Abstract
Purpose
Dairy foods are often a major contributor to dietary saturated fatty acids (SFA) intake. However, different SFA-rich foods may not have the same effects on cardiovascular risk factors. We compared full-fat yogurt with low-fat yogurt and butter for their effects on cardiometabolic risk factors in healthy individuals.
Methods
Randomized, two-period crossover trial conducted from October 2022 to April 2023 among 30 healthy men and women (15 to receive full-fat yogurt first, and 15 to receive low-fat yogurt and butter first). Participants consumed a diet with 1.5-2 servings of full-fat (4%) yogurt or low-fat (< 1.5) yogurt and 10–15 g of butter per day for 4 weeks, with 4 weeks wash-out when they consumed 1.5-2 servings of low-fat milk. At baseline, and the end of each 4 weeks, fasting blood samples were drawn and plasma lipids, glycemic and inflammatory markers as well as expression of some genes in the blood buffy coats fraction were determined.
Results
All 30 participants completed the two periods of the study. Apolipoprotein B was higher for the low-fat yogurt and butter [changes from baseline, + 10.06 (95%CI 4.64 to 15.47)] compared with the full-fat yogurt [–4.27 (95%CI, -11.78 to 3.23)] and the difference between two treatment periods was statistically significant (p = 0.004). Non-high-density lipoprotein increased for the low-fat yogurt and butter [change, + 5.06 (95%CI (-1.56 to 11.69) compared with the full-fat yogurt [change, − 4.90 (95%CI, -11.61 to 1.81), with no significant difference between two periods (p = 0.056). There were no between-period differences in other plasma lipid, insulin, and inflammatory biomarkers or leukocyte gene expression of ATP-binding cassette transporter 1 and CD36.
Conclusion
This study suggests that short-term intake of SFAs from full-fat yogurt compared to intake from butter and low-fat yogurt has fewer adverse effects on plasma lipid profile.
ClinicalTrials.gov
NCT05589350, 10/15/2022.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyslipidemia, particularly high levels of low-density lipoprotein cholesterol (LDL-C), is a cardiovascular disease (CVD) risk factor [1]. High intakes of dietary saturated fatty acids (SFA) are associated with an increase in serum LDL-C [2]. High SFA intake may also decrease insulin sensitivity [3]. It has been shown that reducing SFA intake reduces combined cardiovascular events [4]. As a result, most current dietary guidelines recommend limiting SFA intake. Dairy fat in general is rich in SFAs, and dairy foods are often a major contributor to dietary SFA intake. Therefore, healthy eating guidelines often recommend low-fat or fat-free varieties of milk, cheese, and yogurt instead of high or full-fat ones. However, food products with similar nutrient compositions may not necessarily have the same effect on health. Although SFA intake from meat and processed meat are associated with greater CVD risk [5], the association between SFA intake from dairy sources and CVD risk is inconsistent [6] and some observational studies have shown that higher intakes of cheese, milk, and yogurt were not associated with a significantly increased risk of mortality compared with low intakes [7]. Although butter (consisting of approximately 80% of the milkfat) is a by-product of milk, it is not generally considered a ‘dairy food’ for dietary guideline purposes, and its consumption is not recommended in healthy eating guidelines due to the high content of SFA [8]. However, the results of some meta-analyses suggest small or neutral overall associations of butter with CVD, and diabetes [9]. Yet other studies have demonstrated that butter can increase circulating LDL-C [10, 11]. In addition to these contradictions, there are other issues related to dairy fat, and it has been suggested that with respect to the impact on the risk of CVD and type 2 diabetes, not all dairy products, notably fermented varieties, can be considered equal and consumption of some specific dairy may not be associated with a higher risk of CVD and type 2 diabetes [12]. Studies have shown that cheese which is a relatively higher-fat dairy food does not have the same effect on increasing LDL-C as compared to butter with the same saturated fat content [13,14,15]. Considering some of the controversy surrounding the health effects of dairy fat, some have suggested that guidelines could be food-based rather than nutrient-specific [16]. Cheese is a fermented dairy product and the above findings may also apply to other fermented dairy products.
Yogurt is a fermented dairy product with potential favorable effects on both lipid- and non-lipid-related cardiovascular risk factors and its higher consumption has been associated with lower risk of cardiovascular disease events and mortality [17]. Yogurt contains bacterial cultures, which may produce short-chain fatty acids and bioactive peptides that may modulate the effect of its fat content on lipid and lipoprotein metabolism. Despite the relatively high-fat content of full-fat (3.5% fat) yogurt products, their consumption has not been associated with a higher risk of myocardial infarction [18]. Hence, compared to the fat intake from other dietary sources, the intake of equal amounts of fat from full-fat yogurt may have a different effect on the blood concentration of lipids and lipoproteins.
In the present study, we investigated the effects of two diets, one with full-fat yogurt and the other with low-fat yogurt and butter, on plasma LDL-C (primary outcome) in healthy participants. We hypothesized there would be no differences between the two test diets. Furthermore, effects on other blood lipid profiles, glycemic and inflammatory markers as well as expression of some genes in the blood buffy coats fraction were assessed as secondary outcomes.
Materials and methods
Subjects and study design
This study was a randomized, crossover trial. The study was conducted between October 2022 to April 2023 at the Shahid Beheshti University of Medical Sciences. Participants were adult men and pre-menopausal women who were recruited through public advertisements. The inclusion criteria included: age 20 to 60 years, willingness to consume the provided dairy products for the duration of the study, body mass index (BMI) from 18.5 to 29.9 kg/m2, and stable body weight during the previous month. Participants were excluded from the study if they were taking lipid-lowering drugs, were in pregnancy and breastfeeding periods, had type 2 diabetes or coronary artery disease, were being treated for cancer, had serum creatinine > 1.4 mg/dL, taking any anti-inflammatory medications, or taking calcium or omega-3 supplements. Diet sequences were determined using a computer-generated random list. Simple randomization was performed between two treatments (1:1). Each number was placed in an envelope and was opened sequentially. Eligible subjects were allocated to one of two treatment sequences by the study’s research assistant. The primary outcome was a change in plasma lipid profile. The secondary outcomes were changes in glycemic measures, as measured by plasma fasting blood glucose and insulin, and inflammatory markers as measured by plasma c-reactive protein (CRP) and interleukin-6 (IL-6). In this study, the effect of the consumption of full-fat yogurt was compared with the consumption of low-fat yogurt and butter. The two dietary periods of study, with either full-fat yogurt or low-fat yogurt and butter, were of 4 weeks duration, preceded by a 2-week run-in and separated by a 4-week wash-out period, during which low-fat milk was consumed instead of yogurt. Based on previous studies on dairy fat consumption, it appears that a 4-week period was sufficient to observe a change in plasma lipid profile and allow wash-out of the first treatment [14, 15]. Milk was consumed during run-in and wash-out periods because the daily consumption of dairy products is recommended in the healthy dietary guidelines, and it was not appropriate not to consume an alternative dairy product during these periods. Low-fat milk was chosen since its fat content was low, which reduced the level of fat intake from dairy sources, and its use instead of yogurt was more accepted by the participants than other alternative dairy products, such as cheese. Body weight, physical activity, biochemical factors, and expression of some genes in the blood buffy coats fraction were assessed at the beginning and end of each intervention period. Due to the nature of the diets, the study coordinator and participants could not be blinded to treatments. However, laboratory measurements as well as statistical analysis were undertaken in a blinded fashion.
Diets
The dietary intervention included the consumption of commercially available full-fat (4% fat) yogurt during one period and low-fat (< 1.5%) yogurt along with butter during another study period. First, weight maintenance calories were estimated for each subject (by taking gender, body weight, age, and physical activity level into account) and then macronutrients were calculated (~ 50% from carbohydrates, ~ 15% from protein, and ~ 35% from fat). Then, by using an exchange list for meal planning, the number of servings in food groups was determined. The food plan included daily consumption of 6–9 servings of grains, 2–3 servings of vegetables, 2–3 servings of fruit, and 3–4 servings of chicken, meat, fish or egg. The recommended quantities for daily consumption of dairy products (milk, yogurt, cheese) based on the food guide pyramid is 2–3 servings per day and this quantity was considered in the present study, considering 1.5-2 servings for yogurt and 1 serving for cheese. During the consumption of low-fat yogurt, participants also incorporated butter in their diet (10 g of butter for participants who were consuming 1.5 servings of low-fat yogurt per day, and 15 g of butter for participants who were consuming 2 servings of low-fat yogurt per day). The nutrient composition of the test foods is listed in Table 1. During the two treatment periods, the participants were not allowed to consume milk, but during the run-in and wash-out periods, they consumed low-fat milk instead of yogurt and avoided butter. During all stages of the study, participants avoided kefir and buttermilk. Full-fat yogurt, low-fat yogurt, butter, and low-fat milk were delivered to people for consumption in each of the study periods. In addition, to reduce the variation in the type of fatty acid intake, participants were delivered canola oil (for low-heat cooking and salads) and blended vegetable frying oil (for high-heat cooking) to be consumed during all study periods (including run-in, wash-out, and treatment periods) and the recommended intake was 6 to 9 servings (30 to 45 g) per day depending on daily calories.
The dietary intake of each participant was assessed by taking two 24-hour dietary recalls (one weekday and one weekend) obtained by telephone interview during each of the study periods. The composition of obtained dietary recalls was analyzed by the software (Nutritionist 4, N-Squared Computing, USA). For each individual, the mean of the two 24-hour dietary recalls was calculated and used for data analysis.
At the beginning and end of each intervention period, the participants attended the clinic in the morning after 12 h of fasting, and fasting blood samples were collected.
Anthropometric and physical activity measurement
Weight, height, and physical activity level were determined at the beginning and end of each intervention period. Weight was measured without heavy clothing. Height was measured without shoes. The level of physical activity was determined by using the International Physical Activity Questionnaire [19].
Blood sample collection and analysis
Fasting blood samples were collected into the heparin-containing tubes. The samples were then centrifuged (3000 × g for 10 min) and plasma and buffy coat sub-fractions were separated, collected, and stored at -80 0C. Collected buffy coats were stored along with an RNA-stabilization reagent.
Plasma total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), apolipoprotein B (apo B), C-reactive protein (CRP), and glucose were measured using commercial kits (Delta Darman Part, Tehran, Iran) by an autoanalyzer (Selectra ProXL). Low-density lipoprotein cholesterol (LDL-C) concentration was calculated with the Friedewald equation. Non-HDL-C was calculated as TC minus HDL-C. Plasma concentrations of insulin (Monobind, Inc., Lake Forest, CA, USA) and interleukin-6 (IL-6) (Biolegend, San Diego, USA) were measured in frozen plasma samples by commercially available enzyme-linked immunosorbent assays kits. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated according to the formula: fasting glucose (mg/dL) × fasting insulin (mIU/mL) /405.
The RNA from the buffy-coat fraction was extracted using an RNA extraction kit (Bio Basic, Canada Inc.) in accordance with the manufacturer’s protocol. The concentration and purity were determined using a NanoDrop 2000 UV spectrophotometer (Thermo Scientific). The extracted RNA was then converted to complementary DNA using a commercially available kit (Sinaclon, Tehran, Iran). Quantitative real-time PCR was performed using the SYBR Green PCR master mix (Sinaclon, Tehran, Iran) and gene-specific primers on a Step-one plus system (Applied Biosystems). ATP-binding cassette transporter (ABCA1) and the cluster of differentiation 36 (CD36) expression were measured. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as the housekeeping gene.
Statistical analysis
The change in LDL-C was considered the primary outcome of calculating the sample size. G*Power 3.1 software was used to calculate the sample size. For sample size calculation, we could not find a similar study in which the effect of full-fat yogurt and low-fat yogurt and butter had been compared. Considering the hypothetical mean plasma LDL-C concentration in two periods of yogurt consumption (100 mg/dL at the end of the full-fat yogurt period and 110 mg/dL at the end of the low-fat yogurt and butter period), the standard deviation of 18 to 20 mg/dL, the power of 80%, a significance level of 0.05 and the correlation between treatment values of 0.5, the sample size estimated to be n = 30.
Statistical Package for the Social Sciences software (IBM Corporation, version 26.0) was used. The Kolmogorov-Smirnov test was used to check the normality of continuous variables. Plasma CRP, IL-6, insulin, and non-HDL-C were non-normally distributed and log-transformation was performed before analysis. To check the assumption of no carry-over effect, a pretest of the data was conducted. For this test, the sum of the values measured in periods 1 and 2 was calculated for each participant and compared across the two sequence groups by the independent t-test. The equality of sequence effect was also tested by calculating the difference between periods 1 and 2 for each participant and then comparing the two sequences by the independent t-test. A mixed design analysis of covariance (ANCOVA) was used to evaluate the differences between the full-fat and low-fat yogurt and butter treatments on the fourth-week values of the primary and secondary outcome measures. The models included adjustments for baseline values (values at the beginning of each treatment period) and body weight change (weight change during each treatment period) as fixed effects.
For each outcome, we present mean ± standard deviations (SD), the mean change from baseline (∆) and 95% confidence interval (CI) for each phase, and the difference of the mean changes between the two phases (∆∆). Gene expression data are presented as mean ± standard error and calculated as fold changes in the expression. First, normalized Ct or ΔCt (ΔCt = Ct gene –Ct house-keeping) was calculated and analyzed statistically using ANCOVA. Then fold changes in the expression were calculated as the differences between ΔCts values at baseline and ΔCts values at 4 weeks.
Results
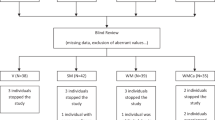
All 30 individuals completed the study periods and were included in the final analysis (Fig. 1). The baseline characteristics of the participants are shown in Table 2. The pretest results to check for the assumption of negligible sequence or carry-over effects indicated no evidence of relevant effects. Therefore, data for both periods were pooled for statistical analysis. Table 3 shows the dietary intake during each of the two periods. The calorie and macronutrient intake were not different between the two dietary periods. The total dietary fat, as well as fatty acid intake, did not differ between the two dietary periods. Self-reported compliance indicated more than 80% compliance with yogurt and butter consumption.
In Table 4, values of body weight and physical activity level are shown. Body weight and physical activity level were not different between the two periods.
Table 5 shows plasma cardiometabolic factors during each of the two periods. Compared to the period of full-fat yogurt, consumption of low-fat yogurt and butter tended to increase in plasma TC (p = 0.075), non-HDL-C (p = 0.056), and the TC to HDL-C ratio (p = 0.095). Low-fat yogurt and butter consumption increased plasma apo B (p = 0.004). There were no differences in plasma TG, LDL-C, and HDL-C levels between the two treatment periods. The dietary interventions had no significant effects on plasma fasting plasma glucose, insulin, and HOMA-IR. The two diets had no significant effect on the measured plasma concentration of systemic inflammatory factors. However, the concentration of IL-6 tended to increase more in the period of low-fat yogurt and butter consumption compared to the high-fat yogurt period (p = 0.052). The concentration of CRP remained unchanged during each of the two study periods.
The leukocyte gene expressions of ABCA1 or CD36 results are shown in Fig. 2. There were no significant changes in gene expressions of ABCA1 or CD36 of participants during the period of full-fat yogurt compared with low-fat yogurt and butter consumption.
Effect of dietary intervention with full-fat yogurt and low-fat yogurt and butter on the expression of leukocyte’s selected genes (Values are the mean ± standard error). ABCA1 or CD36 gene expression was normalized to GAPDH (∆Ct = Ct target gene − Ct GAPDH). Fold change in the gene expression was calculated as the difference between ∆Cts at baseline and ∆Cts after 4 weeks during each of the yogurt consumption. Positive values indicate an increased expression. ABCA1, ATP-binding cassette transporter 1; CD36, the cluster of differentiation 36
Discussion
This randomized, crossover study evaluated the impacts of consuming full-fat yogurt compared with low-fat yogurt and butter on cardiometabolic risk factors in healthy volunteers. We hypothesized there would be no differences between the two test diets, indicating that neither diet would worsen plasma cardiometabolic risk markers. However, the results of our study indicated that compared to the consumption of full-fat yogurt, the consumption of low-fat yogurt along with the incorporation of butter in the daily diet had an increasing effect on plasma apo B concentration. Conversely, there was no difference in plasma levels of other lipids or lipoproteins, and levels of glycemic or inflammatory markers between the two diets.
The plasma apo B may represent atherogenic particles more accurately [1, 20]. Each 10 mg/dL reduction in apo B could reduce 9% coronary heart disease risk by 9% and major cardiovascular disease by 6% [21]. Despite the increase in the concentration of apo B with the consumption of low-fat yogurt and butter, the concentration of LDL-C did not change significantly between treatments. One of the reasons for not reaching a statistically significant difference in plasma LDL-C concentration between the two study periods may be that most of the participants had the optimal baseline LDL-C concentration (100 mg/dL or less) in the present study. Low baseline levels may cause dietary changes to have little effect on circulating LDL-C levels. In a study, it was shown that baseline levels of blood TC, LDL-C, and TG, influence the lipid metabolism response following the consumption of dairy fat and participants who showed the greatest percent reduction in LDL-C had higher baseline LDL-C levels and lower TG levels [22]. Considering that these were healthy subjects, a lack of significant change in LDL-C does not mean that increasing SFA intake from butter is not unhealthy, particularly since total and non-HDL cholesterol concentrations tended to increase with butter and low-fat yogurt consumption. Due to the short duration of the study, consumption in the longer term may have significant effects on LDL-C and other lipid parameters.
In this study, physical activity did not differ between the two treatment periods. Therefore, its influence on the observed changes in plasma lipids and lipoproteins concentrations was probably negligible. Changes in body weight also had a small and non-significant difference between the two periods, and its effect was adjusted by entering it as a confounding factor in the statistical analyses. In addition, the evaluation of the food intake indicates that the intake of calories and macronutrients was not significantly different between the two periods. Some characteristics of dairy foods, including their different food matrix, may partly explain the different effects on blood lipids. The food matrix effect refers to the interaction of food structure and nutrients contained within, which determines the digestion and absorption of nutrients, thereby affecting the food’s nutritional properties. Different food structures of dairy and common processing methods influence interactions between nutrients in the dairy matrix, which may alter the metabolic effects of consumed dairy food [23]. The food matrix in butter and yogurt is different which may affect the rate of absorption of the fat in these products, and thereby concentration of lipoproteins. Fat globules in milk are surrounded by a biophysical membrane called milk fat globule membrane (MFGM). The lipid and protein components of MFGM may have beneficial effects on inflammation and insulin metabolism [24]. Butter is obtained by mechanically agitated cream, which damages the MFGM and separates the fat globules, resulting in the formation of a continuous phase of fat-containing dispersed water droplets, whereas, in yogurt, the fat droplets are dispersed in a loosely gelled protein matrix [25]. Compared to high-fat yogurt, MFGM is reduced in low-fat yogurt, which can reduce the level of bioactive components in low-fat yogurt [26]. The lipids of the MFGM are primarily polar lipids, and sphingomyelin is one of the major types of polar lipids present in the membrane [23]. Sphingomyelin and its metabolites may influence cholesterol absorption, triglyceride hydrolysis, and lipoprotein formation in the gut [27] and in rats, sphingomyelin has dose-dependently lowered the intestinal absorption of fats and cholesterol [28]. The role of the food matrix on the effect of dietary fat on blood lipids has also been considered in other dairy products. Compared to butter, a favorable effect of cheese matrix on LDL cholesterol and total cholesterol has been suggested [29].
In addition to the dairy food matrix, the calcium content of dairy products may also affect blood lipids via increased fecal fat excretion. It has been suggested that the increase in fecal fat loss may be caused by the formation of insoluble calcium soaps or insoluble conjugates of bile acids and calcium-phosphate complexes. Soerensen and colleagues, in a cross-over trial, showed that a 2-week intake of milk and cheese-rich diets (both containing 1700 mg calcium per day) compared to a control diet (containing ~ 500 mg calcium per day and equal fat content from butter) increases fecal fat excretion and reduces SFA-induced increase in serum LDL-C [30]. The formation of calcium soaps in the digestive system due to the consumption of dairy products has also been shown in an in vitro study [31]. In the present study, the amount of yogurt consumption and calcium and phosphate intake were relatively equal in both intervention periods. However, the consumption of low-fat yogurt and butter was not in the same meal. It is possible if calcium and fat do not enter the small intestine at the same time, the interaction between fat and calcium may be limited. Thereby the interaction between calcium in low-fat yogurt and fat in butter may have not been similar to the interaction of calcium and fat in full-fat yogurt. However, it should be noted that the formation of unabsorbed soaps may not be solely responsible for the observed difference in apo B concentration. An RCT by Feeney et al. showed that increasing the level of calcium within the cheese matrix resulted in significantly lower LDL-C when compared to the reduced-calcium cheese or to the same reduced-calcium cheese with matched calcium, delivered as a supplement [32]. Therefore, calcium content may affect the overall physical properties of the matrix and thus the availability of nutrients within the matrix in the dairy product.
Our results indicate the neutral effect of consuming full-fat and low-fat yogurt with butter on circulating CRP and IL-6. This finding is supported by data from studies showing no effect of various dairy products on systemic inflammation [14, 33,34,35]. Our 4-week study also suggested a neutral effect of full-fat yogurt or low-fat yogurt and butter on fasting glucose, insulin, and HOMA-IR. However, fermented dairy products, especially yogurt, may be considered promising foods for reducing the risk of type 2 diabetes [36]. The potentially greater beneficial effect of yogurt on metabolic health may be related to the fermentation process with bacterial strains that may generate bioactive peptides [37]. Consistent with our finding, change (increase or decrease) in the amount of consumption of different dairy products (including yogurt) for 1 month in healthy participants did not affect plasma glucose, insulin, or HOMA-IR [38]. However, daily consumption of 1 serving of conventional yogurt for 24 weeks had a better effect than milk at reducing plasma insulin and improving HOMA-IR in Chinese women with metabolic syndrome and nonalcoholic fatty liver disease [39]. These differential effects may be explained by the study population and study duration. In the current study, the lack of difference in glycemic markers may be related to the fact that in both treatment periods of the study, the participants consumed equal amounts of yogurt, and also there was no difference in the body weight calorie, or carbohydrate intake between the two periods.
As some changes in plasma lipids were observed following two treatment periods, gene expression of ABCA1 and CD36 was also determined in leukocytes. However, the effect of dairy diets on plasma lipids and lipoprotein was not explained by changes in ABCA1 and CD36. The CD36 also known as fatty acid translocase is present on the surface of several cells including monocytes/macrophages. It is involved in fatty acid uptake and also is a scavenger receptor [40]. Intracellular lipids taken up by CD36 may activate pro-inflammatory signals. Blocking CD36 in macrophages has reduced inflammatory cytokine secretion and the formation of foam cells [41]. In the current study, full-fat yogurt or low-fat yogurt and butter had no significant effect on participants’ buffy coats CD36 expression. Results of an animal study have shown that feeding pregnant rats with a high-fat diet containing a large amount of butter has increased the expression of protein CD36 in the liver of the offspring fetuses [42]. In contrast, in a human study, long-chain n-3 polyunsaturated fatty acids supplementation has reduced peripheral blood mononuclear cells’ expression of CD36 [43]. ABCA1, an integral membrane protein, also known as the cholesterol efflux regulatory protein mediates the transfer of free cholesterol and cellular phospholipids to apolipoprotein A-I for generating nascent HDL which then can accept more free cholesterol from peripheral cells to form mature HDL [44]. In the present study, although the concentration of HDL-C was higher after the consumption of full-fat yogurt, it had no significant effect on the expression of the ABCA1 gene of leukocytes. The increase in HDL-C during full-fat yogurt consumption may have not been sufficient to affect the ABCA1 gene expression, especially that was not associated with between periods difference in HDL-C. Furthermore, it should also be considered that buffy coat gene expression may not reflect whole-body lipid metabolism.
This study has some limitations. The use of a short 4-week diet intervention may not have been long enough to fully capture the long-term effects of habitual consumption of either of the test diets. However, it is noteworthy that we were able to detect differences in plasma lipoprotein levels with such short interventions, and this may suggest that longer interventions could lead to greater differences. Furthermore, the sample size was relatively small for the detection of subtle changes, and the power to detect differences between groups was limited. Despite participants reporting good compliance, it was subjective, and the objective compliance biomarkers such as the serum fatty acid study would have benefited the study. Furthermore, we did not standardize on the evening prior to study visits. In addition, evaluating the expression of a limited number of genes and not measuring the expression of different genes involved in lipid metabolism is another limitation of the study. Finally, the study sample included apparently healthy men and women, so these results may not be generalizable to other populations.
Conclusion
This study shows that SFA intake from full-fat yogurt compared to the same intake from butter and low-fat yogurt has fewer adverse effects on plasma lipid profile. The structure or matrix of the yogurt in which these SFAs are present may influence the lipid response. However, it is unclear whether these effects would persist with long-term exposure. A comparison of the effects of consuming full-fat versus low-fat yogurt may have provided more direct results regarding the cardiometabolic health effects of yogurt fat. However, according to dietary guidelines, it is generally recommended to consume low-fat or fat-free variety instead of full-fat or high-fat dairy products. Furthermore, such a comparison may have not properly reflected the possible role of the food matrix containing the SFAs. Based on this finding, in evaluating the effect of ingested SFAs on the concentration of plasma lipids, only estimating the amount of SFA intake may not be sufficient and the type of food source containing SFA may also be important, which may partly be explained by the matrix of food in which SFA is found.
However, as discussed earlier in this review, it is also increasingly recognized that the dairy matrix, or structure, in which these SFAs are contained may influence the blood lipid response.
References
Carr SS, Hooper AJ, Sullivan DR, Burnett JR (2019) Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology 51(2):148–154 https://doi.org/10.1016/j.pathol.2018.11.006. PubMed PMID: 30595507
Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM et al (2017) Dietary Fats and Cardiovascular Disease: a Presidential Advisory from the American Heart Association. Circulation 136(3):e1–e23. 10.1161/CIR.0000000000000510. PubMed PMID: 28620111
Lopez S, Bermudez B, Ortega A, Varela LM, Pacheco YM, Villar J et al (2011) Effects of meals rich in either monounsaturated or saturated fat on lipid concentrations and on insulin secretion and action in subjects with high fasting triglyceride concentrations. Am J Clin Nutr 93(3):494–499. https://doi.org/10.3945/ajcn.110.003251. PubMed PMID: 21209225
Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS (2020) Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 8(8):Cd011737. Epub 20200821. https://doi.org/10.1002/14651858.CD011737.pub3. PubMed PMID: 32827219; PubMed Central PMCID: PMCPMC8092457
de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR Jr., Nettleton JA (2012) Dietary intake of saturated fat by food source and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Am J Clin Nutr 96(2):397–404 Epub 20120703. https://doi.org/10.3945/ajcn.112.037770
Givens DI (2022) Saturated fats, dairy foods and cardiovascular health: no longer a curious paradox? Nutr Bull 47(4):407–422
O’Sullivan TA, Hafekost K, Mitrou F, Lawrence D (2013) Food sources of saturated fat and the association with mortality: a meta-analysis. Am J Public Health 103(9):e31–42 Epub 20130718. https://doi.org/10.2105/ajph.2013.301492
Department of Agriculture US, Department of Health and Human Services US (2020) Dietary guidelines for americans, 2020–2025, 9th edn. US Government Publishing Office, Washington, DC
Pimpin L, Wu JH, Haskelberg H, Del Gobbo L, Mozaffarian D (2016) Is butter back? A systematic review and Meta-analysis of butter consumption and risk of Cardiovascular Disease, Diabetes, and total mortality. PLoS ONE 11(6):e0158118 Epub 20160629. https://doi.org/10.1371/journal.pone.0158118
Engel S, Tholstrup T (2015) Butter increased total and LDL cholesterol compared with olive oil but resulted in higher HDL cholesterol compared with a habitual diet. Am J Clin Nutr 102(2):309–315
Trichia E, Luben R, Khaw KT, Wareham NJ, Imamura F, Forouhi NG (2020) The associations of longitudinal changes in consumption of total and types of dairy products and markers of metabolic risk and adiposity: findings from the European Investigation into Cancer and Nutrition (EPIC)-Norfolk study, United Kingdom. Am J Clin Nutr 111(5):1018–1026. https://doi.org/10.1093/ajcn/nqz335. PubMed PMID: 31915813; PubMed Central PMCID: PMCPMC7198306
Nestel PJ, Mori TA (2022) Dairy foods: is its Cardiovascular Risk Profile changing? Curr Atheroscler Rep 24(1):33–40 Epub 20220119. https://doi.org/10.1007/s11883-022-00984-1
Hjerpsted J, Leedo E, Tholstrup T (2011) Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am J Clin Nutr 94(6):1479–1484. https://doi.org/10.3945/ajcn.111.022426. PubMed PMID: 22030228
Brassard D, Tessier-Grenier M, Allaire J, Rajendiran E, She Y, Ramprasath V et al (2017) Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: a randomized controlled trial. Am J Clin Nutr 105(4):800–809. https://doi.org/10.3945/ajcn.116.150300. PubMed PMID: 28251937
Nestel PJ, Chronopulos A, Cehun M (2005) Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur J Clin Nutr 59(9):1059–1063. https://doi.org/10.1038/sj.ejcn.1602211. PubMed PMID: 16015270
Astrup A, Bertram HC, Bonjour JP, de Groot LC, de Oliveira Otto MC, Feeney EL et al (2019) WHO draft guidelines on dietary saturated and trans fatty acids: time for a new approach? BMJ 366:l4137 Epub 20190703. https://doi.org/10.1136/bmj.l4137
Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R et al (2018) Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet 392(10161):2288–2297 Epub 20180911. https://doi.org/10.1016/s0140-6736(18)31812-9
Kvist K, Laursen ASD, Overvad K, Jakobsen MU (2020) Substitution of milk with whole-Fat Yogurt products or cheese is Associated with a lower risk of myocardial infarction: the Danish Diet, Cancer and Health cohort. J Nutr 150(5):1252–1258. https://doi.org/10.1093/jn/nxz337. PubMed PMID: 31919508
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE et al (2003) International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise. 35(8):1381-95. doi: 10.1249/01.MSS.0000078924.61453.FB. PubMed PMID: 12900694
Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA (2019) Apolipoprotein B particles and Cardiovascular Disease: a narrative review. JAMA Cardiol 4(12):1287–1295. https://doi.org/10.1001/jamacardio.2019.3780. PubMed PMID: 31642874; PubMed Central PMCID: PMC7369156
Robinson JG, Wang S, Jacobson TA (2012) Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol 110(10):1468–1476. https://doi.org/10.1016/j.amjcard.2012.07.007. PubMed PMID: 22906895
O’Connor A, Feeney EL, Bhargava N, Noronha N, Gibney ER (2022) Determination of factors associated with serum cholesterol response to dairy fat consumption in overweight adults: secondary analysis from an RCT. Front Nutr 9:945723 Epub 20220803. https://doi.org/10.3389/fnut.2022.945723
Thorning TK, Bertram HC, Bonjour JP, de Groot L, Dupont D, Feeney E et al (2017) Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr 105(5):1033–1045 Epub 20170412. https://doi.org/10.3945/ajcn.116.151548
Beals E, Kamita SG, Sacchi R, Demmer E, Rivera N, Rogers-Soeder TS et al (2019) Addition of milk fat globule membrane-enriched supplement to a high-fat meal attenuates insulin secretion and induction of soluble epoxide hydrolase gene expression in the postprandial state in overweight and obese subjects. J Nutr Sci 8:e16. PubMed PMID: 31080589; PubMed Central PMCID: PMCPMC6498758
Mulet-Cabero A-I, Brodkorb A (2021) Dairy products. In: Grundy MML, Wilde PJ (eds) Bioaccessibility and digestibility of lipids from Food. Springer International Publishing, Cham, pp 133–149
Ahn Y-J, Ganesan P, Kwak H-S (2011) Composition, structure, and bioactive components in milk fat globule membrane. Food Sci Anim Resour 31(1):1–8
Nilsson A, Duan RD (2006) Absorption and lipoprotein transport of sphingomyelin. J Lipid Res 47(1):154–171 Epub 20051026. https://doi.org/10.1194/jlr.M500357-JLR200
Eckhardt ER, Wang DQ, Donovan JM, Carey MC (2002) Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology 122(4):948–956. https://doi.org/10.1053/gast.2002.32539. PubMed PMID: 11910347
Feeney EL, Barron R, Dible V, Hamilton Z, Power Y, Tanner L et al (2018) Dairy matrix effects: response to consumption of dairy fat differs when eaten within the cheese matrix-a randomized controlled trial. Am J Clin Nutr 108(4):667–674. https://doi.org/10.1093/ajcn/nqy146. PubMed PMID: 30107488
Soerensen KV, Thorning TK, Astrup A, Kristensen M, Lorenzen JK (2014) Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am J Clin Nutr 99(5):984–991 Epub 20140312. https://doi.org/10.3945/ajcn.113.077735
Lamothe S, Rémillard N, Tremblay J, Britten M (2017) Influence of dairy matrices on nutrient release in a simulated gastrointestinal environment. Food Res Int. 92:138 – 46. Epub 20161228. https://doi.org/10.1016/j.foodres.2016.12.026. PubMed PMID: 28290291
Feeney EL, Daly A, Dunne S, Dible V, Barron R, Seratlic S et al (2023) Effect of reduced-calcium and high-calcium cheddar cheese consumption on the excretion of faecal fat: a 2-week cross-over dietary intervention study. Eur J Nutr 62(4):1755–1765 Epub 20230223. https://doi.org/10.1007/s00394-023-03118-8
Raziani F, Tholstrup T, Kristensen MD, Svanegaard ML, Ritz C, Astrup A, Raben A (2016) High intake of regular-fat cheese compared with reduced-fat cheese does not affect LDL cholesterol or risk markers of the metabolic syndrome: a randomized controlled trial. Am J Clin Nutr 104(4):973–981. https://doi.org/10.3945/ajcn.116.134932. PubMed PMID: 27557654
Rancourt-Bouchard M, Gigleux I, Guay V, Charest A, Saint-Gelais D, Vuillemard JC et al (2020) Effects of regular-fat and low-fat dairy consumption on daytime ambulatory blood pressure and other cardiometabolic risk factors: a randomized controlled feeding trial. Am J Clin Nutr 111(1):42–51. https://doi.org/10.1093/ajcn/nqz251. PubMed PMID: 31584063
Schmidt KA, Cromer G, Burhans MS, Kuzma JN, Hagman DK, Fernando I et al (2021) The impact of diets rich in low-fat or full-fat dairy on glucose tolerance and its determinants: a randomized controlled trial. Am J Clin Nutr 113(3):534–547. https://doi.org/10.1093/ajcn/nqaa301. PubMed PMID: 33184632; PubMed Central PMCID: PMCPMC7948850
Companys J, Pla-Pagà L, Calderón-Pérez L, Llauradó E, Solà R, Pedret A, Valls RM (2020) Fermented dairy products, probiotic supplementation, and Cardiometabolic diseases: a systematic review and Meta-analysis. Adv Nutr 11(4):834–863. https://doi.org/10.1093/advances/nmaa030. PubMed PMID: 32277831; PubMed Central PMCID: PMCPMC7360468
Fernandez MA, Panahi S, Daniel N, Tremblay A, Marette A (2017) Yogurt and Cardiometabolic diseases: a critical review of potential mechanisms. Adv Nutr 8(6):812–829 Epub 20171115. https://doi.org/10.3945/an.116.013946
Benatar JR, Jones E, White H, Stewart RA (2014) A randomized trial evaluating the effects of change in dairy food consumption on cardio-metabolic risk factors. Eur J Prev Cardiol 21(11):1376–1386 Epub 20130617. doi: 10.1177/2047487313493567. PubMed PMID: 23774272
Chen Y, Feng R, Yang X, Dai J, Huang M, Ji X et al (2019) Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. Am J Clin Nutr 109(6):1611–1619. https://doi.org/10.1093/ajcn/nqy358. PubMed PMID: 31136662
Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL (2007) CD36 and macrophages in atherosclerosis. Cardiovasc Res 75(3):468–477 Epub 20070314. https://doi.org/10.1016/j.cardiores.2007.03.010
Chávez-Sánchez L, Garza-Reyes MG, Espinosa-Luna JE, Chávez-Rueda K, Legorreta-Haquet MV, Blanco-Favela F (2014) The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans. Hum Immunol 75(4):322–329 Epub 20140130. https://doi.org/10.1016/j.humimm.2014.01.012
Heinecke F, Mazzucco MB, Fornes D, Roberti S, Jawerbaum A, White V (2020) The offspring from rats fed a fatty diet display impairments in the activation of liver peroxisome proliferator activated receptor alpha and features of fatty liver disease. Mol Cell Endocrinol 511:110818 Epub 20200413. https://doi.org/10.1016/j.mce.2020.110818
Bouwens M, van de Rest O, Dellschaft N, Bromhaar MG, de Groot LC, Geleijnse JM et al (2009) Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr 90(2):415–424 Epub 20090610. https://doi.org/10.3945/ajcn.2009.27680
Chen L, Zhao Z-W, Zeng P-H, Zhou Y-J, Yin W-J (2022) Molecular mechanisms for ABCA1-mediated cholesterol efflux. Cell Cycle 21(11):1121–1139. https://doi.org/10.1080/15384101.2022.2042777
Acknowledgements
This research would not have been possible without the contribution of Elaheh Tajeddin as a study research assistant. We thank Dr. Omid Rahnama and also Rahnama laboratory staff for providing the necessary laboratory facilities. Finally, the authors would like to thank the individuals who volunteered to participate in this trial.
Funding
This research is funded by the Shahid Beheshti University of Medical Science, Tehran, Iran (research project code: 32333).
Author information
Authors and Affiliations
Contributions
JN designed the study; YR performed the selection of study volunteers and data collection; JN analyzed and interpreted data and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics statement and consent for participants
This study was conducted by the Helsinki Declaration. All procedures were approved by the Ethics Committees of the National Nutrition and Food Technology Research Institute, and informed consent was taken from the participants. This study was registered at ClinicalTrials.gov (NCT05589350).
Conflict of interest
The authors declare that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ranjbar, Y.R., Nasrollahzadeh, J. Comparison of the impact of saturated fat from full-fat yogurt or low-fat yogurt and butter on cardiometabolic factors: a randomized cross-over trial. Eur J Nutr 63, 1213–1224 (2024). https://doi.org/10.1007/s00394-024-03352-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-024-03352-8