Abstract
Atrial fibrillation (AF) is associated with atrial fibrosis. Inhibition of atrial fibrosis might be a plausible approach for AF prevention and therapy. This study is designed to evaluate the potential role of CD44, a membrane receptor known to regulate fibrosis, and its related signaling in the pathogenesis of atrial fibrosis and AF. Treatment of cultured rat atrial fibroblasts with transforming growth factor-β (TGF-β, a key mediator of atrial fibrosis) led to a higher expression of hyaluronan (HA), CD44, STAT3, and collagen (a principal marker of fibrosis) than that of ventricular fibroblasts. In vivo, TGF-β transgenic mice and AF patients exhibited a greater expression of HA, CD44, STAT3, and collagen in their atria than wild-type mice and sinus rhythm subjects, respectively. Treating TGF-β transgenic mice with an anti-CD44 blocking antibody resulted in a lower expression of STAT3 and collagen in their atria than those with control IgG antibody. Programmed stimulation triggered less AF episodes in TGF-β transgenic mice treated with anti-CD44 blocking antibody than in those with control IgG. Blocking CD44 signaling with anti-CD44 antibody and mutated CD44 plasmids attenuated TGF-β-induced STAT3 activation and collagen expression in cultured atrial fibroblasts. Deletion and mutational analysis of the collagen promoter along with chromatin immunoprecipitation demonstrated that STAT3 served as a vital transcription factor in collagen expression. TGF-β-mediated HA/CD44/STAT3 pathway plays a crucial role in the development of atrial fibrosis and AF. Blocking CD44-dependent signaling may be a feasible way for AF management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is commonly associated with atrial fibrosis [3, 30]. Atrial fibrosis is generally thought to be originated from non-myocyte growth and extracellular matrix protein deposition [3, 30, 35, 39]. Atrial fibroblasts constitute the major component of non-myocytes, and are the main origin of extracellular matrix proteins in the atrium [3, 39]. We and others have demonstrated that atrial fibroblasts exhibit a greater response to fibrotic insult than ventricular fibroblasts either in vitro or in vivo [2, 5, 7, 24, 38, 40, 43]. Conceivably, suppression of the fibrotic response in atrial fibroblasts may offer a potential strategy for the treatment of AF.
Transforming growth factor-β (TGF-β) is generally accepted as a main mediator of atrial fibrosis [3, 39]. TGF-β1 is capable of activating atrial fibroblasts to differentiate into myofibroblasts and promoting them to proliferate, migrate, and generate extracellular matrix protein, such as collagen [3]. In transgenic mice with cardiac over-expression of TGF-β1, their atria display prominent atrial fibrosis and AF property despite equivalent TGF-β1 expression in the atria and ventricles [24, 38]. Collectively, it is conceivable that TGF-β1 over-expression model may be a feasible way for studying atrial fibrosis and AF pathogenesis either in vitro or in vivo.
CD44, a trans-membrane receptor for hyaluronan (HA), is implicated in regulating various adhesion-dependent cellular processes, such as proliferation, migration, and differentiation [26]. It has been demonstrated that HA may mediate signaling interaction between TGF-β and CD44 in some cells [1, 9, 10, 19, 20, 25]. Furthermore, prior studies have provided substantial evidence that CD44 is essential for the development and treatment of fibrosis, including pulmonary and ventricular fibrosis [8, 18, 23]. Because CD44 itself does not possess intrinsic kinase activity, it modulates intracellular signaling by interacting with other pathways, such as receptor or intracellular kinases [26]. Therefore, we hypothesize that CD44 and its related downstream signaling may participate in the pathogenesis of atrial fibrosis and AF.
We first compared in parallel the response of cultured atrial and ventricular fibroblasts to TGF-β1 in HA/CD44 expression. We then utilized gain-of-function and loss-of-function studies to explore the interaction between signaling pathways downstream of TGF-β1 and CD44 and to implicate in the pathogenesis of atrial fibrosis. Furthermore, we sought to determine whether the in vitro findings could be verified in transgenic mice with cardiac over-expression of TGF-β1, especially aiming at the impact of CD44 attenuation on atrial fibrosis and AF development. Finally, the relevant findings obtained from in vitro and animal models were also validated in atrial tissue-samples from AF patients.
Materials and methods
Cell cultures
Fibroblasts were obtained from atria and ventricles of adult male Wistar rats [euthanized with ketamine (100 mg/kg) and xylazine (11.5 mg/kg), IP] using the collagenase and trypsin digestion methods as described [43], and grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS). Death of rats was confirmed by cessation of respiration. Only cells from early passages (1–2) at 80–90% confluence were used. Positive staining for vimentin (>95%) and negative staining for desmin and von Willebrand factor determined the fibroblastic character of cells. In most experiments unless otherwise indicated, the medium was replaced with serum-free DMEM for 24 h and subsequently treated with or without TGF-β1 for an additional 24 h in DMEM with 10% FBS. The animal study was approved by the Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital, and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (the eighth edition, revised 2011).
Western blot analysis
Western blot was performed as described previously [42]. Equal amount of protein in SDS-PAGE sample buffer was sonicated and subjected to electrophoresis on 8% SDS-polyacrylamide gels. After transfer to PVDF membranes (Stratagene, the Netherlands), proteins were incubated with primary antibodies against collagen I, phospho-signal transducer and activator of transcription 3 (p-STAT3, Tyr 705), total form STAT3, CD44, lamin B1, connective tissue growth factor (CTGF), TGF-β1 (Abcam, Cambridge, MA), phospho-Smad3 (p-Smad3), total form Smad3 (R&D Systems, Minneapolis, MN), GAPDH, and tubulin (Santa Cruz, Delaware Avenue, CA). Signals were detected by ECL-detection (Amersham, the Netherlands) and quantified by densitometry. Signal-bands were in the linear immunoreactive range and expressed relative to GAPDH, lamin B, or tubulin.
Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted using TRIzol reagent (Life Technologies, Rockville, MD) and real-time quantitative RT-PCR was performed as described previously [41]. GAPDH mRNA was used as the internal control. Relative expression of hyaluronan synthase (HAS) and hyaluronidase (HYAL-1) was calculated using 2\( ^{{ - \Delta \Delta {\text{C}}_{t} }} \) method by SYBR green detection mechanism.
Immunohisto- and cytochemical analyses
Immunohisto- and cytochemical studies were performed by confocal microscopy using primary antibodies against HA (Abcam), CD44, p-STAT3, collagen I, p-Smad3, CTGF, and TGF-β1 followed by FITC (green) or Cy3 (red, Chemicon, Temecula, CA)-conjugated secondary antibodies. Immunocytochemical cells were grown on 25 mm glass coverslips. The cells treated with or without 5 ng/mL TGFβ1 were removed from the media, and washed in PBS, and fixed in 4% paraformaldehyde for 20 min at room temperature (RT). The coverslips were rinsed in PBS and placed in 0.5% Triton X-100 for 15 min at RT. The cells were then washed twice with PBS and blocked with 2% BSA for 1 h at RT. For immunohistochemical analysis, frozen tissue sections were cut at 5 μm and stored at −80 °C for future usage. Frozen sections were washed with PBS and blocked with 2% BSA for 30 min at RT. Immunohisto- and cytochemical samples were exposed to primary antibodies with 1:100 dilution in BSA at 4 °C over night or at RT for 1 h. The coverslips were washed three times with PBS for 2 min each and then exposed to FITC (green) or Cy3 (red, Chemicon, Temecula, CA)-conjugated secondary antibodies with 1:200 dilution in PBS for 60 min at RT. The coverslips were washed three times with PBS for 2 min, and mounted in chambers for observation. Nuclei were visualized by DAPI-staining. Cardiac fibroblasts were identified by co-localization with vimentin (Santa Cruz). The expression level of target protein was calculated as protein-occupied area in the tissue divided by the nuclear area. Nuclear expression of p-STAT3 and p-Smad3 was calculated as the p-STAT3 and p-Smad3-occupied areas in the nucleus divided by total nuclear area. For each analysis, at least three random fields were chosen to observe >30 cardiac fibroblasts.
Determination of HA concentration and HA fragmentation
Cardiac fibroblasts were grown to confluence in 35-mm dishes. The HA concentration in the supernatant was determined by a commercially available enzyme-linked HA-binding protein assay (R&D). The degree of HA fragmentation in the supernatant was determined by a commercially available hyaluronidase activity ELISA kit (Echelon Biosciences Inc., Salt Lake City, UT) according to manufacturer’s instructions.
Expression vectors, mutants, and small interfering (si) RNA
Wild-type CD44 and its mutants were cloned as described previously [34]. Wild-type CD44 was purchased from Gendiscovery Biotechnology (Taiwan). CD44 mutants with C-terminal deletion (deletion of amino acid 295–361) (ΔC-ter) and N-terminal deletion (deletion of amino acid 24–222) (ΔN-ter) were generated via PCR using wild-type CD44 as a template. Wild-type Smad3 plasmid was purchased from Origene (Rockville, MD). Chemically synthesized siRNAs for Smad3, STAT3, and their control siRNAs were purchased from Dharmacon (Lafayette, CO).
Promoter activity assay
Fragments of rat collagen I promoter (nucleotides −3529 to +109) were amplified by PCR with primers designed from the published nucleotide sequence and were sub-cloned into the pGL3Basic vector (Promega, Madison, WI) at BglII and HindIII sites [43]. Three putative STAT3 binding elements (SBEs, −2759 to −2751, −2683 to −2675, and −1994 to −1986, respectively) were found at the collagen I promoter. Site-directed mutations of these three SBE sites and deletions were constructed by PCR using the same strategy. For transient transfection assays, atrial fibroblasts at 50–60% confluence were transfected with indicated plasmids using TransIT-LT1 reagent (Life Technologies). The transfection efficiency by this method was approximately 60%. After an additional 24 h, samples were sent for measurement of luciferase activity with an assay system (Dual-Luciferase® Reporter, Promega). Co-transfection of a β-galactosidase expression vector was served as an internal control for normalizing the transfection efficiency. Luciferase activities were measured with a luminometer (Luminoskan TL PMS, Thermo Labsystems).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed as described elsewhere [43]. Briefly, serum-deprived atrial fibroblasts were treated with or without TGF-β1 (5 ng/mL) for 24 h, cross-linked with 1% formaldehyde, and sonicated. Soluble chromatin was cleared with 30 μL of 50% protein G (Sigma) containing 5 µg ssDNA at 4 °C for 30 min. Then, the remaining lysate was used for immunoprecipitation with anti-p-STAT3, or rabbit nonspecific IgG (Sigma) as a negative control. After incubation at 4 °C overnight, protein G (30 μL) was added, and then washing the immunocomplexes twice with RIPA A buffer (150 mmol/L NaCl, 1% NP40, 1% deoxycholic acid, 50 mmol/L Tris–Cl pH9.0, 5 mmol/L EDTA, and 0.1% SDS), three times with RIPA B buffer (300 mmol/L NaCl, 1% NP40, 1% deoxycholic acid, 50 mmol/L Tris–Cl pH9.0, 5 mmol/L EDTA, and 0.1% SDS), three times with LiCl wash buffer (150 mmol/L NaCl, 300 mmol/L LiCl, 1% NP40, 1% deoxycholic acid, 50 mmol/L Tris–Cl pH9.0, 5 mmol/L EDTA, and 0.1% SDS), and twice with TE buffer (10 mmol/L Tris–Cl pH8.0 and 1 mmol/L EDTA). The immunocomplexes were eluted twice with 150 μL of elution buffer (50 mmol/L NaHCO3 and 1% SDS) at 37 °C for more than 30 min. For the reversal of cross-links, 18 μL of 5 mol/L NaCl and 1 μL RNase A were added at 67 °C overnight. The DNA was extracted with 300 μL of phenol/chloroform/isoamyl alcohol for more than twice. Then, the supernatant was mixed with 30 μL of 5 mol/L NaCl and 20 μg of glycogen to precipitate the DNA fragment and eluted in TE buffer. The precipitated DNA was quantified by real-time PCR using SYBR Green. A (−2090 to −1885) fragment of the rat collagen I promoter was amplified using a primer pair (5′-GGGTGCTAGATCAGGAGCAG-3′ and 5′-TCCACAGTGGTCAGTTCCAA-3′).
Immunoprecipitation
After 24 h of serum starvation, atrial fibroblasts were treated with or without TGF-β1 (5 ng/mL) for 24 h. The cells were harvested with lysis buffer (25 mmol/L Tris–HCl (pH 7.6), 0.3 mol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.5% Nonidet P-40, and 0.5 mmol/L dithiothreitol) and immunoprecipitated with anti-CD44 antibody and protein G-agarose beads (Sigma). After incubation with 20 μL of protein G-agarose beads for 1 h, beads were collected, washed three times, and resuspended in SDS sample buffer. The immunocomplexes were resolved by SDS-PAGE and analyzed by western blot with anti-STAT3, Src (Millipore), or reblotting with anti-CD44 antibody.
Generation of neutralizing anti-CD44 monoclonal antibody
Rat anti-mouse CD44 blocking antibody recognizing the HA binding domain of CD44N terminal region was generated as described [18, 44]. This antibody was routinely used for blocking HA-related signaling. TIB-240 and CRL-1912 hybridoma cells producing anti-CD44 blocking antibody (KM201) and control rat IgG1 (R187), respectively, were purchased from the American Type Culture Collection. Hybridoma cells were grown in complete DMEM-10/HEPES/pyruvate under conditions that promote log-phase growth. The antibody was purified using saturated ammonium sulfate method. Antibodies precipitated with 45–50% final concentration of ammonium sulfate were desalted and the concentration was measured by Nanodrop system (Thermo). The activity of purified antibodies was assessed by western blot.
Administration of neutralizing anti-CD44 monoclonal antibody
In vivo, anti-CD44 blocking antibody or control IgG (300 μg in 500 μL PBS for each animal) was administered IP every week from the 5th week to the 12th week of age. Experiments were performed at the age of 12 weeks. In vitro, anti-CD44 blocking antibody or control IgG (10 μmol/L) was administered 2 h before TGF-β treatment.
Cell migration assay
Transwell filter chamber (Corning Costar) with 8.0 µm pore size was used for migration assay. Cardiac fibroblasts were seeded at a density of 5 × 105 cells per filter. To initiate the chemotaxis assay, cells in 200 µL DMEM without FBS were added to the upper chamber and the bottom chamber was filled with 600 µL DMEM plus 10% FBS as chemotaxis factor for cell moving. Cardiac fibroblasts were allowed to migrate at 37 °C for 6 h. Cells on the lower aspect of filter membrane were stained with Liu’s stain. Total filter membrane was divided to six fields. Each field was randomly photographed and counted.
MHC-TGFcys33 ser transgenic mice
The MHC-TGFcys33 ser transgenic mice (generous gift from Loren J. Field, James Whitcomb Riley Hospital for Children, IN) are generated by the mouse α-cardiac MHC promoter and sequences encoding the human TGF-β1 cDNA as described [24, 38]. The resulting pups were screened using diagnostic PCR amplification. The DBA/2 strain mice were designated as controls.
Programmed electrical stimulation
Transesophageal stimulation was performed as described previously with modification [24, 38, 41]. After the mice at the age of 12 weeks were anesthetized with IP 50 mg/kg pentobarbital, a 4F electrode catheter (St. Jude Medical, MN, USA) was inserted into the esophagus and connected to an isolated stimulator (SI-200, iWork Systems Inc, WA, USA) for atrial pacing. The pacing programs and EKG recordings were managed by IWX/214 and the LabScribe software (iWork Systems Inc). Amplitude of 1.5× diastolic capture threshold and duration of 2 ms were used. A pre-test burst was done with a cycle length of 100 ms for 10 s to ensure the capture of atrial stimulation, and then the pacing burst with a cycle length of 33 ms for 3 s were repeated for ten times. After these series of burst pacing, AF was defined as a period of rapid irregular atrial rhythm lasting for more than 2 s. If one or more pacing bursts provoked an AF episode, AF was considered to be inducible. The AF inducibility was expressed as a ratio of pacing-triggered AF episodes/ten pacing bursts in each individual mouse. The AF duration was presented as the longest period during AF episode in each individual mouse. The mice under anesthetized condition were killed and the atria were collected and stored in liquid nitrogen for further experiments.
Patients
Right atrial appendages were obtained from ten patients with AF and ten controls with sinus rhythm (SR) undergoing open-heart surgery (Supplemental Table 1). After excision, atrial appendages were immediately frozen in liquid-N2 and stored at −85 °C. The investigation followed the principles of the Declaration of Helsinki. The Institutional Review Board approved the study and all patients gave written informed consent.
Statistical analysis
All values were expressed as the mean ± SEM. Differences between two groups were compared by unpaired t test. Two-way repeated measures ANOVA models followed by post hoc Bonferroni test were applied with factors including cardiac regions and treatment groups. For multiple groups without repeated measures, one-way ANOVA with post hoc Scheffe test was used. A two-tailed value of p < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism 4.0 (GraphPad, San Diego, CA) software.
Results
TGF-β induced a higher HA/CD44/STAT3 signaling in cultured atrial fibroblasts
Our prior study demonstrated that TGF-β induced a greater activation of its canonical downstream target (Smad3) and expression of fibrosis-related protein (collagen) in atrial fibroblasts than in ventricular fibroblasts [43]. To explore whether CD44 is involved in the pathogenesis of atrial fibrosis, we first compare the effect of TGF-β on CD44 expression between cultured atrial and ventricular fibroblasts. Treatment of atrial and ventricular fibroblasts with TGF-β1 increased CD44 expression in a concentration-dependent manner. Nevertheless, atrial fibroblasts showed a stronger response to TGF-β1 in expressing CD44 than ventricular fibroblasts (Fig. 1a, b).
Effect of TGF-β1 on CD44/STAT3 signaling in vitro. a After 24 h of serum deprivation, cardiac fibroblasts were treated with indicated concentrations of TGF-β1 for 24 h. The expression of CD44, p-STAT3, total-STAT3, and tubulin protein was evaluated by western blot as described in “Materials and methods”. The expression of tubulin was used as an internal control. b The relative expression level of each protein was quantified by densitometry and normalized to the control level, which was set at 1.0. Each value represents the mean ± SE of four independent experiments. p < 0.05; asterisk, plus sign, hash: the different symbols represent the significant difference among groups. c After 24 h of serum deprivation, cardiac fibroblasts were treated with or without 5 ng/mL TGF-β1 for 24 h. The expression of p-STAT3 in the nuclear extract of cardiac fibroblasts was evaluated by western blot. The expression of lamin B was used as an internal control. Each value represents the mean ± SE of four independent experiments. The symbol (asterisk) represents the significant difference. d Co-immunoprecipitation was performed using anti-CD44 antibody to immunoprecipitate cell lysates from atrial fibroblasts pretreated with control IgG or anti-CD44 blocking antibody for 2 h followed by treatment with or without 5 ng/mL TGF-β1 for 24 h. The immunocomplexes were resolved by western blot with anti-STAT3, Src, or reblotting with anti-CD44 antibody. The picture is a representative of three blots from three independent experiments
Previous studies have reported that STAT3 might act as a downstream target of CD44 [13, 15, 16, 32]. Classically, STAT3 is activated via intracellular kinases, including JAK2 and Src, to phosphorylate STAT3 at tyrosine residue 705, leading to its translocation into the nucleus for transcriptional regulation [16]. To document the contributing role of CD44 in atrial fibrosis, we further assessed whether TGF-β may produce a similar effect on STAT3 activation. Western blot revealed that TGF-β1 concentration-dependently enhanced STAT3 (phospho and total form) expression, with a more prominent effect in atrial fibroblasts (Fig. 1a, b). Furthermore, TGF-β1 induced a higher expression of p-STAT3 in the nuclei of atrial fibroblasts than in those of ventricular fibroblasts (Fig. 1c). These data implicated that TGF-β provoked more activated STAT3 signaling in atrial fibroblasts than in ventricular fibroblasts. In agreement with previous notion, co-immunoprecipitation assay revealed that the interaction among CD44, Src, and STAT3 was absent in the untreated atrial fibroblasts, whereas TGF-β1 treatment promoted this association (Fig. 1d).
TGF-β has been reported to enhance HA production via the up-regulation of hyaluronan synthase (HAS) mRNA in human fibroblasts [33]. We next evaluated whether the atrium-selective effect of TGF-β is also observed in the expression of HA and HAS. Time-dependent experiment showed that treatment of atrial fibroblasts with TGF-β1 resulted in a higher expression of HAS mRNA than that of ventricular fibroblasts, with the most obvious change occurring at 2 h (Fig. 2a). Consistently, TGF-β1 also promoted a higher secretion of HA in atrial fibroblasts than in ventricular fibroblasts (Fig. 2b–d). Furthermore, hyaluronidases (HYAL-1) that digest high molecular weight HA into smaller fragments can generate HA digestion products with unique biological activities [22, 31]. We also found that TGF-β1 induced a higher degree of HYAL-1 expression and activity in atrial fibroblasts than in ventricular fibroblasts (Fig. 2a, e).
Effect of TGF-β1 on HA, HAS, and expression in vitro. a After 24 h of serum deprivation, cardiac fibroblasts were treated with 5 ng/mL TGF-β1 for indicated times. The level of HAS1, 2, 3, and HYAL-1 mRNA was analyzed by quantitative real-time RT-PCR using GAPDH as an internal control. b Confocal immunocytochemistry shows HA deposition in growth-arrested cardiac fibroblasts treated with or without 5 ng/mL TGF-β1 for 24 h. c Relative intensity of HA deposition was quantified. Each value represents the mean ± SE of four independent experiments. d Growth-arrested cardiac fibroblasts were treated with indicated concentrations of TGF-β1 for 24 h. The secretion of HA was analyzed by method as described in “Materials and methods”. e Growth-arrested cardiac fibroblasts were treated with 5 ng/mL TGF-β1 for 24 h. The degree of HA fragmentation (HYAL-1 activity) was analyzed by method as described in “Materials and methods”. Each value represents the mean ± SE of four independent experiments. p < 0.05; asterisk, plus sign: the different symbols represent the significant difference among groups
TGF-β induced a higher HA/CD44/STAT3 signaling in the atrium
The next experiment was designed to determine whether the in vitro findings could be applicable to TGF-β1 over-expression mice. We and others have found that MHC-TGFcys33 ser transgenic mice exhibit a higher degree of fibrosis in the atrium than in the ventricle [24, 38, 43]. Western blot and immunohistochemical analyses in TGF-β transgenic mice revealed that the expression of CD44 and STAT3 was more prominent in the atrium than in the ventricle (Fig. 3a–e), which was reflected by a higher production of collagen (Fig. 3a, b) and nuclear translocation of p-STAT3 in the atrium (Fig. 3c, e). Furthermore, immunohistochemistry revealed a higher secretion of HA in the atrium than in the ventricle (Fig. 3c, e). Taken together, these data suggested that TGF-β provokes a more activated HA/CD44/STAT3 signaling to induce fibrosis in the atrium than in the ventricle.
Effect of TGF-β on HA/CD44/STAT3 signaling in vivo. a The expression of TGF-β1, CD44, p-STAT3, total-STAT3, and collagen in the atria and ventricles of MHC-TGFcys33 ser transgenic mice (TGF transgene) and wild-type controls (WT) was detected by western blot. b The relative expression level of each protein was quantified by densitometry and normalized to the control level, which was set at 1.0. Each value represents the mean ± SE of six mice. The symbol (asterisk) represents the significant difference. c Confocal immunohistochemical analysis shows HA deposition, CD44 expression, and p-STAT3 nuclear translocation (arrows) in the atria and ventricles of TGF-β transgenic mice (TGF transgene) compared to controls (wild type, WT). In p-STAT3 staining (red, C3), green color (FITC) indicates vimentin-expressing cells and purple color indicates nuclear translocation of p-STAT3. d The negative control for immunofluorescence staining using the respective IgG is shown. e Relative intensities of HA deposition, CD44 expression, and p-STAT3 nuclear translocation were quantified and normalized to the control level. Each value represents the mean ± SE of six mice. The symbol (asterisk) represents the significant difference. A atrium, V ventricle
TGF-β induced collagen expression via CD44/STAT3-dependent signaling
To further clarify the essential role of CD44/STAT3 in TGF-β-induced collagen expression, we then utilized an anti-CD44 blocking antibody to block CD44-related signaling. First of all, we found that administration of anti-CD44 antibody, but not control isotype IgG, to TGF-β1-treated atrial fibroblasts blocked the promoting effect of TGF-β1 on CD44/STAT3 interaction (Fig. 1d), STAT3 activation/expression (Fig. 4a; Supplemental Figure 1), and collagen expression [Fig. 4a (lines 3 and 4)]. Furthermore, we used a wild-type CD44 plasmid to increase CD44 expression and two CD44 deletion mutants with N- and C-terminal deletions to assess the relative contribution of CD44 domain to TGF-β-elicited STAT3 association. It has been indicated that N-terminal region of CD44 is essential for its binding with HA and STAT3, whereas C-terminal region is critical for CD44 internalization [15]. Transfection of wild-type CD44 plasmid in atrial fibroblasts augmented basal and/or TGF-β-induced STAT3 activation/expression and collagen expression [Fig. 4b (lines 3 and 4); Supplemental Figure 2]. Contrarily, transfection of N-terminal deletion reduced the TGF-β-stimulated effects, whereas transfection of C-terminal deletion did not alter the effect of TGF-β [Fig. 4b (lines 5–8); Supplemental Figure 2]. Furthermore, silencing of STAT3 with its respective siRNA attenuated the up-regulation of collagen by TGF-β1 [Fig. 4c (lines 3 and 4)]. Moreover, inhibition of HAS1 with 4-methyl-umbelliferone (4-MU, 2 mmol/L) and knockdown of HAS1 using siRNA could prevent TGF-β1-mediated CD44/STAT3 signaling (Supplemental Figure 3).
Effect of CD44 blocking on STAT3/collagen signaling in vitro a Growth-arrested atrial fibroblasts were pre-incubated with control IgG or anti-CD44 blocking antibody for 2 h and subsequently treated with or without 5 ng/mL TGF-β1 for 24 h. The expression of indicated proteins was evaluated by western blot (left panels). The relative expression level of each protein was quantified by densitometry and normalized to the control level (right panels). After deprivation of serum for 24 h and followed by transfection of indicated plasmids (b) and siRNAs (c) for 24 h, atrial fibroblasts were subsequently treated with or without 5 ng/mL TGF-β1 for 24 h. The expression of indicated proteins was evaluated by western blot (left panels). The relative expression level of each protein was quantified by densitometry and normalized to the control level (right panels). The expression of myc and the suppression of STAT3 and Smad3 indicate the transfection efficiency. Each value represents the mean ± SE from four independent experiments. p < 0.05; asterisk, plus sign, hash: the different symbols represent the significant difference among groups
TGF-β induced collagen transcription via CD44/STAT3-dependent signaling
Our previous study in atrial fibroblasts demonstrates that TGF-β1 up-regulates collagen expression at the transcriptional level, which is mainly mediated via Smad-dependent pathways [43]. The next experiments were designed to investigate whether TGF-β also induced collagen transcription in a CD44/STAT3-dependent manner. Over-expression of CD44 in atrial fibroblasts with a wild-type CD44 plasmid enhanced basal and TGF-β1-induced collagen I promoter activities [Fig. 5a (lines 3 and 4)]. In contrast, transfection of N-terminal deletion, but not C-terminal deletion, attenuated the promoting effect of TGF-β1 on collagen promoter activity [Fig. 5b (lines 3–6)]. Consistently, treatment of atrial fibroblasts with an anti-CD44 blocking antibody also alleviated TGF-β1-induced collagen transcriptional activity [Fig. 5c (lines 3 and 4)]. Furthermore, STAT3 knockdown with its respective siRNA diminished the up-regulation of collagen by TGF-β1 at the transcriptional level [Fig. 5d (lines 3 and 4)]. Bioinformatic analysis identified three putative STAT3-binding elements in the promoter region of collagen I gene. Deletion and mutational analyses of the collagen I promoter showed that TGF-β1-increased transcriptional activity was evident only when the promoter constructs contained the proximal and middle STAT3-binding elements (Fig. 5e). To directly test in vivo binding of STAT3 to the collagen I promoter, chromatin immunoprecipitation assay was performed using a specific p-STAT3 antibody. TGF-β1 produced a large amount of p-STAT3 bound to the collagen I promoter, whereas concurrent administration of an anti-CD44 blocking antibody intervened this binding (Fig. 5f). These findings indicate that, beyond the canonical Smad signaling, TGF-β also provokes collagen expression via CD44/STAT3-mediated signaling.
Effect of CD44/STAT3 on collagen transcription. a, b After 24 h of serum deprivation, atrial fibroblasts were transfected with indicated plasmids and plasmid containing collagen I promoter for 24 h followed by treatment with or without 5 ng/mL TGF-β1 for 24 h. The total amount of DNA was kept constant by adding the control plasmid. The luciferase activity was assayed as described in “Materials and methods”. c Growth-arrested atrial fibroblasts were pretreated with control IgG or anti-CD44 blocking antibody for 2 h followed by treatment with or without 5 ng/mL TGF-β1 for 24 h. The luciferase activity was assayed. d After 24 h of serum deprivation, atrial fibroblasts were transfected with indicated siRNAs and plasmid containing collagen I promoter for 24 h followed by treatment with or without 5 ng/mL TGF-β1 for 24 h. The luciferase activity was assayed. e A schematic linear map of the putative three SBEs at the 5′ end point of the collagen I gene and its mutated luciferase (Luc) constructs are shown in left panels. Growth-arrested atrial fibroblasts were transfected with mutants of the promoter constructs and treated with or without 5 ng/mL TGF-β1 for 24 h. The luciferase activity was assayed. Each value (mean ± SE, n = 4) is expressed as a fold of luciferase activity relative to the control condition. f ChIP assays were performed from soluble chromatins of atrial fibroblasts treated with or without 5 ng/mL TGF-β1 for 24 h, and immunoprecipitated with antibodies against p-STAT3, or nonspecific IgG. A representative PCR at 40 cycles of amplification using primers covering the collagen I promoter is shown (upper panels). Real-time PCR was used to quantify the PCR signals (bottom panels). The picture is a representative of four independent experiments. p < 0.05; asterisk, plus sign, hash: the different symbols represent the significant difference among groups
Interaction between Smad3 and STAT3 pathways in mediating TGF-β-induced effects
The next experiments investigated whether there is a crosstalk between the canonical Smad and CD44/STAT3 pathways in mediating TGF-β effects. First of all, our loss-of-function studies showed that treatment of anti-CD44 blocking antibody and transfection of a CD44 plasmid with N-terminal deletion did not affect the activation (phosphorylation and nuclear translocation) of Smad3 (Fig. 4a, b; Supplemental Figure 4). Second, silencing of Smad3 signaling could not alter TGF-β1-induced STAT3 expression/activation [Fig. 4c (lines 5 and 6); Supplemental Figure 5]. Third, CD44 suppression by either blocking antibody or plasmid transfection produced a more inhibitory effect on STAT3 expression than on collagen expression (Fig. 4a, b). Fourth, our gain-of-function study showed that co-transfection of CD44 and Smad3 expression vectors augmented the basal collagen transcriptional activity relative to that of individual plasmid [Fig. 5a (lines 3 and 5)]. However, transfection of both expression vectors did not further enhance the inducing effect of TGF-β on collagen transcriptional activity, possibly due to the reach of a maximal effect when both signaling pathways were activated [Fig. 5a (lines 7 and 8)]. Contrarily, in loss-of function study, silencing both STAT3 and Smad3 with their respective siRNAs could completely suppress the increasing effect of TGF-β1 on collagen expression [Figs. 4c (lines 7 and 8) and 5d (lines 7 and 8)]. Finally, mutations of STAT3 binding sites failed to show a complete inhibition of TGF-β1-stimulated collagen transcription, suggesting the contribution of Smad signaling (binding at −2194 to −2189 and −2084 to −2079) (Fig. 5e). Taken together, these findings indicate the irrelevance between Smad3 and CD44/STAT3 pathways in mediating TGF-β-induced collagen expression.
TGF-β induced atrial fibroblast migration via CD44
Because CD44 has been shown to direct cell migration [26], we next evaluate the role of CD44 in TGF-β-stimulated atrial fibroblast migration, another important process in the pathogenesis of atrial fibrosis. Consistently, migratory assay showed that TGF-β1 promoted a greater migratory activity in atrial fibroblasts than in ventricular fibroblasts (Supplemental Figure 6). Furthermore, administration of an anti-CD44 blocking antibody could abolish the promoting effect of TGF-β1 on cardiac fibroblast migration (Supplemental Figure 6).
Neutralizing anti-CD44 antibody repressed inducibility of AF
We then utilized the anti-CD44 blocking antibody to investigate the essential role of CD44 in STAT3 activation, atrial fibrosis, and the consequent AF occurrence in the MHC-TGFcys33 ser transgenic mice. In consistence with in vitro findings, administration of anti-CD44 blocking antibody in TGF-β over-expression mice reduced atrial STAT3 activation/expression and collagen expression, but not Smad3 activation (Fig. 6a–d). Furthermore, there was no left–right difference (Fig. 6c, d). Previous study has showed that these transgenic mice exhibited an increase of vulnerability to AF during esophageal pacing [38]. Notably, we found that treatment of anti-CD44 blocking antibody could diminish pacing-induced AF occurrence in these transgenic mice with cardiac over-expression of TGF-β (Fig. 7).
Effect of CD44 blocking on STAT3/collagen signaling in vivo. The expression of indicated proteins in the atria of MHC-TGFcys33 ser transgenic mice (TGF transgene) treated with control IgG or anti-CD44 blocking antibody was detected by western blot. a The relative expression level of each protein was quantified by densitometry and normalized to the levels of control. Each value represents the mean ± SE of six mice. b Confocal immunohistochemical analysis shows CD44 expression, p-STAT3 nuclear translocation (purple, arrows), and collagen I deposition in the atria of MHC-TGFcys33 ser transgenic mice (TGF transgene). LA left atrium, RA right atrium. c Relative intensity of each protein was quantified and normalized to the levels of control, which was set at 1.0. Each value represents the mean ± SE of six mice. The symbol (asterisk) represents the significant difference
Effect of transesophageal burst pacing on AF occurrence. a Representative EKG tracing of burst pacing-induced AF episode is shown. b The inducibility of AF was quantified by the ratio of pacing-induced AF episode in ten pacing bursts in each mouse. c The duration of AF was quantified by the longest period during AF episode in each mouse. Each value represents the mean ± SE of ten mice. p < 0.05; asterisk: the different symbol represents the significant difference among groups
CD44 and STAT3 increase in the atria of AF patients
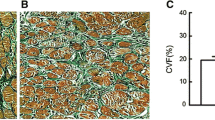
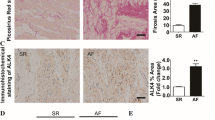
We then evaluated whether these changes observed in vitro could be also detectable in atrial tissues from AF patients. Accordingly, we assessed the expression of CD44 and STAT3 in the atria of AF patients versus sinus rhythm controls. Supplemental Table 1 displays the baseline characteristics of ten AF patients and ten controls. Immunohistochemistry revealed that AF patients exhibited a higher expression of HA, CD44, STAT3 activation/expression, and collagen in their atria than control subjects (Fig. 8a, b). In consistent with previous report [4], AF atria also had a greater expression of TGF-β1 and CTGF than controls (Fig. 8a, b). Furthermore, western blot quantitatively documented the changes in immunohistochemistry (Fig. 8c, d). Finally, trichrome staining, another standard method for detecting atrial fibrosis, confirmed the changes in tissue sections from transgenic mice and patients (Supplemental Figure 7).
HA deposition, STAT3 activation, and CD44/collagen/CTGF/TGF-β1 expression in human atrial tissues. a Representative confocal images show the expression of HA, CD44, p-STAT3 (purple, arrows), total-STAT3, collagen I, CTGF, and TGF-β1 in the atria of sinus rhythm (SR) subjects and AF patients. In p-STAT3 staining (red, C3), green color (FITC) indicates vimentin-expressing cells and purple color indicates nuclear translocation of p-STAT3. b Relative intensities of HA, CD44, STAT3, collagen I, CTGF, and TGF-β1 were quantified. Data are mean ± SE (at least five random fields were chosen to observe >30 cells with scanning and averaging). The symbol (asterisk) represents the significant difference. c The expression of indicated proteins in the atria of sinus rhythm (SR) subjects and AF patients was detected by western blot. d The relative expression level of each protein was quantified by densitometry and normalized to the levels of control. Each value represents the mean ± SE of ten subjects. The symbol (asterisk) represents the significant difference
Discussion
In this study, we provide several lines of evidence to demonstrate the pivotal role of CD44 in the pathogenesis of atrial fibrosis and AF. We utilize TGF-β, a key mediator of fibrosis, to explore the importance of CD44 in AF development in either in vitro or in vivo models. This study follows our and others’ previous studies regarding the susceptibility of atrial fibroblasts to TGF-β-mediated effect [24, 38, 43]. Now, we further extend the prior knowledge to show that TGF-β treatment induced a higher HA/CD44/STAT3 signaling in the atrium than in the ventricle, which is reflected by a more severe collagen deposit in the atrium. Furthermore, we find that the CD44/STAT3 signaling, independent of the canonical Smad pathways, is involved in TGF-β-induced up-regulation of collagen. Third, we demonstrate that CD44 suppression with an anti-CD44 antibody could not only attenuate atrial fibrosis but also diminish AF occurrence in transgenic mice with cardiac over-expression of TGF-β. Finally, the relevant findings have been verified in atrial tissues of AF patients.
Structural remodeling derived from atrial fibrosis is an important feature of AF [3, 30]. A variety of signaling systems, particularly TGF-β-related pathways, are associated with the pathogenesis of atrial fibrosis [3, 30, 39]. Beyond the Smad targets, we further demonstrated the crucial role of CD44/STAT3 signaling in mediating the effect of TGF-β on inducing extracellular matrix protein (collagen) deposit and atrial fibrosis. In comparison with ventricular fibrosis, studies concerning atrial fibrosis are relatively limited. Most notions concerning atrial fibrosis-promoted AF development are originated from ventricular fibrosis. With respect to arrhythmogenesis, atrial fibrosis is more prone to develop arrhythmia than ventricular fibrosis [3, 30]. Atrial fibrosis may induce local conduction slowing, resulting in conduction heterogeneity and producing unidirectional conduction block and macro-reentry [3, 6, 30]. Our findings provide a new therapeutic direction for the treatment of atrial fibrosis and AF.
Several investigations have associated CD44 with the pathogenesis of fibrosis in other organs, including kidney, bone marrow, lung, and articular cartilage [12, 17, 18, 27]. The knowledge about CD44 function in cardiovascular diseases is mostly limited to ventricular and vascular levels. For instance, CD44 has been found to be involved in the dysfunction of ventricular fibroblasts within the infarcted myocardium [8, 23]. In this study, we further indicate that CD44 contributes to the development of atrial fibrosis. Some mechanisms may be responsible for the contributing role of CD44 in atrial fibrosis. CD44 might participate in various functions of atrial fibroblasts. Utilizing a blocking anti-CD44 antibody, we first document the critical role of CD44 in TGF-β-induced migration of atrial fibroblasts. Furthermore, the promoting effect of CD44 on atrial fibrosis might be attributed to its action on stimulating extracellular matrix protein (collagen) deposit. However, our previous report has shown the failure of TGF-β to modulate phenotypic transit and promote proliferation in cultured atrial fibroblasts [43]. It is conceivable that the TGF-β/CD44 signaling could not regulate all features of atrial fibroblasts to the same degree.
STAT3 is a transcription factor that regulates the cellular response to diverse cytokines and growth factors [16]. Prior studies have identified STAT3 as an important contributor to cancer growth and invasion by interacting with CD44 [13, 15, 16, 32]. In comparison with its role in oncogenesis, little is known about the involvement of CD44/STAT3 signaling in fibrosis. Our findings imply that CD44 may contribute to atrial fibrosis by activating STAT3 signaling. This conclusion is drawn on the basis of three main findings either in vivo or in vitro. First of all, we find that TGF-β, a major mediator of atrial fibrosis, enhances the expression of CD44 and STAT3. Second, the inducing effect of TGF-β on the activation/expression of STAT3, the association between CD44 and STAT3, and the expression of fibrosis-related protein (collagen) could be abrogated by knockdown of CD44. Third, STAT3 is essential for the transcriptional regulation of collagen by TGF-β. Our findings accord with prior reports showing the crucial role of STAT3 in angiotensin II-induced atrial fibrosis and promoting fibrotic substrate [4, 37]. Conceivably, the induction of CD44/STAT3 signaling by TGF-β is a critical process in atrial fibrosis and AF development.
The complicated interaction among TGF-β, HA, and CD44 still remain unresolved. A study in metastatic breast cancer cells finds that HA/CD44 binding may stimulate TGF-β receptor (TβRI) serine/threonine kinase activity including Smad2/3 phosphorylation [1]. However, another group shows that HA/CD44 interaction down-regulates TGF-β/Smads-dependent events in immortalized renal cells [9, 10, 19, 20]. The discrepancy may arise from differences in experimental design and culture conditions. Our study demonstrated that blocking CD44 signaling could not affect the phosphorylation of Smad3 by TGF-β, implicating that TGF-β-induced CD44/STAT3 signaling is independent of Smad3. Because CD44 may carry different variants in tumor cells [26], our study using primary culture in atrial fibroblasts may be more representative for the exact role of CD44 in the pathogenesis of atrial fibrosis.
To our knowledge, this is the first study to use a monoclonal antibody in intervening or preventing atrial fibrosis and AF development. Several studies have shown that administration of anti-CD44 antibody may inhibit tumor growth/progression and inflammatory processes [36]. Consequently, anti-CD44 antibodies have been demonstrated to abrogate arthritis, lymphoma, and leukemia [11, 14, 21]. Although most implications are applied in animal models, some clinical trials using these anti-CD44 antibodies are indeed in progress to treat breast and head/neck carcinoma patients [28, 29]. Our results implicate that blocking CD44-related signaling might be a feasible way for the prevention or management of AF. Nevertheless, whether the inference obtained from our in vitro and animal models is applicable in clinic merits further investigation.
In conclusion, this study has clarified the critical role of TGF-β-mediated CD44/STAT3 signaling in the pathogenesis of atrial fibrosis and/or AF development, which is mediated by enhancing the extracellular collagen deposit in the atrium. Notably, our findings implicate that attenuating the pathways involved in CD44-dependent actions is postulated to be a reasonable approach for the treatment of atrial fibrosis and AF.
References
Bourguignon LY, Singleton PA, Zhu H, Zhou B (2002) Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem 277:39703–39712. doi:10.1074/jbc.M204320200
Burstein B, Libby E, Calderone A, Nattel S (2008) Differential behaviors of atrial versus ventricular fibroblasts. A potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 117:1630–1641. doi:10.1161/CIRCULATIONAHA.107.748053
Burstein B, Nattel S (2008) Atrial fibrosis: mechanism and clinical relevance in atrial fibrillation. J Am Coll Cardiol 51:802–809. doi:10.1016/j.jacc.2007.09.064
Chen Y, Surinkaew S, Naud P, Qi XY, Gillis MA, Shi YF, Tardif JC, Dobrev D, Nattel S (2017) JAK-STAT signalling and the atrial fibrillation promoting fibrotic substrate. Cardiovasc Res 113:310–320. doi:10.1093/cvr/cvx004
Hanna N, Cardin S, Leung TK, Nattel S (2004) Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res 63:236–244. doi:10.1016/j.cardiores.2004.03.026
Hoffmann S, Clauss S, Berger IM, Weiß B, Montalbano A, Röth R, Bucher M, Klier I, Wakili R, Seitz H, Schulze-Bahr E, Katus HA, Flachsbart F, Nebel A, Guenther SP, Bagaev E, Rottbauer W, Kääb S, Just S, Rappold GA (2016) Coding and non-coding variants in the SHOX2 gene in patients with early-onset atrial fibrillation. Basic Res Cardiol 111:36. doi:10.1007/s00395-016-0557-2
Hong CS, Cho MC, Kwak YG, Song CH, Lee YH, Lim JS, Kwon YK, Chae SW, Kim DH (2002) Cardiac remodeling and atrial fibrillation in transgenic mice overexpressing junction. FASEB J 16:1310–1312. doi:10.1096/fj.01-0908fje
Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG (2008) CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol 180:2625–2633. doi:10.4049/jimmunol.180.4.2625
Ito T, Williams JD, Fraser D, Phillips AO (2004) Hyaluronan attenuates transforming growth factor-beta1-mediated signaling in renal proximal tubular epithelial cells. Am J Pathol 164:1979–1988. doi:10.1016/S0002-9440(10)63758-3
Ito T, Williams JD, Fraser DJ, Phillips AO (2004) Hyaluronan regulates transforming growth factor-beta1 receptor compartmentalization. J Biol Chem 279:25326–25332. doi:10.1074/jbc.M403135200
Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE (2006) Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 12:1167–1174. doi:10.1038/nm1483
Kers J, Xu-Dubois YC, Rondeau E, Claessen N, Idu MM, Roelofs JJ, Bemelman FJ, ten Berge IJ, Florquin S (2010) Intragraft tubular vimentin and CD44 expression correlate with long-term renal allograft function and interstitial fibrosis and tubular atrophy. Transplantation 90:502–509. doi:10.1097/TP.0b013e3181e86b42
Khurana SS, Riehl TE, Moore BD, Fassan M, Rugge M, Romero-Gallo J, Noto J, Peek RM Jr, Stenson WF, Mills JC (2013) The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem 288:16085–16097. doi:10.1074/jbc.M112.445551
Krause DS, Lazarides K, von Andrian UH, Van Etten RA (2006) Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med 12:1175–1180. doi:10.1038/nm1489
Lee JL, Wang MJ, Chen JY (2009) Acetylation and activation of STAT3 mediated nuclear translocation of CD44. J Cell Biol 185:949–957. doi:10.1083/jcb.200812060
Levy DE, Darnell JE Jr (2002) Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3:651–662. doi:10.1038/nrm909
Li J, Gorski DJ, Anemaet W, Velasco J, Takeuchi J, Sandy JD, Plaas A (2012) Hyaluronan injection in murine osteoarthritis prevents TGFbeta 1-induced synovial neovascularization and fibrosis and maintains articular cartilage integrity by a CD44-dependent mechanism. Arthr Res Ther 14:R151. doi:10.1186/ar3887
Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW (2011) Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208:1459–1471. doi:10.1084/jem.20102510
Meran S, Luo DD, Simpson R, Martin J, Wells A, Steadman R, Phillips AO (2011) Hyaluronan facilitates transforming growth factor-β1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J Biol Chem 286:17618–17630. doi:10.1074/jbc.M111.226563
Meran S, Thomas DW, Stephens P, Enoch S, Martin J, Steadman R, Phillips AO (2008) Hyaluronan facilitates transforming growth factor-beta1-mediated fibroblast proliferation. J Biol Chem 283:6530–6545. doi:10.1074/jbc.M704819200
Mikecz K, Brennan FR, Kim JH, Glant TT (1995) Anti-CD44 treatment abrogates tissue oedema and leukocyte infiltration in murine arthritis. Nat Med 1:558–563
Misra S, Hascalll VC, Markwald RR, Ghatak S (2015) Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol 6:201. doi:10.3389/fimmu.2015.00201
Müller J, Gorressen S, Grandoch M, Feldmann K, Kretschmer I, Lehr S, Ding Z, Schmitt JP, Schrader J, Garbers C, Heusch G, Kelm M, Scheller J, Fischer JW (2014) Interleukin-6-dependent phenotypic modulation of cardiac fibroblasts after acute myocardial infarction. Basic Res Cardiol 109:440. doi:10.1007/s00395-014-0440-y
Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ (2000) Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res 86:571–579. doi:10.1161/01.RES.86.5.571
Nataatmadja M, West J, West M (2006) Overexpression of transforming growth factor-beta is associated with increased hyaluronan content and impairment of repair in Marfan syndrome aortic aneurysm. Circulation 114(Suppl I):I371–I377. doi:10.1161/CIRCULATIONAHA.105.000927
Ponta H, Sherman L, Herrlich PA (2003) CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4:33–45. doi:10.1038/nrm1004
Rameshwar P, Chang VT, Gascón P (1996) Implication of CD44 in adhesion-mediated overproduction of TGF-beta and IL-1 in monocytes from patients with bone marrow fibrosis. Br J Haematol 93:22–29. doi:10.1046/j.1365-2141.1996.4631004.x
Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S (2008) Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol 44:823–829. doi:10.1016/j.oraloncology.2007.10.009
Rupp U, Schoendorf-Holland E, Eichbaum M, Schuetz F, Lauschner I, Schmidt P, Staab A, Hanft G, Huober J, Sinn HP, Sohn C, Schneeweiss A (2007) Safety and pharmacokinetics of bivatuzumab mertansine in patients with CD44v6-positive metastatic breast cancer: final results of a phase I study. Anticancer Drugs 18:477–485. doi:10.1097/CAD.0b013e32801403f4
Schotten U, Verheule S, Kirchhof P, Goette A (2011) Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 91:265–325. doi:10.1152/physrev.00031.2009
Sherman LS, Matsumoto S, Su W, Srivastava T, Back SA (2015) Hyaluronan synthesis, catabolism, and signaling in neurodegenerative diseases. Int J Cell Biol 2015:368584. doi:10.1155/2015/368584
So JY, Smolarek AK, Salerno DM, Maehr H, Uskokovic M, Liu F, Suh N (2013) Targeting CD44-STAT3 signaling by Gemini vitamin D analog leads to inhibition of invasion in basal-like breast cancer. PLoS ONE 8:e54020. doi:10.1371/journal.pone.0054020
Stuhlmeier KM, Pollaschek C (2004) Differential effect of transforming growth factor beta (TGF-beta) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-beta-induced hyaluronan synthase 1 activation. J Biol Chem 279:8753–8760. doi:10.1074/jbc.M303945200
Su YJ, Lai HM, Chang YW, Chen GY, Lee JL (2011) Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J 30:3186–3199. doi:10.1038/emboj.2011.211
Sun Z, Zhou D, Xie X, Wang S, Wang Z, Zhao W, Xu H, Zheng L (2016) Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res Cardiol 111:63
Toole BP (2009) Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res 15:7462–7468. doi:10.1158/1078-0432.CCR-09-0479
Tsai CT, Lai LP, Kuo KT, Hwang JJ, Hsieh CS, Hsu KL, Tseng CD, Tseng YZ, Chiang FT, Lin JL (2008) Angiotensin II activates signal transducer and activators of transcription 3 via Rac1 in atrial myocytes and fibroblasts. Circulation 117:344–355. doi:10.1161/CIRCULATIONAHA.107.695346
Verheule S, Sato T, Everett T, Engle SK, Otten D, der Rubart-von LM, Nakajima HO, Nakajima H, Field LJ, Olgin JE (2004) Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res 94:1458–1465. doi:10.1161/01.RES.0000129579.59664.9d
Wang Q, Yu Y, Zhang P, Chen Y, Li C, Chen J, Wang Y, Li Y (2017) The crucial role of activin A/ALK4 pathway in the pathogenesis of Ang-II-induced atrial fibrosis and vulnerability to atrial fibrillation. Basic Res Cardiol 112:47. doi:10.1007/s00395-017-0634-1
Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC Jr, Kasi VS, Hoit BD, KeshelavaG Zhao H, Capecchi MR, Bernstein KE (2004) Mice with cardiac restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol 165:1019–1032. doi:10.1016/S0002-9440(10)63363-9
Yeh YH, Hsu LA, Chen YH, Kuo CT, Chang GJ, Chen WJ (2016) Protective role of heme oxygenase-1 in atrial remodeling. Basic Res Cardiol 111:58. doi:10.1007/s00395-016-0577-y
Yeh YH, Kuo CT, Chang GJ, Chen YH, Lai YJ, Cheng ML, Chen WJ (2015) Rosuvastatin suppresses atrial tachycardia-induced cellular remodeling via Akt/Nrf2/heme oxygenase-1 pathway. J Mol Cell Cardiol 82:84–92. doi:10.1016/j.yjmcc.2015.03.004
Yeh YH, Kuo CT, Chang GJ, Qi XY, Nattel S, Chen WJ (2013) Nicotinamide adenine dinucleotide phosphate oxidase 4 mediates the differential responsiveness of atrial versus ventricular fibroblasts to transforming growth factor-β. Circ Arrhythm Electrophysiol 6:790–798. doi:10.1161/CIRCEP.113.000338
Zheng Z, Katoh S, He Q, Oritani K, Miyake K, Lesley J, Hyman R, Hamik A, Parkhouse RM, Farr AG, Kincade PW (1995) Monoclonal antibodies to CD44 and their influence on hyaluronan recognition. J Cell Biol 130:485–495. doi:10.1083/jcb.130.2.485
Acknowledgements
We thank Mr. Chih-Chun Chen for his technical assistance in confocal microscopy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by grants from Chang Gung Research Grant Foundation (CMRPG 3B1691-3, 3D1331-3, 3D1631-3, 3F0991-3, and 3F2281) and from Ministry of Science and Technology, Taiwan (102-2628-B-182-011-MY3, 104-2314-B-182A-135-MY2, and 104-2314-B-182-052-MY3).
Conflict of interest
US patent is issued for the method in treating and/or preventing AF patients with a pharmaceutical composition containing an anti-CD44 neutralizing antibody or an antigen binding portion.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, SH., Yeh, YH., Lee, JL. et al. Transforming growth factor-β-mediated CD44/STAT3 signaling contributes to the development of atrial fibrosis and fibrillation. Basic Res Cardiol 112, 58 (2017). https://doi.org/10.1007/s00395-017-0647-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-017-0647-9