Abstract
Chronic hypertension is associated with left ventricular (LV) hypertrophy and LV diastolic dysfunction with impaired isovolumic relaxation and abnormal LV filling. Increased heart rate (HR) worsens these alterations. We investigated whether the I f channel blocker ivabradine exerts beneficial effects on LV filling dynamic. In this setting, we also evaluated the relationship between LV filling and isovolumic contraction as a consequence of contraction-relaxation coupling. Therefore, hypertension was induced by a continuous infusion of angiotensin II during 28 days in 10 chronically instrumented pigs. LV function was investigated after stopping angiotensin II infusion to offset the changes in loading conditions. In the normal heart, LV relaxation filling, LV early filling, LV peak early filling rate were positively correlated to HR. In contrast, these parameters were significantly reduced at day 28 vs. day 0 (18, 42, and 26 %, respectively) despite the increase in HR (108 ± 6 beats/min vs. 73 ± 2 beats/min, respectively). These abnormalities were corrected by acute administration of ivabradine (1 mg/kg, iv). Ivabradine still exerted these effects when HR was controlled at 150 beats/min by atrial pacing. Interestingly, LV relaxation filling, LV early filling and LV peak early filling were strongly correlated with both isovolumic contraction and relaxation. In conclusion, ivabradine improves LV filling during chronic hypertension. The mechanism involves LV contraction-relaxation coupling through normalization of isovolumic contraction and relaxation as well as HR-independent mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The left ventricle (LV) mainly fills during early diastole when the mitral valve opens (accounting for 70–80 % of stroke volume) and during late diastole when the atrium contracts [23, 38]. In the normal heart, myocardial relaxation is a well known determinant of LV filling. When heart rate (HR) increases during exercise or adrenergic stimulation, its acceleration creates a more pronounced mitral valve inflow to maintain or increase stroke volume despite reduced LV filling duration [4, 5, 32, 34]. Chronic hypertension associated with LV hypertrophy and diastolic dysfunction elicits impaired LV isovolumic relaxation and subsequent reduced LV early filling which is often compensated by increased atrial filling [10, 16, 36]. In this setting, an increase in HR worsens LV diastolic dysfunction as it reduces LV filling duration and it exacerbates maladaptive LV relaxation and abnormal LV filling [9]. Therefore, reducing HR appears to be an interesting strategy to improve LV relaxation and to correct LV filling during chronic hypertension.
We previously investigated LV function during chronic hypertension and LV hypertrophy induced by angiotensin II infusion in the instrumented conscious pigs and observed maladaptive responses of isovolumic relaxation during tachycardia [30, 31]. Acute reduction in HR with ivabradine, a selective I f channel blocker, corrected these abnormal responses of the cardiac cycle phases by restoring a normal profile for isovolumic relaxation [31]. However, this previous study rather focused on isovolumic contraction and relaxation, i.e., it did not investigate in detail LV filling dynamic with its components, but only a temporal parameter with its overall duration. Accordingly, the first aim of this study was to investigate the effects of HR reduction with ivabradine on LV filling dynamic during chronic hypertension. Moreover, we previously reported that physiological and pathophysiological modifications of LV isovolumic relaxation were closely linked to LV isovolumic contraction. Therefore, we also examined the relationship between LV filling dynamics and both LV isovolumic contraction, and LV isovolumic relaxation in response to ivabradine under spontaneous and controlled heart rate.
Methods
The experiments were conducted in accordance with the European directive for the protection of vertebrate animals used for experimental and other scientific purposes and were agreed by the animal ethical committee ComEth AFSSA-ENVA-UPEC [#11-0059].
Surgical instrumentation
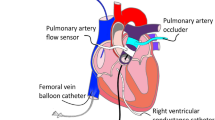
Ten female pigs (Landrace Large White crossed, 20–30 kg, Lebeau, Gambais, France) underwent left thoracotomy under anesthesia. Fluid-filled Tygon catheters were implanted in the descending thoracic aorta, left atrium and pulmonary artery for measurement of arterial blood pressures and angiotensin II infusion, respectively. A solid state pressure transducer (Konigsberg Instruments, Pasadena, CA, USA) was introduced into the left ventricle. A flow probe (Transonic Systems Inc, Ithaca, NY, USA) was implanted around the aortic root. A pair of piezoelectric crystals was implanted on opposed LV anterior and posterior endocardial surfaces to measure LV internal diameter. Two electrodes were fixed on the left atrial appendage for pacing in 6 pigs. All catheters and wires were exteriorized between the scapulae. For postoperative care, animals were treated with diazepam (0.2–0.4 mg/kg iv), buprenorphine (0.3 mg sc during 5 days), enrofloxacine (2 mg/kg/day during 10 days, im) and long acting amoxicillin (15 mg/kg every 2 days during 10 days, im). The position of all catheters and crystals was confirmed at autopsy.
Hemodynamic measurements
Hemodynamic signals were digitized and analyzed using HEM v4.2 software (Notocord Systems, Croissy sur Seine, France). Aortic and left atrial pressures were measured with P23XL pressure transducers (Becton–Dickinson, Franklin Lakes, NJ, USA). Cardiac output was measured using a T206 blood flowmeter (Transonic Systems Inc., Ithaca, NY, USA). LV pressure was cross-calibrated with the left atrial and aortic pressures. The change in LV pressure over time (dP/dt) was computed from the LV pressure signal. The change in LV internal diameter over time (dD/dt) was computed from the LV internal diameter signal. In 2 animals, the signals of LV internal diameter and aortic flow were lost during the protocol due to defective crystals and flow probes.
As illustrated in Fig. 1 and previously described [26, 27], LV end-diastole, aortic valve opening and closure, mitral valve opening were identified by crossing LV, aortic and atrial pressure waveforms. Diastasis was defined as the period where LV pressure remains unchanged, i.e., when dP/dt = 0. Atrial systole was defined as the period between the upstroke of LV pressure tracing after diastasis and LV end-diastole.
a Left panel: representative waveforms of left ventricular, aortic and left atrial pressures used to determine isovolumic contraction, ejection, isovolumic relaxation and total filling durations. b Right panel: representative waveforms of left ventricular filling pressures (bottom) and corresponding left ventricular internal diameter (middle) and LV filling rate (top) during left ventricular filling
Cardiac cycle phases
Isovolumic contraction time was calculated as the time interval between end-diastole and aortic valve opening. Ejection time was measured by the time interval between aortic valve opening and closure. Isovolumic relaxation time was the period between aortic valve closure and mitral valve opening. Total filling time was defined as the duration between mitral valve opening and end-diastole.
Early filling was defined as the period between mitral valve opening and the beginning of diastasis. Early filling was described using its duration and the internal diameter course during this time interval. Peak early filling rate was measured as the maximal dD/dt during early filling.
Within this early phase, relaxation filling was defined as the period between mitral valve opening and LV minimal pressure. Relaxation filling was described using its duration and the internal diameter course during this time interval.
Finally, atrial filling was defined as the difference between the values of LV internal diameter at the end-diastole and the end-diastasis. Peak atrial filling rate was measured as the maximal dD/dt during atrial filling.
Protocol
The experiments were conducted 2–3 weeks after surgery. Pigs were introduced in a sling in upright position. At day 0, to examine the influence of HR on LV filling parameters, we first performed a 2 h recording to observe spontaneous variations in HR. We defined three ranges, i.e., 70, 90, and 110 beats/min. Once this average value was detected, parameters were calculated and averaged for a period of 30 s. Then we performed baseline recordings when animals were kept calm and repeated the measurements after administration of ivabradine (1 mg/kg, iv bolus). Recordings were also performed under controlled heart rate with short sequences of atrial pacing (150 beats/min) to offset the HR-reducing properties of ivabradine.
Thereafter, pigs received a continuous infusion of angiotensin II (30 ng/kg/min) during 4 weeks using external peristaltic portable pumps to induce chronic hypertension and LV hypertrophy with an increase in LV to body weight ratio (3.6 ± 0.1) as compared to control animals used in previous studies [30]. In the present study, LV to body weight ratio at day 28, responses to ivabradine were again evaluated under spontaneous and controlled HR with atrial pacing. These recordings were performed 1 h after stopping angiotensin II infusion to minimize the impact of changes in loading conditions, i.e., to evaluate the intrinsic LV contractile properties.
Statistical analyses
All results are mean ± SEM. Statistical analysis was performed with StatView (version 5.0, Abacus Concept, Berkeley, CA) using two-way analyses of variance for repeated measures. If needed, individual comparisons were performed by Student’s t test with Bonferroni correction. Statistical significance was accepted at p < 0.05.
Results
LV filling maladaptive responses to increased HR during chronic hypertension
In the normal heart at day 0, the values of LV relaxation filling, LV early filling and LV peak early filling rate were increased with spontaneous increases in HR as illustrated by Fig. 2 (left panels). These changes in overall LV filling reflect the normal adaptation of the heart to the rise in HR. In contrast, as illustrated in Fig. 2 (right panel), parameters describing LV filling were paradoxically decreased after 4 weeks of continuous angiotensin II infusion (day 28) as compared to day 0 despite a significant increase in HR (108 ± 6 vs. 73 ± 2, respectively).
Left panel: relationships between left ventricular (LV) relaxation filling (top), LV early filling (middle), LV peak early filling rate (bottom) and heart rate measured during a prolonged recording that includes spontaneous changes in heart rate at day 0. Right panel: relationship between LV relaxation filling (top), LV early filling (middle), LV peak early filling rate (bottom) and heart rate observed at day 0 (open square) and at day 28 (close square). *p < 0.05 vs. day 0
Moreover, the time to LV peak early filling rate was significantly increased (64 ± 3 ms at day 28 vs. 48 ± 3 ms at day 0). This was accompanied by a reduced contribution of the early phase to LV filling, but an increased contribution of LV atrial filling as suggested by the decreased ratio of LV peak early to late filling rates (2.5 ± 0.3 at day 28 vs. 4.0 ± 0.3 at day 0, p < 0.05). These changes reflect a maladaptive diastolic function during tachycardia after 4 weeks of continuous infusion of angiotensin II.
Selective heart rate reduction with ivabradine improves LV filling during chronic hypertension
At day 0, the acute administration of ivabradine reduced HR by 26 % without significant changes in LV pressure, dP/dtmax and cardiac output (Table 1). As illustrated in Fig. 3 (upper panel), this bradycardia was accompanied by significant increases in LV relaxation filling (38 %), LV early filling (18 %) and little changes in LV peak filling rate.
Left ventricular (LV) relaxation filling, LV early filling and LV peak early filling rate were evaluated at baseline (full line bars) and after ivabradine administration (dotted line bars) both at day 0 (open symbols) and day 28 (dashed symbols). Measurements were performed at spontaneous heart rate (top) and under controlled heart rate at 150 beats/min with atrial pacing (bottom). *p < 0.05 vs. day 0. † p < 0.05 vs. baseline
At day 28, administration of ivabradine still reduced HR by 26 %. The effects on other hemodynamic parameters were similar to those observed at day 0. As shown in Fig. 3, ivabradine increased LV relaxation filling, LV early filling and LV peak early filling rate by 102, 32, and 30 %, respectively (all p < 0.05). This led to the normalization of these parameters to their respective baseline levels. Similarly, LV total filling time was also significantly increased from 242 ± 14 ms to 462 ± 38 ms at day 28, reaching its respective baseline value at day 0 (458 ± 22 ms). Moreover, ivabradine significantly reduced the time to LV peak early filling rate (53 ± 5 ms vs. 64 ± 3 ms without ivabradine). This was accompanied by a concomitant increase in the ratio of LV peak early filling rate over LV peak atrial filling rate (4.1 ± 0.6 vs. 2.5 ± 0.3 without ivabradine). Thus, administration of ivabradine corrected the abnormal LV filling dynamic induced by chronic angiotensin II infusion.
Heart rate independent beneficial effects of ivabradine on LV filling during chronic hypertension
To determine whether the improvement of LV filling by ivabradine was only due to HR reduction, we analyzed the effects of ivabradine under controlled HR by atrial pacing at 150 beats/min. At day 0 and day 28, ivabradine still increased LV relaxation filling, LV early filling and LV peak filling rate indicating HR-independent effects of ivabradine (Fig. 3, lower panel).
Involvement of contraction-relaxation coupling in the modulation of LV filling during chronic hypertension and heart rate reduction with ivabradine
Isovolumic relaxation is known to be a determinant of LV filling. Despite the increase in HR at day 28, we observed that isovolumic relaxation time failed to reduce at day 28 (70 ± 2 ms and 69 ± 2 ms with HR values at 73 ± 2 beats/min and 108 ± 6 beats/min, respectively at days 0 and 28). Administration of ivabradine significantly shortened isovolumic relaxation time both at days 0 and 28 (65 ± 2 ms and 62 ± 4 ms, respectively at days 0 and 28). Moreover, as illustrated in Fig. 4, all values of LV relaxation filling, LV early filling and LV peak early filling rate measured at baseline and after ivabradine at both day 0 and 28 were negatively correlated to the duration of isovolumic relaxation.
Considering the concept of contraction-relaxation coupling, we also investigated the relationship between LV filling and myocardial contraction during its isovolumic phase. Indeed, as illustrated in Fig. 5, isovolumic contraction and relaxation times were tightly coupled. Similar to isovolumic relaxation, isovolumic contraction failed to reduce despite the increase in HR at day 28 (52 ± 1 ms with HR at 108 ± 6 beats/min vs. 53 ± 2 ms with HR at 73 ± 2 at day 0). Administration of ivabradine shortened isovolumic contraction time (45 ± 3 ms and 43 ± 2 ms at days 0 and 28, respectively). Interestingly, as illustrated in Fig. 6, all values of LV relaxation filling, LV early filling and LV peak filling rate measured at baseline and after ivabradine both at day 0 and 28 were negatively correlated to the duration of isovolumic contraction. Thus, as observed with isovolumic relaxation, reducing isovolumic contraction time with ivabradine was associated with the improvement of LV filling.
Discussion
In the present study, chronic hypertension secondary to angiotensin II infusion induced LV diastolic dysfunction with abnormal LV isovolumic relaxation and altered LV filling dynamic. HR reduction with the I f channel blocker ivabradine corrected the abnormal LV filling dynamic in this experimental setting by improving LV early filling and reducing the atrial contribution during the late filling phase. These beneficial effects of ivabradine during chronic hypertension were related to enhanced LV myocardial relaxation with a shortened isovolumic period and a greater LV relaxation filling after mitral valve opening. Experiments with controlled HR by atrial pacing revealed that HR-independent mechanisms participated to the effects of ivabradine on LV filling dynamic. Interestingly, examining contraction-relaxation coupling based on the concept of cardiac muscular pump [3] revealed that the improvement in LV filling dynamic was associated with changes in myocardial contraction.
In our experimental conditions, we observed a concomitant alteration in global LV filling characterized by reduced filling during its early phase and a compensated increase in atrial contribution during its late phase as shown by the reduction in LV early filling to atrial filling ratio. These alterations are well known as hallmarks of LV diastolic dysfunction [25, 37]. Within the LV early filling phase, we also paid a specific attention on LV relaxation filling that starts at the end of isovolumic relaxation when mitral valve opens, to examine the impact of LV myocardial relaxation on LV filling. At first during this phase, LV fills as the myocardium continues to relax. Increased HR has been demonstrated to be an aggravating factor of LV dysfunction by impacting LV relaxation, and thereby LV filling, especially for the hypertrophied heart [9].
In the normal heart, LV myocardial relaxation plays a key role in LV filling. During an increase in HR, its acceleration during the isovolumic phase leads to a more pronounced mitral valve inflow thereby preserving LV filling and counteracting the reduction in filling duration. In the hypertrophic heart, abnormal subendocardial blood flow favors the occurrence of myocardial ischemia and LV systolic dysfunction with exacerbated postsystolic wall thickening, contributing to slowed myocardial relaxation [8, 14]. In this setting, increase in HR aggravates the unbalance between the cardiac cycle phases by prolonging LV isovolumic relaxation, and thus, reducing LV early filling. With all these considerations, HR reduction seemed to be a valid pharmacological strategy to correct LV filling abnormalities during chronic hypertension. Indeed at day 28, HR reduction with ivabradine concomitantly normalized LV early filling, LV relaxation filling and LV peak early filling rate as compared to baseline at day 0. The contribution of atrial contraction was thereby reduced, leading to the normalization of the ratio of LV peak early filling rate to LV peak atrial filling rate. Previous pharmacological strategies have attempted to correct LV filling during diastolic dysfunction associated with myocardial hypertrophy by reducing HR especially with β-blockade [15] or calcium channel inhibition [2]. Contrasting with these strategies, ivabradine does not exert significant negative inotropic or lusitropic effects that could be deleterious on lusitropism [6, 7].
In our study, we observed maladaptive responses of LV isovolumic relaxation and LV filling to accelerated HR after 4 weeks of angiotensin II infusion. It should be stressed that angiotensin II may not only induce hypertension, but also other biochemical changes, e.g., reactive oxygen species formation, nitric oxide metabolism through AT2 receptor [21]. Nevertheless, at day 0, LV isovolumic relaxation accelerated with tachycardia to enhance LV early filling with increases in LV relaxation filling and LV peak early filing rate. In contrast at day 28, LV isovolumic relaxation failed to reduce its duration with increased HR. This was associated with reduced LV relaxation filling, LV early filling and LV peak early filling rate. Interestingly, these changes were closely correlated to LV isovolumic relaxation time, indicating that ivabradine exerted simultaneous beneficial effects on both LV filling and isovolumic relaxation. When we examined whether the beneficial effects of ivabradine on LV filling were also associated to concomitant changes in the contraction phase, we observed a strong relationship between isovolumic relaxation and contraction times. This is in agreement with our previous report using the same model of chronic hypertension [30]. Here, we further demonstrated that alterations in LV filling dynamic were tightly coupled to changes in LV isovolumic contraction time. In the normal heart, isovolumic contraction was shortened when HR increases, and reducing isovolumic contraction with ivabradine was associated with an improvement in LV filling. Conversely, LV filling dynamic was altered when isovolumic contraction time failed to reduce in response to increased HR. This suggests a close contraction-relaxation coupling from the onset of isovolumic contraction to an early phase of LV filling that persists during chronic hypertension.
Contraction-relaxation coupling has been previously described in the cellular [17], hemodynamic [3] and echocardiographic studies [27–29]. The balance between contraction and relaxation in the normal myocardium is constant and not significantly impacted by the conditions of length, frequency or isoproterenol. The maximal positive and negative derivatives of myofilament force are tightly correlated. This coupling is not affected by changes in excitation–coupling and the underlying calcium transient shape does not uncouple this relationship. It seems rather result from the structural property of the sarcomere [17, 18]. The processes that affect contraction-relaxation coupling are solely influenced by the myofilament matrix, e.g., overexpressing or knocking out SERCA does not alter this coupling [18], and this contraction-relaxation balance is completely maintained in mdx mice [19]. This coupling affects cardiac performance including LV filling both at rest and during adrenergic stimulation [24]. Chronic hypertension has been demonstrated to be associated with abnormal isovolumic contraction duration [22] and with impaired filling [35], especially with altered restoring forces during isovolumic relaxation and early phase of diastole [33] when HR increases. Because both isovolumic contraction and relaxation are closely linked to LV filling, it is likely that the beneficial effects of ivabradine on LV filling may be related to its concomitant effects on isovolumic contraction and relaxation. This further suggests that ivabradine did not uncouple contraction and relaxation.
The effects of ivabradine on LV filling persisted when HR was maintained constant at 150 beats/min by pacing to offset HR reduction, suggesting HR-independent pleiotropic properties of ivabradine [11] similar to those reported during ischemia–reperfusion in other circumstances [12]. It also suggests these HR-independent effects of ivabradine did not uncouple contraction and relaxation. Such effects could involve ventricular I f-channels and/or other pleiotropic effects that remain to be determined. Indeed chronic hypertension in SHR rats has been associated with ventricular re-expression of hyperpolarization-activated cyclic nucleotide-gated channels (which carry the I f current) [39] and increased I f-channels HCN-2 and -4 were observed in mice treated chronically with angiotensin II [1]. This could contribute to calcium overload [26]. An anti-reactive oxygen species (ROS) independent from heart rate reduction by ivabradine might also been involved [20]. In mitochondria isolated from mouse hearts, ivabradine had no impact on complex I respiration, but reduced ROS formation and improved ATP production and calcium retention capacity. Finally, although we did not specifically investigated this issue, we cannot rule out that the beneficial effect of ivabradine were related to improved coronary blood flow as a result of increased diastolic time with bradycardia. In addition, heart rate reduction with ivabradine would have reduced myocardial oxygen demand [7, 11]. Both properties are particularly beneficial in the context of LV hypertrophy which is known to produce abnormal subendocardial blood flow and limited coronary reserve [13].
In conclusion, we demonstrated that the alterations in LV filling dynamic during chronic hypertension were corrected by the selective I f channel inhibitor ivabradine. Beyond selective HR reduction, the mechanisms involve HR-independent pleiotropic effects. These beneficial effects were tightly associated with improvement of both isovolumic contraction and relaxation. As isovolumic contraction is an important phase of global LV contraction, our data suggest that early alteration of the contractile function, despite preserved global systolic function, is associated with LV diastolic dysfunction. Correction of isovolumic contraction duration with ivabradine improved LV filling dynamic. The close correlation between isovolumic contraction duration and LV relaxation suggests the existence of a wide contraction-relaxation coupling from the onset of isovolumic contraction to an early phase of ventricular filling. Thus, targeting myocardial contraction might be helpful for improving the LV diastolic dysfunction.
References
Becher PM, Lindner D, Miteva K, Savvatis K, Zietsch C, Schmack B, Van Linthout S, Westermann D, Schultheiss HP, Tschope C (2012) Role of heart rate reduction in the prevention of experimental heart failure: comparison between If-channel blockade and beta-receptor blockade. Hypertension 59:949–957. doi:10.1161/HYPERTENSIONAHA.111.183913
Bonow RO, Rosing DR, Epstein SE (1983) The acute and chronic effects of verapamil on left ventricular function in patients with hypertrophic cardiomyopathy. Eur Heart J 4 Suppl F:57–65. doi:10.1093/eurheartj/4.suppl_F.57
Brutsaert DL (2006) Cardiac dysfunction in heart failure: the cardiologist’s love affair with time. Prog Cardiovasc Dis 49:157–181. doi:10.1016/j.pcad.2006.08.010
Cheng CP, Igarashi Y, Little WC (1992) Mechanism of augmented rate of left ventricular filling during exercise. Circ Res 70:9–19. doi:10.1161/01.RES.70.1.9
Colan SD, Borow KM, Neumann A (1985) Effects of loading conditions and contractile state (methoxamine and dobutamine) on left ventricular early diastolic function in normal subjects. Am J Cardiol 55:790–796. doi:10.1016/0002-9149(85)90158-4
Colin P, Ghaleh B, Hittinger L, Monnet X, Slama M, Giudicelli JF, Berdeaux A (2002) Differential effects of heart rate reduction and beta-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol 282:H672–H679. doi:10.1152/ajpheart.00547.2001
Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A (2004) Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther 308:236–240. doi:10.1124/jpet.103.059717
Duncker DJ, Ishibashi Y, Bache RJ (1998) Effect of treadmill exercise on transmural distribution of blood flow in hypertrophied left ventricle. Am J Physiol 275:H1274–H1282
Geskin G, Schulman DS (1997) Relation of changes in left ventricular peak filling rate during exercise to exercise performance in systemic hypertension and in healed myocardial infarction. Am J Cardiol 80:1144–1149. doi:10.1016/S0002-9149(97)00630-9
Hartford M, Wikstrand J, Wallentin I, Ljungman S, Wilhelmsen L, Berglund G (1984) Diastolic function of the heart in untreated primary hypertension. Hypertension 6:329–338. doi:10.1161/01.HYP.6.3.329
Heusch G (2008) Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol 153:1589–1601. doi:10.1038/sj.bjp.0707673
Heusch G, Skyschally A, Gres P, van Caster P, Schilawa D, Schulz R (2008) Improvement of regional myocardial blood flow and function and reduction of infarct size with ivabradine: protection beyond heart rate reduction. Eur Heart J 29:2265–2275. doi:10.1093/eurheartj/ehn337
Hittinger L, Mirsky I, Shen YT, Patrick TA, Bishop SP, Vatner SF (1995) Hemodynamic mechanisms responsible for reduced subendocardial coronary reserve in dogs with severe left ventricular hypertrophy. Circulation 92:978–986. doi:10.1161/01.CIR.92.4.978
Hittinger L, Shannon RP, Kohin S, Manders WT, Kelly P, Vatner SF (1990) Exercise-induced subendocardial dysfunction in dogs with left ventricular hypertrophy. Circ Res 66:329–343. doi:10.1161/01.RES.66.2.329
Hittinger L, Shen YT, Patrick TA, Hasebe N, Komamura K, Ihara T, Manders WT, Vatner SF (1992) Mechanisms of subendocardial dysfunction in response to exercise in dogs with severe left ventricular hypertrophy. Circ Res 71:423–434. doi:10.1161/01.RES.71.2.423
Inouye I, Massie B, Loge D, Topic N, Silverstein D, Simpson P, Tubau J (1984) Abnormal left ventricular filling: an early finding in mild to moderate systemic hypertension. Am J Cardiol 53:120–126. doi:10.1016/0002-9149(84)90695-7
Janssen PM (2010) Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am J Physiol Heart Circ Physiol 299:H1092–H1099. doi:10.1152/ajpheart.00417.2010
Janssen PM (2010) Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol 299:H1741–H1749. doi:10.1152/ajpheart.00759.2010
Janssen PM, Hiranandani N, Mays TA, Rafael-Fortney JA (2005) Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol 289:H2373–H2378. doi:10.1152/ajpheart.00448.2005
Kleinbongard P, Gedik N, Witting P, Freedman B, Klöcker N, Heusch G (2015) Pleiotropic, heart rate-independent cardioprotection by ivabradine. Br J Pharmacol 172:4380–4390. doi:10.1111/bph.13220
Kleinbongard P, Heusch G (2015) Extracellular signalling molecules in the ischaemic/reperfused heart–druggable and translatable for cardioprotection? Br J Pharmacol 172:2010–2025. doi:10.1111/bph.12902
Kono M, Kisanuki A, Ueya N, Kubota K, Kuwahara E, Takasaki K, Yuasa T, Mizukami N, Miyata M, Tei C (2010) Left ventricular global systolic dysfunction has a significant role in the development of diastolic heart failure in patients with systemic hypertension. Hypertens Res 33:1167–1173. doi:10.1038/hr.2010.142
Little WC, Downes TR (1990) Clinical evaluation of left ventricular diastolic performance. Prog Cardiovasc Dis 32:273–290. doi:10.1016/0033-0620(90)90017-V
Liu J, Murata K, Fujino T, Ueda K, Kimura K, Wada Y, Oyama R, Tanaka N, Matsuzaki M (2003) Effect of dobutamine on regional diastolic left ventricular asynchrony in patients with left ventricular hypertrophy. Circ J 67:119–124. doi:10.1253/circj.67.119
Lorell BH, Apstein CS, Weinberg EO, Cunningham MJ (1990) Diastolic function in left ventricular hypertrophy: clinical and experimental relationships. Eur Heart J 11 Suppl G:54–64. doi:10.1093/eurheartj/11.suppl_G.54
Michels G, Brandt MC, Zagidullin N, Khan IF, Larbig R, van Aaken S, Wippermann J, Hoppe UC (2008) Direct evidence for calcium conductance of hyperpolarization-activated cyclic nucleotide-gated channels and human native If at physiological calcium concentrations. Cardiovasc Res 78:466–475. doi:10.1093/cvr/cvn032
Notomi Y, Martin-Miklovic MG, Oryszak SJ, Shiota T, Deserranno D, Popovic ZB, Garcia MJ, Greenberg NL, Thomas JD (2006) Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation 113:2524–2533. doi:10.1161/CIRCULATIONAHA.105.596502
Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, Shiota T, Greenberg NL, Thomas JD (2008) Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol 294:H505–H513. doi:10.1152/ajpheart.00975.2007
Opdahl A, Remme EW, Helle-Valle T, Edvardsen T, Smiseth OA (2012) Myocardial relaxation, restoring forces, and early-diastolic load are independent determinants of left ventricular untwisting rate. Circulation 126:1441–1451. doi:10.1161/CIRCULATIONAHA.111.080861
Rienzo M, Bize A, Pongas D, Michineau S, Melka J, Chan HL, Sambin L, Su JB, Dubois-Rande JL, Hittinger L, Berdeaux A, Ghaleh B (2012) Impaired left ventricular function in the presence of preserved ejection in chronic hypertensive conscious pigs. Basic Res Cardiol 107:298. doi:10.1007/s00395-012-0298-9
Rienzo M, Melka J, Bize A, Sambin L, Jozwiak M, Su JB, Hittinger L, Berdeaux A, Ghaleh B (2015) Ivabradine improves left ventricular function during chronic hypertension in conscious pigs. Hypertension 65:122–129. doi:10.1161/HYPERTENSIONAHA.114.04323
Sabbah HN, Stein PD (1981) Pressure-diameter relations during early diastole in dogs. Incompatibility with the concept of passive left ventricular filling. Circ Res 48:357–365. doi:10.1161/01.RES.48.3.357
Takeuchi M, Borden WB, Nakai H, Nishikage T, Kokumai M, Nagakura T, Otani S, Lang RM (2007) Reduced and delayed untwisting of the left ventricle in patients with hypertension and left ventricular hypertrophy: a study using two-dimensional speckle tracking imaging. Eur Heart J 28:2756–2762. doi:10.1093/eurheartj/ehm440
Udelson JE, Bacharach SL, Cannon RO 3rd, Bonow RO (1990) Minimum left ventricular pressure during beta-adrenergic stimulation in human subjects. Evidence for elastic recoil and diastolic “suction” in the normal heart. Circulation 82:1174–1182. doi:10.1161/01.CIR.82.4.1174
Vignon P, Mor-Avi V, Weinert L, Koch R, Spencer KT, Lang RM (1998) Quantitative evaluation of global and regional left ventricular diastolic function with color kinesis. Circulation 97:1053–1061. doi:10.1161/01.CIR.97.11.1053
Wachtell K, Smith G, Gerdts E, Dahlof B, Nieminen MS, Papademetriou V, Bella JN, Ibsen H, Rokkedal J, Devereux RB (2000) Left ventricular filling patterns in patients with systemic hypertension and left ventricular hypertrophy (the LIFE study). Losartan Intervention for Endpoint. Am J Cardiol 85:466–472. doi:10.1016/S0002-9149(99)00773-0
Wan SH, Vogel MW, Chen HH (2014) Pre-clinical diastolic dysfunction. J Am Coll Cardiol 63:407–416. doi:10.1016/j.jacc.2013.10.063
Yellin EL, Nikolic S, Frater RW (1990) Left ventricular filling dynamics and diastolic function. Prog Cardiovasc Dis 32:247–271. doi:10.1016/0033-0620(90)90016-U
Yu X, Chen XW, Zhou P, Yao L, Liu T, Zhang B, Li Y, Zheng H, Zheng LH, Zhang CX, Bruce I, Ge JB, Wang SQ, Hu ZA, Yu HG, Zhou Z (2007) Calcium influx through If channels in rat ventricular myocytes. Am J Physiol Cell Physiol 292:C1147–C1155. doi:10.1152/ajpcell.00598.2005
Acknowledgments
We thank the Laboratoire Roche for providing diazepam (Valium®) and Servier for the gift of ivabradine. This work was supported by grants from INSERM (Mario Rienzo, poste d’accueil 2008), the Société Française d’Hypertension Artérielle (2010), the Fondation de l’Avenir (ET9-529, ET2-648), the Région Ile de France (CODDIM) and UPEC (Université Paris Est Créteil). Jonathan Melka received a fellowship Grant from the GRRC/SFC (France).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jonathan Melka was a previous fellow of Servier Research Institute (9 months during 2011). Alain Berdeaux has received honoraria for lectures and has served as consultant to Servier. A Grant from Servier was obtained by Bijan Ghaleh for another unrelated project. The other authors have no conflict of interest to declare. No direct pharmaceutical grant was associated with this work.
Rights and permissions
About this article
Cite this article
Melka, J., Rienzo, M., Bizé, A. et al. Improvement of left ventricular filling by ivabradine during chronic hypertension: involvement of contraction-relaxation coupling. Basic Res Cardiol 111, 30 (2016). https://doi.org/10.1007/s00395-016-0550-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-016-0550-9