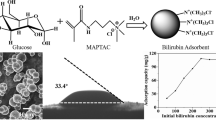
Abstract
The development and design of adsorbents with high adsorption performance is the actual aim for successful toxin removal. In this work, we report the performance of new guanidine-containing polymers as a shell orientated around silica particles with following application for effective bilirubin removal. To evaluate polymer-shell modification, Fourier transform infrared spectroscopy and high-resolution electron microscopy were performed. Changes in surface morphology of polymer-coated silicas were detected. It was shown that polymeric shell completely covers silica surface. The grafting amount was evaluated by thermogravimetric and was in 18–34 % range. Incorporation of guanidine containing polymers into silica matrix leads to the great increase in adsorption capacity According to the Langmuir model, the maximum adsorption capacity for bilirubin was 43.47 mg/g. We also demonstrated that prepared polymer-coated silicas could well adsorb bilirubin from the bilirubin-albumin solution at low concentration of albumin. Therefore, this study revealed that guanidine-containing polymers have great potential to serve as effective agents for improving of the adsorbent for bilirubin removal.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, a variety of nanomaterials with different structures have been applied in biomedicine because of their unique characteristics inherent to the nanoscale [1–3]. For example, mesoporous materials have been widely investigated and effectively used in drug delivery systems; different metallic nanoclusters based on gold, iron, silver nanodots, and its hybrids have found potential application in sensing and magnetic resonance imagining (MRI) [4, 5].
Preparation of nanoscale systems with new functional properties is a major challenge; however, silica nanoparticles remain widely used due to their unique features such as a high surface area and thermal and chemical stabilities [6]. The unique compatibility and possible size control in synthesis are allowed to realize different combinations of silica particles with other available materials. One of such approaches based on using silica as a scaffold for templating another material such as dyes, quantum, or magnetic dots [7, 8]. Here, silica is used as a shell for the protection of encapsulated materials. In another way, silica nanoparticles can be considered as seeds or cores around which other materials can be grafted (Fig. 1b). In this field, different functional polymers are being involved for surface modification of silica core [9]. Functional groups in polymeric chain provide excellent binding properties of modified silica surface for effective captures of different small objects: magnetite or fluorescent nanoparticles, drugs, etc. A number of polymers have been already synthesized and applied for silica functionalization. The most common studies include poly(acrylamide) [10], polyethilenglycol [11], and polyacrilic acid [12]. For this reason, a new kind of polymers should be examined for developing of new surface properties of polymer-coated silica particles. Taking into account the published results about polymer science and biotechnology, we suggest considering new guanidine-containing polymers based on polydiallyl and polymethacryloyl guanidine (Fig. 1a) as one of the most promising candidates for silica modification due to their low hemotoxicity and high ionic properties [13, 14]. There are only few studies concerning investigation and application of guanidine-containing polymers excepting polythexamethylen guanidine and its analogues [15]. Besides, It was reported that guanidine fragment in polymeric chain is more promising than their amine equivalents due to capability of guanidine groups bind stronger with negative molecules compared to amine groups [16].
a Structures of diallyl guanidine acetate (DAGA), polydiallyl guanidine acetate (PDAGA) and polymethacryloyl guanidine hydrochloride (PMCGH). b Schematic illustration of modification of silica core. c Structure formula of bilirubin in “ridge-tile” 5Z, 15Z conformation stabilized by six intramolecular hydrogen bonds
Polymer-coated silica particles have received tremendous attention as adsorbents for many organic compounds [17]. At present, synthesis of highly effective adsorbents has become a promising technique for blood detoxification, especially for bilirubin removal [18]. Bilirubin (Fig. 1c) is a dicarboxylic acid and circulates in human blood plasma where it is bound to serum albumin to form a water-soluble complex [19]. It is transported to the liver as a complex with albumin, where it is normally conjugated with glucuronic acid and excreted into bile [20]. However, bilirubin can be accumulated in blood at high concentration once patients suffer from a liver disease (hyperbilirubinemia) [21]. Several techniques have been employed for removal of the high concentration of free bilirubin from plasma in order to prevent hepatic and brain damage [22]. However, hemoperfusion treatment based on application of hemoadsorbents is one of the most effective techniques [23, 24]. Many kinds of adsorbents (carbon nanotubes, mesoporous silica, graphene oxide) have been exploited. All of them have some drawbacks related to the low adsorption capacity, biocompatibility, or high toxicity [21]. Therefore, the search and synthesis of new adsorbents for efficient bilirubin removal is very demanding.
In this work, polymer-coated silica particles were synthesized via sol-gel method in the presence of guanidine-containing polymer. A subsequent treatment of silica particles with positively charged guanidine-containing polymers leads to the great increase in adsorption capacity for bilirubin compared to unmodified silica. We demonstrated successful modification of silica core by guanidine-containing polymers using electron microscope, Fourier transform infrared (FT-IR) spectroscopy, and thermal analysis.
Experimental section
Chemicals
TEOS (Si(OC2H5)4, 98 %) and ammonia solution (25 wt%) were purchased from commercial chemical company “Ecos-1” (Russian Federation). Bilirubin (M w = 584.7 g/mol) and human serum albumin (HSA, > 97 % pure by gel electrophoresis, 66.478 kDa) were purchased from Sigma-Aldrich (USA). The guanidine-containing polymers, PDAGA (M w = 1594 g/mol) and PMCGH (M w = 15,051 g/mol), were provided by the department of macromolecular compounds of the Kabardino-Balkar State University and by the department of chemistry of polyelectrolytes and biomedical polymers of A.V. Topchiev Institute of Petrochemical Synthesis, Russian Academy of Science. All chemicals were of analytical grade and used without further treatment.
Preparation of polymer-coated silica particles
The silica particles (SiPs) were prepared according to the sol-gel method [25]. Briefly, for the synthesis of uncoated SiP, 8.57 mL of TEOS was mixed with 20 mL of water-ethanol (14 %) solution and stirred for 5 h. The ammonia solution (pH 9) was added as a base catalyst. Polymer-coated silica particles were synthesized via Stöber method: 10 mL of water solution containing the polymer at three different concentrations (42, 56, and 78 mg/mL) was mixed with 8.57 mL of TEOS to obtain a reaction mixture which was stirred for 5 h under basic condition (pH 9). Then, this mixture was transferred into a Petri dish and was aged until the formation of the solid products. The solid phase was separated by centrifugation and washed with distilled water to remove unreacted reagents. After that, it was dried under vacuum at 105 °C for 3 days. Such synthetic procedure allows obtaining 1.61 g of solid product (yield, ~ 70 %). The silica particles with the lowest and highest amount of grafted polymer were labeled “a” and “c,” respectively.
Bilirubin adsorption experiments
Ten milligrams of bilirubin was dissolved in 1 mL of 0.1 M NaOH solution. Then, the bilirubin solution was diluted to a definite concentration using 10 mM phosphate buffer (PBS, pH 7.4). For each experiment (static and dynamic bilirubin adsorption), 25 mg of polymer-coated silicas was incubating with 5 mL of bilirubin PBS solution with an initial concentration of 200 mg/L. In case of dynamic adsorption, 1 mL of suspension (which was centrifuged at 132,000 rpm before) was tested by UV-visible spectrometer at definite time and then returned to the initial tube. As for static experiment of bilirubin adsorption, the solution was separated by centrifugation at 132,000 rpm after the adsorption equilibrium has been attained (4 h). For obtaining adsorption isotherm, adsorption experiments were performed with different initial concentration of bilirubin at 30 °C for 4 h. All adsorption experiments were performed in a dark room. The concentration of bilirubin was determined by Varian Cary Bio UV-Visible Spectrophotometer at 438 nm. The amount of bilirubin adsorbed was calculated with following equation:
Where C o and C are the initial and the residual bilirubin concentrations in solution (mg/L), V is the volume of bilirubin solution (in L), m is the mass of the adsorbent (in mg), respectively.
For study of bilirubin adsorption in the presence of HSA solution, 25 mg of polymer-coated silicas was mixed with 5 mL of bilirubin PBS solution (C bilirubin = 200 mg/L) containing different initial concentration of HSA (from 4.1 to 49 g/L) at 30 °C for 4 h. The extent of bilirubin adsorption at the presence of HSA was determined as
The concentrations of bilirubin and HSA were analyzed via UV-vis spectroscopy at 460 and 280 nm, respectively.
Characterization
The images of uncoated and polymer-coated silica particles were obtained using LEO 1550 Scanning Electron Microscope (SEM) which is available in Centre of MicroNano Technology (CMi) of Ecole Polytechnique Fédérale de Lausanne (EPFL). Before examining, all samples were mixed with ethanol to prepare suspension. The drop of the suspension was adhesive onto copper surface and dried. SEM imaging was performed under a working distance between 3 and 4 mm with acceleration voltages of 3–5 kV. The chamber vacuum was 10−7 mbar. We used SE2 signal (topography visualization) for SEM imaging. Fourier transform infrared (FT-IR) spectra of the samples were recorded on a Nicolet TM 4700 FTIR spectrometer (“Nicolet,” USA) using KBr technique. Thermal gravimetric (TG) analysis was used to determine the content of grated polymer into silica core and conducted via STA 449 F3 Jupiter (Netzsch, Germany) in argon atmosphere from room temperature up to 900 °C with a ramp rate of 10 °C/min. An alumina crucible with a cover was used during thermal analysis. The zeta-potential of prepared samples were measured using Brookhaven ZetaPlus zeta potential analyzer. Zeta potential measurements were repeated three times. UV-visible spectra were taken with Varian Cary Bio UV-Visible Spectrophotometer. The pH values were measured by pH meter (Mettler Toledo, Swiss).
Results and discussion
Characteristics of polymer-coated silica particles
PDAGA and PMCGH are cationic polymers containing guanidine groups which are very strong bases in water (pKa = 13.6) [26]. In contrast, silica surface is negatively charged in wide range of pH values due to the existence of silanol groups (Fig. S1, supporting information). Therefore, the mixture of them can form a complex via electrostatic interactions and hydrogen bonds forming a strong polymeric shell around silica nanoparticles during the sol-gel process. However, in order to remove weak unbonded polymers, the washing procedure was repeated until the weight loss of polymer-grafted silicas (calculated via TG analysis) stopped changing. According to the TG analysis, it was determined that the maximum concentration of polymer should be up to 78 mg/mL; otherwise, it becomes difficult to clean the result material from weakly adsorbed polymer. In this work, the polymer-coated silica particles were prepared with three different graft ratios (evaluated by TG analysis (Fig. 2b, c)) in order to evaluate the effect of guanidine-containing polymers on surface morphology and adsorption capacity.
One of the effective methods to identify the successful modification of silica core by polymer is FT-IR spectroscopy. IR-spectra of samples with maximum content of grafted polymer are presented in Fig. 2a. The IR-spectra of samples with lower content of polymer are available as a supporting information (Fig. S2). Analysis of FTIR spectra revealed the broad band in the range 3600–3300 cm−1 corresponding to the O–H stretching bands of hydrogen-bonded water molecules and SiO–H stretching vibrations [27]. The corresponding Si–OH bending mode is found around 950 cm−1. The band at 1620 cm−1 is an indication of adsorbed water, which is also present on the bare silica, because of its difficult removal in conditions of experiments. The “bulk” vibrational modes corresponding to SiO4 groups are observed at 1087–1095 and 800 cm−1 (antisymmetric and symmetric Si-O-Si vibrations, respectively), with the bending vibrations near to 460 cm−1 [28].
The FTIR spectra of polymer-coated silicas show the described modes of amorphous silica and some new frequencies due to presence of guanidine-containing polymers. Minor similar changes were observed in low frequency range (1000–450 cm−1) for all modified samples: broadening and light shifts and intensity increasing of peaks. In a high frequency range the new bands between 2949 and 2851 cm−1 corresponding to intensive asymmetric and symmetric C–H stretching vibrations were emerged. Their discussion is obstructed because of strong overlapping by –OH and N–H stretching modes of primary amines (3400 and 3200 cm−1). The latter are clear seen in spectra of modified silica as a shoulder on the left side of the broad band caused by O–H stretching vibrations (between 3120 and 3275 cm−1) and can serve as an evident of successful inclination of functionalize groups into silicas.
Perceptible differences were observed in 1700–1400 cm−1 regions. Here, polymer-coated silicas demonstrated a development of number weak bands at 1397, 1455, and 1550 cm−1 while 1620 cm−1 band strong increased, up-shifted and in case of SiP-PDAGA-c. The band around 1410 cm−1 can be assigned to the combination mode of ν(C-O) + ν(C-C) vibration. The peak new at 1550 cm−1 corresponds to the δ(N-H2) bending vibrations. The bands in 1700–1600 cm−1 range are responsible for carboxylic and amine bonds which are present in all functional groups applied. Thus, FT-IR results clearly show the presence of different chemical groups corresponding to the functional groups of grafted polymer and consequently confirm a successful silica surface modification with guanidine containing polymers.
Then, we used TG analysis to evaluate thermal stability of each polymers grafted on silica core and calculate the grafting yield. The TG curves of the polymer-coated silicas show the mass loss of the organic functional groups as it decomposes upon heating (Fig. 2b). The weight loss at temperatures below 150 °C is due to the removal of physically adsorbed water and surface hydroxyl groups [29, 30]. In case of SiP-PDAGA, the TG shows one strong weight loss which starts at 150 °C and mostly finishes near 400 °C. Around 250 °C, the inclination was slightly changed that assumes another process. The long tail after 400 °C was due to the carbonization of the decomposed products to ash. It is interesting that the weight lost below 150 °C due to presence of water on surface is negligible and not influenced by content of polymer. In contrast, the SiP-PMCGH has a quantity of water comparable with uncoated silica which increases with content of grafted polymer. Also on TG curves of these samples (SiP-PMCGH), there are distinguishable few steps of mass loss and the temperature of polymer decomposition is much higher compared to SiP-PDAGA.
The amount of grafted polymers has been calculated using the following equation:
where W 1 and W 0 represent the weight loss of initial and grafted substrate, respectively.
The grafting yield for SiP-PDAGA and SiP-PMCGH (Fig. 2c) ranges from ~17.8–33.4 %. We can conclude that it was managed to obtain polymer-coated silica particles with three different grafting yields (~18, 23, and 33 %) that will further help us to investigate the effect of polymer grafting on bilirubin adsorption.
We next performed SEM analysis to observe changes in surface morphology between uncoated and polymer-coated silicas. Special SE2 detector was applied to observe the morphology surface of uncoated and polymer-coated silica particles. The results are shown in Fig. 3. It can be seen that SiP (Fig. 3a) exhibits a good uniform and monodisperse smooth spherical morphology with an average particle diameter of 200–250 nm. As shown in the SEM images (Fig. 3b–d), polymer-coated silicas (SiP-PDAGA-c and SiP-PMCGH-c) also demonstrate spherical morphology but with rough surface and an average diameter in 210–280 nm range. Such obvious changes in surface morphology of polymer-coated silica particles confirm successful surface modification of silica core using PDAGA and PMCGH as a polymeric shell. There were no significant differences in SEM images for polymer-coated silica particles with change of polymer and its content. On that reason, we presented it as supporting information (Fig. S3).
Adsorption properties of polymer-coated silica particles
The effect of polymer content on the bilirubin adsorption was investigated (Fig. 4a (inset)). As it can be seen, the adsorption capacity for bilirubin is significantly improved with increasing content of grafted polymers. For silica particles coated with 17 % of PDAGA and PMCGH, the adsorption capacity is 5.3 ± 0.3 and 7.9 ± 0.7 mg/g, respectively, whereas the adsorption capacity of silica particles coated with 33 % of PDAGA and PMCGH is much higher (18.3 ± 1.1 and 31.4 ± 1.9 mg/g). These results strongly illustrate the important role of guanidine-containing polymers in bilirubin removal. The zeta potential measurements (Table S1) indicate that polymer-coated silica particles become positively charged with incorporation of PDAGA and PMCGH. Oppositely, SiP exhibited negative charges at wide range of pH values (Fig. S1, Table S1). This change suggested that guanidine groups of PDAGA and PMCGH were protonated in acidic and neutral medium and as a result, polymer-coated surface of silica is positively charged. At the same time, it is well-known that bilirubin molecule contains two carboxylic groups which are deprotonated at pH 7.4 and thus is negatively charged at pH 7.4. Therefore, the increasing tendency in adsorption capacity of polymer-coated silica particles can be explained by the electrostatic attraction between negatively charged carboxylic groups of bilirubin and positively charged guanidine groups of polymer as illustrated in Fig. 4c. Besides, hydrogen bonding also takes place in additional bilirubin binding. This principle of electrostatic interaction also explains the low adsorption capacity of SiP (1.4 ± 0.2 mg/g): the surface of SiP is negatively charged at pH 7.4 because of the existence of silanols groups which are deprotonated at pH 7.4; therefore, a strong electrostatic repulsion between negatively charged surface of silica and carboxylic groups of bilirubin are generated which leads to the great decrease in adsorption capacity for bilirubin. Only hydrogen bonds can play some role in bilirubin binding (Fig. 4c).
a Adsorption isotherm of bilirubin for SiP-PMCGH-c, effect of polymer content on bilirubin adsorption (inset). b Adsorption capacity of bilirubin on uncoated and polymer-coated silicas as a function of contact time. c Photograph of bilirubin adsorption using uncoated and polymer-coated silica particles. d The effect of HSA concentration on adsorption capacities (mean ± SD, n = 3)
As shown before, the amount of grafted polymer plays the crucial role in the adsorption of bilirubin; for that reason, SiP-PMCGH-c and SiP-PDAGA-c (GY ~33 %) were selected for our following studies. We investigated the kinetic adsorption of bilirubin. Figure 4b represents the adsorption rate curves which were obtained by following decrease of bilirubin concentration within the samples with time. For SiP, bilirubin adsorption rate is fast while in case of SiP-PDAGA-c and SiP-PMCGH-c, adsorption capacity increased quickly in the first 1 h and then reached maximum values only within 3 h. This fact might be explained by the influence of electrostatic interactions between positively charged guanidine polymers and negatively charged bilirubin and hydrogen bonding, which could require a longer time to reach equilibrium conditions.
The dependence between the equilibrium concentration of bilirubin and the equilibrium adsorption amount of bilirubin for SiP-PMCGH-c is shown in Fig. 4a. The adsorption values of bilirubin increased as the bilirubin concentration increased and finally reached a saturation level at high concentrations. Two adsorption isotherm models (Langmuir and Freundlich) have been applied to fit experimental dots. All parameters calculated from Langmuir and Freundlich equations are presented as supporting information (Table S2). According to the values of correlation coefficients (R 2), The Langmuir model shows the best fit for all samples. The maximum adsorption capacity obtained from Langmuir model for SiP-PMCGH-c was 43.47 mg/g. The maximum adsorption capacity of polymer-coated silica particles (SiP-PMCGH-c) is quite comparable with other available adsorbents including albumin-modified membrane (25 mg/g) [31], nanocrystaline titania films (24.5 mg/g) [32], poly(pyrrole-3-carboxylic acid)-alumina composite membrane (32.4 mg/g) [18], polyethylenimine-grafted affinity adsorbents (23.6 mg/g) [33], and heparip-modified chitosan/graphene oxide (GO) (92.6 mg/g) [21]. Although the last adsorbent shows higher adsorption capacity, however, GO exhibits a dose-dependent toxicity and the leakage of GO into blood can be harmful to patients [21].
In blood plasma, bilirubin generally exists in unconjugated and conjugated forms. The conjugated form is thought to be bilirubin-conjugated with glucuronic acid which makes it soluble in water, while unconjugated form is bound to the albumin [34]. It was reported in several papers that some adsorbents can remove only free bilirubin [18]. Therefore, we performed experiment of bilirubin adsorption in the presence of HSA in order to detect the impact of albumin on extent of bilirubin removal. According to the reference, the normal concentration for albumin in serum is approximately 35–50 g/L (3.5–5.0 g/dL) and thereby, we performed experiments at different concentrations of HSA up to 50 g/L. The effect of HSA concentration on bilirubin adsorption was shown in Fig. 4d. These results clearly demonstrate that adsorption capacity of polymer-coated silica particles is greatly affected by HSA concentration and decreased with increasing of HSA concentration: the extent of bilirubin removal for Si-PMCGH-c in the presence of 4 g/L of HSA equals 58 % while in the presence of 49 g/L, it is already 20 %. The similar results are obtained in case of SiP-PDAGA-c. It is generally accepted that there are two binding sites present in each HSA molecule for bilirubin [35]. The second binding is relatively weak and slow as compared with the first binding. The adsorption capacity of each adsorbent is limited by the biding constant of HSA-bilirubin determining selectivity of each adsorbent. As it can be seen from obtained data, good adsorption selectivity for bilirubin is observed until 10 g/L of HSA; after this concentration, the amount of adsorbed bilirubin becomes very low. Thus, the polymer-coated silica particles can be efficiently applied for bilirubin removal at low concentration of albumin. However, it should be mentioned that binding constant of HSA-bilirubin can vary considerably among different persons due to generic, clinical, and physiologic variability [35]; therefore, the influence of HSA should be investigated in blood plasma for each individual person to provide accurate results.
Conclusion
In this work, silica nanoparticles were successfully functionalized with guanidine-containing polymers via sol-gel method. The polymer-coating process allowed obtaining polymeric shell-silica core structure with spherical morphology and rough surface. Incorporation of polymers (PDAGA and PMCGH) into silica core significantly improved the adsorption capacity for bilirubin though it was shown that adsorption capacity for bilirubin is limited by high concentration of albumin. Despite this fact, the results of static and dynamic adsorption experiments showed that prepared polymer-coated silica particles can efficiently remove bilirubin. The principle of electrostatic binding was predominant and beneficial for bilirubin adsorption in case of polymer-coated silicas. Although, we chose the silica as a matrix for polymer modification, we should emphasize that other available matrixes based on titania, carbon, and graphene oxide can be easily used for surface functionalization with PDAGA and PMCGH. Therefore, we expect that such guanidine-containing polymers could be further considered in wide range of affinity systems.
References
Piao Y, Burns A, Kim J, Wiesner U, Hyeon T (2008) Designed fabrication of silica-based nanostructured particle systems for nanomedicine application. Adv Funct Mater 18:3745–3758
Busseron E, Ruff Y, Moulin E, Giuseppone N (2013) Supramolecular self-assemblies as functional nanomaterials. Nanoscale 5:7098–7140. doi:10.1039/c3nr02176a
Rudzka K, Viota JL, Munoz-Gamez JA, Carazo A, Ruiz-Extremera A, Delgado ÁV (2013) Nanoengineering of doxorubicin delivery systems with functionalized maghemite nanoparticles. Colloids Surf B Biointerfaces 111:88–96
Qi W, Shen M, Zhao T, Xu Y, Lin J, Duan Y, Gu H (2015) Low toxicity and long circulation time of polyampholyte-coated magnetic nanoparticles for blood pool contrast agents. Sci Rep 5:7774
Yoon Hee Jang Y, Jang J, Kochuveedu ST, Byun M, Lin Z, Kim DH (2014) Plasmonic dye-sensitized solar cells incorporated with Au-TiO2 nanostructes with tailored configurations. Nanoscale 6:1823
Baeza A, Guisasola E, Ruiz-Hernández E, Vallet-Regí M (2012) Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem Mater 24:517–524
Kim Y-T, Han JH, Hong BH, Kwon Y-U (2010) Electrochemical synthesis of CdSe quantum dot array on graphene basal plane using mesoporous silica thin templates. Adv Mater 22(4):515–518
Stillrich H, Frömsdorf A, Pütter S, Oepen HP (2008) Sub-20 nm magnetic dots with perpendicular magnetic anisotropy. Adv Funct Mater 18(1):76–81
Zou H, Sh W, Shen J (2008) Polymer/silica nanocomposites: preparation, characterization, properties, and applications. Chem Rev 108:3893–3957
Baljit S, Chauhan GS, Kumar S, Chauhan N (2007) Synthesis, characterization and swelling responses of pH sensitive psyllium and polyacrylamide based hydrogels for the use in drug delivery. Carbohydr Polym 67(2):190–200
Knop K, Hoogenboom R, Fischer D, Schubert US (2010) Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed 49:6288–6308
Müller C, Leithner K, Hauptstein S, Hintzen F, Salvenmoser W, Bernkop-Schnürch A (2013) Preparation and characterization of mucus-penetrating papain/poly(acrylic acid) nanoparticles for oral drug delivery applications. J Nanoparticle Res 15:1353
Locock KE, Michl TD, Valentin JD, Vasilev K, Hayball JD, Qu Y, Traven A, Griesser HJ (2013) Guanylated polymethacrylates: a class of potent antimicrobial polymers with low hemolytic activity. Biomacromolecules 14(11):4021–4031
Timin AS, Solomonov AV, Rumyantsev EV (2014) Polyacrylate guanidine and polymethacrylate guanidine as novel cationic polymers for effective bilirubin binding. J Polym Res 21:400–409
Sivov NA (2006) Biocide guanidine containing polymers: synthesis, structure and properties. CRC Press, p 151
Carmona-Ribeiro AM, Melo Carrasco LD (2013) Cationic antimicrobial polymers and their assemblies. Int J Mol Sci 14:9906–9946. doi:10.3390/ijms14059906
Bhattarai B, Muruganandham M, Suri RP (2014) Developments of high efficiency silica coated cyclodextrin polymeric adsorbent for the removal of emergining contaminants of concern from water. J Hazard Mater 273:146–154
Shi W, Cao H, Song C, Jiang H, Wang J, Jiang Sh TJ, Ge D (2010) Poly(pyrrole-3-carboxylic acid)-alumina composite membrane for affinity adsorption of bilirubin. J Membr Sci 353:151–158
Baydemir G, Andac M, Bereli N (2007) Selective removal of bilirubin from human plasma with bilirubin imprinted particles. Ind Eng Chem 46:2843–2852
Rad AY, Yavuz H, Kocakulak M, Denizli A (2003) Bilirubin removal from human plasma with albumin immobilised magnetic poly(2-hydroxyethyl methacrylate) beads. Macromol Biosci 3:471–476
Wei H, Han L, Tang Y, Ren J, Zhao Z, Jia L (2015) Highly flexible heparin-modified chitosan/graphene oxide hybrid hydrogel as a super bilirubin adsorbent with excellent hemocompatibility. J Mater Chem B. doi:10.1039/c4tb01673d
Limin G, Lingxia Z, Jiamin Z, Jian Z (2009) Hollow mesoporous carbon spheres—an excellent bilirubin adsorbent. Chem Commun 40:6071–6073
Holubek WJ, Hoffman RS, Goldfarb DS, Nelson LS (2008) Use of hemodialysis and hemoperfusion in poisoned patients. Kidney Int 74:1327–1334
Tyagi PK, Winchester JF, Feinfeld DA (2008) Extracorporeal removal of toxins. Kidney Int 74:1231–1233
Timin AS, Solomonov AV, Musabirov II, Sergeev SN, Ivanov SP, Goncharenko A, Rumyantsev EV (2014) Immobilization of bovine serum albumin onto porous poly(vinylpyrrolidone)-modified silicas. Ind Eng Chem Res 53(35):13699–13710
Piletsky SA, Whitcombe MJ (2012) Designing receptors for the next generation of biosensors. Springer, Heidelberg. doi:10.1007/978-3-642-32329-4
Mellaerts R, Jammaer JAG, Speybroeck MV, Chen H, Humbeeck JV, Augustijins P, Mooter GV, Martens JA, Langmuir 24 (16): 8651–9.
Zhou J, Zhou L, Dong C, Fend Y, Wei S, Shen J, Wang X (2008) Preparation and photodynamic properties of water-soluble hypocrellin A-silica nanospheres. Mater Lett 62:2910–2913
Kassaee MZ, Masrouri H, Movahed F (2011) Sulfamic acid-functionalized magnetic Fe < sub > 3 </sub > O < sub >4 </sub > nanoparticles as an efficient and reusable catalyst for one-pot synthesis of α-amino nitriles in water. Appl Catal A 2011(395):28–33
Zhuravlev LT (2000) The surface chemistry of amorphous silica. Colloids Surf A 173:1–38
Avramescu ME, Sager WFC, Bornerman Z, Wessling M (2004) Adsorption membranes for bilirubin removal. J Chromatogr B 803:215–223
Yang Z, Si S, Fung Y (2007) Bilirubin adsorption on nanocrystaline titania films. Thin Solid Films 515:3344–3351
Arica MY, Yalcin E, Bayramoglu G (2005) Polyethylenimine-grafted and HAS-immobilized poly(GMA-MMA) affinity adsorbents for bilirubin removal. Polym Int 54:153–160
Roca L, Calligaris S, Wennberg RP, Ahlfors CE, Malik SG, Ostrow JD, Tiribelli C (2006) Factors affecting the binding of bilirubin to serum albumins: validation and application of the peroxidase method. Pediatr Res 60(6):724–728
Sun H, Nie Z, Fung YS (2010) Determination of free bilirubin and its binding capacity by HSA using a microfluidic chip-capillary electrophoresis device with a multi-segment circular-ferrofluid-driven micromixing injection. Electrophoresis 31:3061–3069
Acknowledgments
We are grateful for the support via a stipend of the President of the Russian Federation for study abroad in 2014–2015 (http://www.president-mobility.ru/node/14) and the Ministry of Education and Science of the Russian Federation, special thanks to Aleksandra Radenovic (Laboratory of Nanoscale Biology, EPFL, 1015 Lausanne, Switzerland) and Centre Interdisciplinaire de Microscopie Electronique (CIME) at EPFL for access to electron microscope (SEM).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 968 kb)
Rights and permissions
About this article
Cite this article
Timin, A.S., Khashirova, S.Y., Zhansitov, A. et al. Synthesis and application of silica hybrids grafted with new guanidine-containing polymers as highly effective adsorbents for bilirubin removal. Colloid Polym Sci 293, 1667–1674 (2015). https://doi.org/10.1007/s00396-015-3555-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3555-2