 6a, an increase of CTAB concentration in binary, the CTAB/H2O lyotropic system leads to an increase of the refractive index values. Behavior of the n = n(T) dependences for S1a–S5a samples can be characterized by the y = −2.6·10−4·x +1.3710 equation. The addition of DeOH in lyotropic system with constant CTAB/H2O ratio does not lead to a significant change of the refractive index values in L 1 phase for S6a–S10a samples (Fig. 6b). The effect of CTAB addition in lyotropic system is more significant for variation of the refractive index than effect of aliphatic alcohol. Thus, by variation of amphiphile concentration in the CTAB/H2O lyotropic system is possible to regulate the refractive properties of L 1 phase in comparison with variation of aliphatic alcohol concentration in such lyotropic systems.
Investigations of the temperature and concentration dependences of the refractive index for N C mesophase (S1b–S5b samples) showed that the refractive index increases with an increase of CTAB concentration in the CTAB/H2O lyotropic system (Fig. 7). As is seen in Fig. 7a, the n = n(T) dependences for S1b–S5b samples exhibit linearly a decrease with an increase of temperature. Besides, these dependences exhibit the jump-like change at definite temperature. Investigations of the thermotropic properties of S1b–S5b samples showed that at these temperatures, the N C mesophase–isotropic liquid (N C –I) thermotropic phase transition and appearance of the heterophase regions of this transition took place. Thus, the jump-like change of the refractive index in S1b–S5b samples corresponds to the phase transition from N C mesophase to an isotropic liquid.
Investigations showed that the addition of DeOH in lyotropic system with constant CTAB/H2O ratio did not lead to a significant change of the refractive index values in N C mesophase for S6b–S10b samples (Fig. 7b). As it is noted above, the addition of DeOH in lyotropic system with constant CTAB/H2O ratio did not lead also to a significant change of the refractive index values in L 1 phase. The effect of CTAB addition in lyotropic system is more significant for variation of the refractive index in L 1 phase and N C mesophase than effect of aliphatic alcohol. As known, an increase of amphiphile concentration in lyotropic system leads to an increase of number of micelles in volume of liquid crystalline system and to a decrease of distance between micelles [4, 38, 39]. Such effect leads to changes of interaction between micelles. Additionally, as is known, an increase of amphiphile concentration in lyotropic system leads to an increase of the order degree of polar parts and non-polar chains of amphiphile molecules in micelles [1, 38, 40]. Obviously, such effects lead to change of the refractive index in L 1 phase and N C mesophase. Thus, by variation of amphiphile concentration in the CTAB/H2O, one can regulate the refractive properties of L 1 phase and N C mesophase in comparison with variation of aliphatic alcohol concentration in such lyotropic system.
Concentration dependences of the refractive index n = n(c) for the CTAB/H2O lyotropic system at constant temperature conditions are presented in Fig. 8. As seen in this figure, the refractive index of L 1 phase at constant temperature condition exhibits the linear behavior. The linear behavior of the refractive index for these temperatures exhibits also N C mesophase. However, the tilt angles for L 1 phase (θ 1) and N C mesophase (θ 2) are quite different. Results, which are presented in Fig. 8, show that interval of change of the refractive index in L 1 phase is larger than such interval in N C mesophase. Besides, investigations showed that change of the tilt angle value for dependences, which are presented in Fig. 8, is corresponds to the L 1 phase–N C mesophase (L 1–N C) lyotropic phase transition in the CTAB/H2O lyotropic system, i.e., in the L 1–N C lyotropic phase transition region, the n = n(c) dependences exhibit an alteration of the tilt angle. Thus, by the studies of the temperature and concentration dependences of the refractive index in lyotropic liquid crystalline systems, information about temperature and concentration phase transitions between lyotropic phases and mesophases can be obtained.
Summary
In this work, we are interested in the thermotropic and optical refractive properties of optical isotropic micellar L 1 phase and optical anisotropic nematic-calamitic N C mesophase in the CTAB/H20 and CTAB/H20/DeOH lyotropic liquid crystalline systems. The effect of amphiphile, water, and aliphatic alcohol on the thermo-morphologic and optical refractive properties of the abovementioned lyotropic systems have been investigated. For control and confirmation of availability of the spherical and disc-like micelles in the corresponding concentration regions, the shapes of structural units were estimated. The method of the electrical conductivity anisotropy in the orientational shear flow has been used.
The results, obtained in this work may be shortly summarized as follows:
-
An increase of CTAB concentration in the binary CTAB/H2O lyotropic system leads to an increase of the refractive index values in L 1 phase and N C mesophase. Such change of the refractive index value with change of CTAB concentration is obviously connected with the fact that an increase of CTAB concentration leads to an increase of the order of polar parts and non-polar chains of amphiphile molecules in micelles and also is connected with the fact that such increase in concentration leads to change of number of micelles in volume of lyotropic system.
-
The addition of DeOH in the CTAB/H2O lyotropic system with constant CTAB/H2O ratio does not lead to a significant change of the refractive index values in L 1 phase and N C mesophase. Obviously, the addition of non-polar solvent (decanol) in lyotropic system does not lead to change of order of amphiphile molecules in micelles and also does not lead to change of interaction between micelles.
-
By change of CTAB concentration in both the CTAB/H2O and CTAB/H2O/DeOH lyotropic systems, the control of the refractive index values in L 1 phase and N C mesophase can be carried out.
-
Jump-like changes of the refractive index have been observed in the regions of the N C–I thermotropic and L 1–N C lyotropic phase transitions. Such changes of the refractive index are connected with transformation of partially ordered structure with definite spatial symmetries of micelles to disordered state at the N C–I phase transition and with transformation of the optical isotropic state to the optical anisotropic state in the L 1–N C phase transitions.
-
Estimation of structural units of L 1 phase N C mesophase showed that such units in L 1 phase are the isometric spherical micelles and in N C mesophase are anisometric rod-like micelles. Additionally, L 1 phase is characterized by the absence of any electrical conductivity anisotropy, and N C mesophase is characterized by definite electrical conductivity anisotropy in the mutually perpendicular directions (X-, Y-, and Z-directions).
References
Friberg S (1992) Organized solutions: surfactants in science and technology. CRC Press, New York
Ekwall P (1975) Composition, properties and structures of liquid crystalline phases in systems of amphiphilic compounds. In: Brown GH (ed) Advances in Liquid Crystals Volume 1. Academic Press
Petrov AG (1999) The lyotropic state of matter: molecular physics and living matter physics. Gordon & Breach Science Publishers, London – New York
Figueiredo Neto AM, Salinas SRA (2005) The physics of lyotropic liquid crystals: phase transitions and structural properties. Oxford University Press, Oxford
Nesrullajev A (2007) Lyotropic liquid crystalline systems: amphiphilic systems. Mugla University Press, Mugla
Bartolino R, Meuti M, Chidichimo G, Ranieri GA (1985) In: V. Degiorgio V, Corti M (ed) Physics of amphiphiles: micelles, vesicles and microemulsions. North Holland Press, Amsterdam/Oxford/New York/Tokyo, pp 524
Kuzma MR, Saupe A (1997) In: Collings PJ, Patel JS (ed)Handbook of Liquid Crystal. Oxford University Press, New York/Oxford, pp 237
Özden P, Nesrullajev A, Oktik Ş (2010) Phase states and thermomorphologic, thermotropic, and magnetomorphologic properties of lyotropic mesophases: sodium lauryl sulphate–water–1-decanol liquid-crystalline system. Phys Rev E 82:061701. doi:10.1103/PhysRevE.82.061701
Nesrullajev A (2014) Comparative investigations of phase states, mesomorphic and morphologic properties in hexadecyltrimethyl ammonium bromide/water and hexadecyltrimethyl ammonium bromide/water/1-decanol lyotropic liquid crystalline systems. J Mol Liq 200:425. doi:10.1016/j.molliq.2014.10.036
Hoffmann H, Oetter G, Scwandner B (1987) The aggregation behaviour of tetradecyldimethylaminoxide. Prog Coll Polym Sci 73:95–106. doi:10.1007/3-798-50724-4_68
Santin Fulho O, Itri R, Amaral LQ (2000) Decanol effect on the structure of the hexagonal phase in a lyotropic liquid crystal. J Phys Chem B 104:959. doi:10.1021/jp993240d
Amaral LQ, Santos OR, Braga WS, Kimura NM, Palangana AJ (2015) Biaxial phase and coexistence of the two uniaxial nematic phases in the system sodium dodecyl sulphate–decanol–D2O. Liq Crys 42:240–247. doi:10.1080/02678292.2014.981604
Nesrullajev A, Alippi A, Bertolotti M, Ferrari A, Sibilia C (1987) Optical nonlinear behavior of lyotropic liquid crystal systems. Mol Cryst Liq Cryst 152:105. doi:10.1080/00268948708070945
Nesrullajev A (1988) The surface wave excitation in lyotropic liquid crystals. Lett JTF (Sov) 13:428
Mattoussi H, Srinivasarao M, Kaatz PG, Berry GC (1992) Refractive-indexes dispersion and order of lyotropic liquid-crystal polymers. Macromolecules 25:2860–2868. doi:10.1021/ma00037a012
Gomati R, Gharbia M, Gharbi A (2002) Refractive index variation in swollen lyotropic lamellar liquid crystal. Opt Commun 111:71
Pereira JR, Palangana AJ, Mansanares AM, da Silva EC, Bento AC, Baesso ML (2000) Inversion in the change of the refractive index and memory effect near the nematic-isotropic phase transition in a lyotropic liquid crystal. Phys Rev E 61:5410–3. doi:10.1103/PhysRevE.61.5410
Alves S, Cuppo FLS, Figueiredo Neto AM (2006) Determination of the nonlinear refractive index of lyotropic mixtures with and without ferrofluid doping: a time-resolved Z-scan experiment in millisecond time scales. J Opt Soc Amer B 23:67–74. doi:10.1364/JOSAB.23.000067
Mitra M, Gupta S, Paul R, Paul S (1991) Determination of orientational order parameter from optical studies for a homologous series of mesomorphic compounds. Mol Cryst Liq Cryst 199:257–266. doi:10.1080/00268949108030937
Pan R-P, Tsai T-R, Chen C-Y, Wang C-H, Pan C-L (2004) The refractive indices of nematic liquid crystal 4'-n-pentyl-4-cyanobiphenyl in the THz frequency range. Mol Cryst Liq Cryst 409:137–144. doi:10.1080/15421400490431039
Kumar A (2013) Determination of orientational order and effective geometry parameter from refractive indices of some nematics. Liq Cryst 40:503–510. doi:10.1080/02678292.2012.761355
Götz KG, Heckmann K (1958) The shape of soap micelles and other polyions as obtained from anisotropy of electrical conductivity. J Colloid Sci 13:266–272. doi:10.1016/0095-8522
Heckmann K, Götz KG (1958) Die bestimmung der form gelöster polyionen aus dem leitfähigkeitsanisotropie-effekt. Z Elektrochem 62:281–288. doi:10.1002/bbpc.19580620312
Rehage H (1982) Rheologische untersuchungen an viscoelastischen tensidlösungen. Bayreuth University, Dissertation
Schwarz G (1958) Zur theorie der leifahigkeitsanisotropie von polyelektrolyten in lösung. Z Phys 145:563–584
Nesrullajev A (1992) Lyotropic mesomorphism and electro-physics of lyotropic liquid crystalline systems. Dissertation, Baku Academy of Sciences
Heckmann K (1958) General discussion. Discuss Faraday Soc 25:71–73. doi:10.1039/DF9582500059
Voytilov VV, Trusov AA (1985) Investigation of the polarizability of the aqueous solution of palygorskite by electrooptical methods. Kolloid J 67:455–461
Frolov YG (1982) Colloid chemistry. Chemistry Publ, Moscow
Hoffmann H, Oetter G, Schwandner B (1987) The aggregation behaviour of tetradecyldimethylaminoxide. Progr Colloid Polym Sci 73:95–106. doi:10.1007/3-798-50724-4_68
Hertel G, Hoffmann H (1988) Lyotropic nematic phases of double chain surfactants. Progr Colloid Polym Sci 76:123–131. doi:10.1007/BFb0114182
Kuzma MR, Saupe A (1997) Structure and phase transitions of amphiphilic lyotropic liquid crystals. In: Collings PJ, Patel JS (eds) Handbook of liquid crystal research. Oxford University Press, New York/Oxford, pp 237–258
Nesrullajev A, Tepe M, Kazancı N, Çakmak HM, Abukay D (2000) Surface-induced textures in lyotropic liquid crystalline mesophases. Mater Chem Phys 65:125–129. doi:10.1016/S0254-0584(00)00225-X
Nesrullajev A, Oktik S (2007) Texture transformations and orientational properties of lyotropic nematics in magnetic field. Cryst Res Techn 42:44–49. doi:10.1002/crat.200610768
Sampaio AR, Palangana AJ, Visconini RC (2004) Investigation of uniaxial and biaxial lyotropic nematic phase transitions by means of digital image processing. Mol Cryst Liq Cryst 408:45–51. doi:10.1080/15421400490425838
Nesrullajev A (2010) Shape and sizes of micelles in nematic-calamitic and nematic-discotic mesophases: sodium lauryl sulphate/water/decanol lyotropic system. Mater Chem Phys 123:546–550. doi:10.1016/j.matchemphys.2010.05.012
Tsvetkov VN (1986) Hard-chain polymer molecules. Science Pub, Moscow
Sonin AS (1987) Lyotropic nematics Sov. Phys Usp 30:875–912. doi:10.1070/PU1987v030n10ABEH002967
Nesrullajev A (2013) Structural peculiarities of micelles in lamellar mesophase of lyotropic liquid crystalline systems: shape, sizes and anisometricity. Journ Mol Liq 187:337–342. doi:10.1016/j.molliq.2013.08.017
Yu LJ, Saupe A (1982) Deuteron resonance of D2O of nematic disodium cromoglycate-water systems. Mol Cryst Liq Crys 80:129–134. doi:10.1080/00268948208071026
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozden, P., Nesrullajev, A. Comparative investigations of the thermotropic and optical refractive properties in micellar isotropic phase and nematic-calamitic mesophase of hexadecyltrimethyl ammonium bromide/water and hexadecyltrimethyl ammonium bromide/water/1-decanol lyotropic liquid crystalline systems. Colloid Polym Sci 293, 3295–3303 (2015). https://doi.org/10.1007/s00396-015-3673-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3673-x