Abstract
Purpose
Bacillus coagulans GBI-30, 6086 (BC30) was previously shown to improve nutrient digestibility and amino acid absorption from milk protein in vitro. However, the effect of supplementation with this probiotic on lactose digestibility has not yet been evaluated in vivo.
Methods
Wistar female rats were exposed to an acute high-lactose diet (LD; 35% lactose) meal challenge after 7 days of administration of BC30 (LD-BC; n = 10) or vehicle (LD-C; n = 10). Rats treated with vehicle and exposed to control diet (CD; 35% corn starch) meal were used as controls (CD-C; n = 10). Carbohydrate oxidation (CH_OX) and lipid oxidation (L_OX) were monitored by indirect calorimetry before and after lactose challenge. After the challenge, rats were treated daily with vehicle or probiotic for an additional week and were fed with CD or LD ad libitum to determine the effects of BC30 administration in a lactose-induced diarrhoea and malnutrition model.
Results
LD-C rats showed lower CH_OX levels than CD rats, while LD-BC rats showed similar CH_OX levels compared to CD rats during the lactose challenge, suggesting a better digestion of lactose in the rats supplemented with BC30. BC30 completely reversed the increase in the small intestine length of LD-C animals. LD-BC rats displayed increased intestinal mRNA Muc2 expression. No significant changes were observed due to BC30 administration in other parameters, such as serum calprotectin, intestinal MPO activity, intestinal A1AT and SGLT1 levels or intestinal mRNA levels of Claudin2 and Occludin.
Conclusion
Treatment with BC30 improved the digestibility of lactose in an acute lactose challenge and ameliorated some of the parameters associated with lactose-induced malnutrition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that lactose represents approximately 6% of the carbohydrates consumed in Western countries [1]. Although dairy product consumption is much higher in developed countries than in developing countries, the share in developing countries is continuously increasing [1, 2].
Lactose is primarily digested in the small intestine, where it is hydrolysed by the enzyme lactase to yield the monosaccharides glucose and galactose, which are then actively transported into epithelial cells (enterocytes) by the sodium(+)/glucose (galactose) cotransporter (SGLT1) [3, 4]. Most mammals present high concentrations of intestinal lactase at birth and during suckling. However, lactase expression declines after weaning, which can result in lactose malabsorption (LM) and can eventually lead to lactose intolerance (LI), characterized by symptoms such as diarrhoea, abdominal pain or bloating [4]. Currently, management of LM and LI mainly consists of avoiding lactose intake [5]. Although lactase supplementation can improve some symptoms, clinical studies show significant variability in patient outcome [6]. Another approach is treatment with probiotics with lactase activity, such as Lactobacillus spp., Bifidobacterium longum or Bifidobacterium animalis, with the aim of colonizing the intestinal tract with lactase-producing bacteria [4]. Although a recent systematic review indicates an overall positive effect of this approach, most clinical studies present sample size limitations, and further studies are needed to elucidate the efficacy of these treatments [7].
One of the probiotic strains with a proven capacity to digest lactose is Bacillus coagulans GBI-30, 6086 (BC30) [8]. BC30 is a lactic acid-producing, spore-forming bacterial species. Due to its spore-forming ability, it can survive in the acidic environment of the stomach and colonize the gut, and this ability ensures high viability in food products throughout the shelf life [9]. A variety of positive effects related to its consumption have been demonstrated, such as improvement in abdominal pain and bloating in patients with irritable bowel disease (IBS) or postprandial intestinal gas-related symptoms [10, 11], anti-inflammatory and immune-modulating effects [12, 13] or improvement in symptoms related to induced colitis in mice [14]. Moreover, BC30 has been shown to improve nutrient digestibility and amino acid absorption from milk protein [15]. However, to date, the effect of supplementation with this probiotic on lactose digestibility or its mechanisms of action have not yet been evaluated in vivo.
Adult rats represent a natural model of LM, since their intestinal lactase activity is drastically decreased after weaning, reaching residual levels (approximately 8 and 1 nmol/min/mg at the top and bottom segments of the small intestine, respectively) at 30 days after birth [16]. In fact, it has been reported that rats fed ad libitum with diets with more than 20% lactose content show LI-characteristic symptoms, such as diarrhoea, malnutrition and intestinal damage [17,18,19,20]. The measurement by indirect calorimetry of carbohydrate oxidation (CH_OX) levels after a lactose oral load is considered a surrogate marker of lactose digestion and absorption, since lower digestion levels of lactose are translated into a lower utilization of carbohydrates as an energy source and consequently lower CH_OX measured levels. Therefore, lactose digestion and absorption can be indirectly evaluated by comparing CH_OX in rats consuming a lactose-based meal with rats consuming an isocaloric meal containing a different carbohydrate source [21]. CH_OX levels after a lactose meal have also been shown to correlate with lactose absorption in humans, since lactose malabsorbers present lower levels of CH_OX after a lactose load than lactose absorbers [22].
Accordingly, we hypothesized that supplementation with the probiotic BC30 will have beneficial effects on lactose digestibility and will improve some of the negative effects associated with the intake of lactose-rich diets in a natural model of lactose-intolerant rats. Thus, the aims of the present study were to evaluate whether the oral administration of the probiotic BC30 counteracted or ameliorated LM in rats challenged with a high-lactose diet and to shed some light on the underlying mechanisms.
Materials and methods
Experimental diets
A custom control diet (CD; D18080205) and an isocaloric high-lactose diet (LD; D18080206) were designed for this study (Research Diets, Inc., New Brunswick, New Jersey, USA) (Table 1). Both diets contained 21% protein, 63% carbohydrates and 7% fat. Both diets were identical except that the CD contained 35% corn starch, providing 56.4% of the carbohydrates of the diet, while in the LD, all the corn starch was replaced by lactose.
Animal procedure
The Animal Ethics Committee of the Technological Unit of Nutrition and Health of EURECAT (Reus, Spain) and the Generalitat de Catalunya approved all procedures (code 10279). The experimental protocol followed the “Principles of Laboratory Care” and was carried out in accordance with the European Communities Council Directive (86/609/EEC). A total of 30 12-week-old female Wistar rats (Envigo, Barcelona, Spain) were used. All animals were housed individually at 22 °C with a light/dark cycle of 12 h (lights on at 8:00 a.m.) and were given access to food and water ad libitum during the entire experiment. After one week of acclimatization, animals were assigned to the different experimental groups to obtain groups with similar mean body weights.
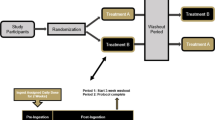
The whole study was planned in two differentiated phases (Fig. 1): in the first experimental phase, animals received the CD ad libitum for 1 week and were orally administered daily with 361 µl of vehicle (water) (n = 20) or 361 µl of the vehicle containing 109 colony forming units (CFUs) of BC30 (Kerry Inc, Beloit, Wisconsin, USA) (n = 10). On the eighth day of study, the animals were exposed to a lactose challenge. In the second experimental phase, beginning after the lactose challenge, rats were fed ad libitum with the CD or LD until Day 15 and received the corresponding treatment until Day 14. Body weight was recorded at the beginning and end of each experimental phase. On Day 15, animals were euthanized under anaesthesia (pentobarbital sodium; 80 mg/kg body weight) after 6 h of diurnal fasting. Blood was collected by cardiac puncture, and serum was obtained and stored at −80 °C until analysis. The liver, caecum, colon, small intestine and spleen were rapidly removed, weighed, frozen in liquid nitrogen, and stored at −80 °C. Small intestine and colon lengths were recorded.
Lactose challenge and indirect calorimetry measurements
For the lactose challenge, a previously described protocol was followed [21], with some modifications. Briefly, at 4:30 a.m. on the seventh day of the study, animals were placed in an Oxylet ProTM System (PANLAB, Cornellà, Spain) in individual acrylic boxes (Oxylet LE 1305 Physiocage; PANLAB) with no food and free access to water. After 16 h of fasting, CD-C and LD-C animals received vehicle treatment, and LD-BC animals received BC30 treatment. After treatment, a 4-g CD meal was placed in each cage for CD-C animals, and a 4-g LD meal was placed in each cage for LD animals. All animals were maintained in the calorimetric system for 6 h after treatment and meal exposure. At the end of the challenge, all feed offered was ingested by all rats. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured in ml/min/kg0.75 every 15 min throughout the period that the animals were inside the system by an O2 and CO2 analyser (Oxylet LE 405 gas analyser; PANLAB) at a controlled flow rate of 600 ml/min (Oxylet LE 400 air supplier, PANLAB). At each point of analysis, the software program Metabolism 2.1.02 (PANLAB) automatically calculated the respiratory quotient (RQ) as the VCO2/VO2 ratio and the energy expenditure (EE) in kcal/day/kg0.75 as VO2 × 1.44 × [3.815 + (1.232 × RQ)], according to the Weir formula [23], and converted it to kJ/day/kg0.75 by multiplying the values by 4.184. To calculate the rates of lipid oxidation (L_OX) and carbohydrate oxidation (CH_OX), VO2 and VCO2 measurements were used, and the stoichiometric equations of Frayn [24], which define the oxidation of carbohydrates (g/min) as 4.55 × VCO2 − 3.21 × VO2 − 2.87 n and the oxidation of fat (g/min) as 1.67 × VO2 − 1.67 × VCO2 − 1.92 n, were applied. According to Carraro et al. [25], a nitrogen excretion rate (n) of 135 μg/kg/min was assumed. To obtain the EE from fat and carbohydrate in kJ, the fat and carbohydrate rates were multiplied by 37 and 16, respectively, using the Atwater general conversion factor [26].
Stool collection and assessment of qualitative score and water content
On the ninth and 14th days of the study, fresh stool was obtained from rats under manual restraint by spontaneous or perianal-stimulated defecation. The qualitative appearance of the stool was assessed on a 3-point scale [where the grades were normal (1), semisolid (2), and diarrhoea (3)], as previously described [27]. Stool samples were weighed and dried at 70 °C for 24 h. Dry stools were weighed again to determine the percentage of the wet weight of the stool. A portion of fresh stool was homogenized in PBS at 10 mg/ml and centrifuged at 1500×g for 10 min, and the supernatant was used to perform calprotectin determination.
Calprotectin levels in faeces and serum
Calprotectin levels were measured in faeces homogenates and serum samples obtained during the sacrifice of the animals using the Rat Calprotectin ELISA Kit (E-EL-R2389, Elabscience Biotechnology Inc., Houston, Texas, USA) according to the manufacturer’s protocol.
SGLT1 and A1AT levels in jejunum
Fifty milligrams of jejunum sections were homogenized in 0.5 ml of phosphate buffered saline (PBS). Homogenates were centrifuged at 10,000×g for 20 min, and supernatants were collected and stored at −80 °C until analysis. SGLT1 and Alpha-1 antitrypsin (A1AT) levels were measured in the supernatants using the Rat Sodium/Glucose Cotransporter 1 ELISA Kit (MBS765055, MyBioSource) and the Rat Alpha 1-Antitrypsin ELISA Kit (E-EL-R2420, Elabscience), according to the manufacturers’ protocols. Protein concentration was determined using a BCA kit (BCA Pierce-23225, Thermo Fisher Scientific, Waltham, Massachusetts, USA) to normalize SGLT1 and A1AT levels against the total protein content.
Myeloperoxidase (MPO) activity in jejunum
Fifty milligrams of jejunum sections were homogenized, and MPO activity was determined in the supernatant using a previously described kinetic method based on the formation of a measurable product at 460 nm after the reaction of o-dianisidine dihydrochloride with MPO in the presence of hydrogen peroxide [28, 29]. A unit of MPO is defined as the degradation of 1 µmol of H2O2 per min at 25 °C.
RNA extraction and gene expression analysis
Total RNA from jejunum samples was extracted using Tripure Reagent (Roche, Barcelona, Spain) and purified using RNeasy Mini Kit spin columns (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The RNA yield and quality were determined in a Nanodrop ND 1000 spectrophotometer. cDNA was synthesized from 1 µg of total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, Massachusetts, USA) and was subjected to q-PCR using the LightCycler 480 II System with SYBR Green I Master Mix (Roche Diagnostic, Barcelona, Spain) with specific primers (0.2 µM) (Table 2). The relative expression of each mRNA was calculated using the 2ΔΔCt method [30], with Ubiquitin C (Ubc) as the reference gene. Each PCR was performed at least in triplicate.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Grubbs’ test was used to detect outliers, which were discarded before subsequent analyses. The differences between groups in continuous measures were analysed using one-way analysis of variance (ANOVA). When the one-way ANOVA was significant (p < 0.05), Fisher’s least significant difference (LSD) post hoc analysis was performed. The differences between groups in parameters measured over time, such as RQ, CH_OX, L_OX and EE, were analysed using repeated-measures ANOVA (rmANOVA) with group (G) and time (t) as fixed factors. The effect of the interaction between group and time factors (G × t) was also analysed in the rmANOVA. Student’s t test was used for single statistical comparisons. All statistical tests were performed with the statistical software IBM Statistical Package for the Social Sciences (SPSS) Statistics v26. The level of statistical significance was set at p < 0.05. GraphPad Prism v. 9.4.1 was used to generate the figures.
Results
Bacillus coagulans GBI-30, 6086 administration increases carbohydrate oxidation in rats exposed to a high-lactose meal
Indirect calorimetry measurements showed that animals exposed to a high-lactose meal and treated with vehicle (LD-C group) presented lower CH_OX and RQ levels during the following 2-h postchallenge compared to levels in animals exposed to a control meal with matched amounts of carbohydrates (CD-C group) (Fig. 2A, B, E, F), as expected. Interestingly, animals exposed to the high-lactose meal and previously treated with BC30 (LD-BC group) showed significantly higher mean levels of CH_OX compared to those in LD-C animals in the following 2 h after the lactose challenge (p = 0.048; ANOVA post hoc LSD), with similar CH_OX compared to animals exposed to a control meal. A similar pattern was observed in the RQ evolution and RQ mean values, with a clear trend of LD-BC animals towards increased levels of this quotient compared to those of the LD-C group (p = 0.051; ANOVA post hoc LSD), indicating an increased carbohydrate/lipid oxidation ratio. No significant changes (one-way ANOVA) between groups in L_OX and EE were observed (Fig. 2C, D and G, H).
Evolution (A–D) and 2-h mean values (E–H) after lactose challenge of the respiratory quotient (RQ) (A, E), carbohydrate oxidation (B, F), fat oxidation (C, G), and energy expenditure (D, H) during lactose challenge. Data are expressed as the mean ± SEM (n = 10). G group effect; t time effect (p < 0.05; rmANOVA). ab, values with unlike letters are significantly different among groups (one-way ANOVA and LSD post hoc comparison, p < 0.05)
Biometric parameters measurements after ad libitum exposure to lactose diet
Animals from both groups fed a high-lactose diet ad libitum for one week showed a significantly lower body weight gain compared to that of CD-C animals, as expected, with no significant effects of BC30 treatment. No differences were observed in the colon length and the weight of the liver, spleen and colon (Table 3). Interestingly, small intestines from LD-C animals were significantly longer than those from CD-C animals, while BC30 treatment displayed a reverting effect on this parameter, since LD-BC animals showed similar small intestine lengths to CD-C animals. LD-C and LD-BC animals showed increased caecum weights (2.75-fold and 2.43-fold, respectively) compared to CD-C animals (Table 3). Although animals treated with BC30 showed a numerically lower caecum weight compared to LD-C animals, these differences were not significant (p = 0.103; post hoc LSD).
Stool consistency and water content
The stool consistency and stool water content were determined one day and six days after ad libitum high-lactose feeding. One day after high-lactose diet exposure (Day 9 of the study), LD-C and LD-BC animals showed significantly higher water contents than that of CD-C animals (65.4% and 62.6%, respectively, vs 46.2%).
On study Day 14, when animals had been exposed to a lactose diet for 6 days, the stool was more consistent in LD-C and in LD-BC animals compared to their values on Day 9 of the study, although the water content remained higher than that in CD-C animals (Table 4). Although the stool water content values in LD-BC animals were numerically lower than the LD-C values on Days 9 and 14, those differences were not significant (p > 0.05).
SGLT1, calprotectin, A1AT and MPO levels
To detect possible changes in a key membrane transporter for the absorption of glucose and galactose, SGLT1 levels were analysed in jejunum homogenates. Although the LD-C group showed the highest mean intestinal SGLT1 levels, no significant differences were detected among groups in the ANOVA (Fig. 3A).
Levels of SGLT1 in jejunum homogenates (A) and levels of serum calprotectin (B), intestinal MPO activity (C) and intestinal A1AT (D). Data are expressed as the mean ± SEM (n = 10). ab, values with unlike letters are significantly different among groups (one-way ANOVA and LSD post-hoc comparison, p < 0.05)
Calprotectin levels were analysed in serum and faeces as a marker of intestinal inflammation. Despite the fact that it was not possible to detect calprotectin in faecal homogenates (LOD = 62.5 ng/mg), analysis in serum samples showed a significant 38.8% increase in LD-C calprotectin levels vs. CD-C. No significant difference between the LD-C and LD-BC groups was observed concerning this parameter (Fig. 3B).
MPO activity was analysed in the jejunum as a marker of intestinal inflammation and neutrophil infiltration. Animals from both groups fed with the LD showed significantly higher levels of MPO activity in the jejunum than levels in the CD-C group, with no significant differences associated with BC consumption (Fig. 3C).
Intestinal A1AT levels were measured as a marker of gut permeability (Fig. 3D), and although no significant changes were observed between groups in the ANOVA, the LD-C and LD-BC groups showed numerically higher intestinal A1AT levels than the CD-C group, and a trend was observed in the CD-C vs. LD-C comparison (p = 0.062; Student’s t test).
Intestinal gene expression of Claudin2, Occludin and Muc2
Claudin2 (Cldn2) and Occludin (Ocln) gene expression levels were analysed as markers of intestinal tight junction permeability. Although no significant changes among groups were observed in the Cldn2 and Ocln mRNA levels in jejunum sections (Fig. 4A, B), a trend towards different Cldn2 gene expression levels was observed (ANOVA; p = 0.097), showing a 1.81-fold reduction in LD-C animals vs. CD-C animals, while LD-BC animals showed similar Cldn2 expression compared to that of CD-C animals (Fig. 4A).
Mucin 2 (Muc2) mRNA levels were analysed as a marker of mucin production, indicative of the state of the intestinal mucosa layer. Interestingly, BC30-treated animals showed a significant 1.77-fold induction of Muc2 gene expression in the jejunum compared to that of LD-C animals (p = 0.033; post hoc LSD) and CD-C animals (p = 0.035; post hoc LSD), while no changes due to the lactose diet were observed in this gene (Fig. 4C).
Discussion
In the present study, we evaluated the effectiveness of BC30 supplementation on the reversal of the symptoms caused by a one-time intake of a lactose-rich meal and continuous exposure to a lactose-rich diet in a natural model of lactose-intolerant rats. Similar to the study published by Alexandre et al. [21], the calorimetry results showed that animals exposed to a lactose-rich meal displayed lower levels of RQ and CH_OX following meal intake in comparison to animals that consumed an isocaloric control meal with the same amount of carbohydrates, indicating a less efficient use of the carbohydrate fraction from lactose and confirming the usefulness of indirect calorimetry measurements as an indicator of lactose digestion. The carbohydrate oxidation and respiratory quotient measured by indirect calorimetry have been shown to significantly correlate with traditional markers of lactose digestion, such as the hydrogen breath test, blood glycaemia, and even hypogastric pain in humans [22]. Interestingly, animals supplemented with BC30 oxidized a similar amount of carbohydrates after lactose intake as animals exposed to the nonlactose control meal, suggesting that animals supplemented with BC30 successfully digested lactose almost entirely. This finding is in accordance with the work of Maathuis et al., who reported the capacity of BC30 to digest lactose in an in vitro model of the stomach and small intestine [8]. After ad libitum exposure to the LD, small intestines from LD-C animals were significantly longer than those from CD-C animals, a fact that has been associated with a compensatory mechanism to increase the absorption of nutrients in cases of malnutrition [31] and could be due to the lower levels of carbohydrate absorption in LD-C animals. In line with this hypothesis, LD-BC-treated animals exhibited a reversal of the increase in small intestine length, presumably because of their major levels of carbohydrate utilization and lactose absorption.
The stool consistency analysis showed that animals consuming the LD excreted wetter faeces, as previously described [32], showing some degree of adaptation to the diet, since the stool water content of these animals was significantly lower after 1 week of consuming the diet in comparison to 1 day after the first exposure. BC30 supplementation seemed to slightly ameliorate stool consistency at both analysed times, even though these differences did not reach statistical significance.
Caecal distension and an increase in the caecal content have been widely described in rats exposed to lactose [32,33,34], as we report in the present study, a fact presumably related to the increase in caecal fermentation due to lactose [21]. Despite the fact that BC30-supplemented rats showed a trend towards a slightly lower caecum weight compared to LD-C animals, this effect was not enough to revert the increase in caecum weight caused by LD. In this sense, it would be interesting for further studies to determine the effect of BC30 supplementation on the caecal content of rats exposed to lactose. These results, together with the nondifferences observed in body weight compared to LD-C animals, suggest that although BC30 consumption enhanced lactose oxidation in a one-time 4-g meal containing 35% lactose, the amelioration in lactose oxidation capacity is not enough to counteract the negative effects of a continuous ad libitum high-lactose diet of approximately 20 g per day.
SGLT1 is the key transporter for the absorption of glucose and galactose in the gastrointestinal tract [35]. Numerically higher levels of this parameter were observed in the LD-C animals, which suggests that these animals increased the production of this transporter as a compensation mechanism for the lack of glucose and galactose absorption due to their incapacity to digest lactose and that the rats supplemented with BC30 did not need to increase SGLT1 production. However, these differences were not significant, and further studies are needed to confirm this hypothesis.
Calprotectin is a calcium- and zinc-binding protein that accounts for 40–60% of the protein mass within the cytosol of neutrophils. The presence of calprotectin in faeces is directly proportional to neutrophil migration towards the intestinal tract, which is related to the inflammatory process [36,37,38]. In the present study, faecal calprotectin levels were below the limit of detection. However, serum calprotectin has been shown to positively correlate with faecal calprotectin, and it has also been proposed as a novel diagnostic marker for IBS [39]. Intestinal MPO activity was also analysed as an indicator of neutrophil accumulation and intestinal inflammation [40]. Thus, the increase in serum calprotectin and intestinal MPO activity observed in rats exposed to lactose suggests a state of intestinal inflammation and neutrophil activation in these animals, and BC30 supplementation was not sufficient to revert these factors.
A1AT is a serum protein that acts as a protease inhibitor. In cases of intestinal barrier dysfunction, A1AT can be localized in the gut [41]. Claudin 2 and Occludin are tight-junction membrane proteins that are key markers of intestinal barrier permeability [42, 43]. Although ANOVA did not show significant differences in intestinal A1AT levels and mRNA levels of Cldn2 among groups, an exploratory Student’s t test between the LD-C and CD-C rats showed a significant reduction in Cldn2 gene expression in the jejunum of LD-C animals vs. CD-C and a trend towards increased intestinal A1AT levels in LD-C vs CD-C rats, thus suggesting a decrease in intestinal permeability due to continuous exposure to lactose [44]. The LD-BC group showed intermediate levels of Cldn2 expression between the CD-C and LD-C groups, suggesting a partial recovery of intestinal barrier function due to BC30 supplementation.
Finally, the significant increase in Muc2 intestinal gene expression in animals supplemented with BC30 would indicate an increase in the production of mucin, one of the main components forming the mucous layer of the intestinal barrier [45]. This finding is in accordance with several other studies that showed increased intestinal Muc2 gene expression after supplementation with several probiotics, such as Propionibacterium freudenreichii, Lactococcus lactis, Lactobacillus salivarius, Lactobacillus rhamnosus or different combinations of probiotics [46,47,48,49,50,51].
Conclusions
Globally, the results of the present study showed interesting beneficial effects of the administration of Bacillus coagulans GBI-30, 6086 in a rat model of lactose intolerance exposed to a lactose-rich meal, as evidenced by the increase in the oxidation of carbohydrates and the improvement in parameters associated with lactose-induced malnutrition, such as small intestine lengthening, as well as improved intestinal barrier function by increasing Muc2 expression. Despite these positive effects, BC30 administration did not counteract some of the effects of a one-week continuous high-lactose diet, such as intestinal inflammation, minor weight gain, watery faeces, or a high caecum weight. However, BC-30 supplementation could represent a promising approach to relieving or preventing some of the negative effects associated with lactose-induced malabsorption.
Data availability
The datasets used and analysed in this current study are available from the corresponding author (JT) upon reasonable request.
References
Ugidos-Rodríguez S, Matallana-González MC, Sánchez-Mata MC (2018) Lactose malabsorption and intolerance: a review. Food Funct 9:4056–4068. https://doi.org/10.1039/C8FO00555A
Silanikove N, Leitner G, Merin U (2015) The interrelationships between lactose intolerance and the modern dairy industry: global perspectives in evolutional and historical backgrounds. Nutrients 7:7312. https://doi.org/10.3390/NU7095340
Chen L, Tuo B, Dong H (2016) Regulation of intestinal glucose absorption by ion channels and transporters. Nutrients. https://doi.org/10.3390/NU8010043
Misselwitz B, Butter M, Verbeke K, Fox MR (2019) Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut 68:2080–2091. https://doi.org/10.1136/GUTJNL-2019-318404
Yang J, Deng Y, Chu H et al (2013) Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. https://doi.org/10.1016/J.CGH.2012.11.034
Ibba I, Gilli A, Boi MF, Usai P (2014) Effects of exogenous lactase administration on hydrogen breath excretion and intestinal symptoms in patients presenting lactose malabsorption and intolerance. Biomed Res Int. https://doi.org/10.1155/2014/680196
Oak SJ, Jha R (2019) The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr 59:1675–1683. https://doi.org/10.1080/10408398.2018.1425977
Maathuis AJH, Keller D, Farmer S (2010) Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef Microbes 1:31–36. https://doi.org/10.3920/BM2009.0009
Tripathi MK, Giri SK (2014) Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods 9:225–241. https://doi.org/10.1016/J.JFF.2014.04.030
Dolin BJ (2009) Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find Exp Clin Pharmacol 31:655–659. https://doi.org/10.1358/MF.2009.31.10.1441078
Kalman DS, Schwartz HI, Alvarez P et al (2009) A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol 9:85. https://doi.org/10.1186/1471-230X-9-85
Jensen GS, Benson KF, Carter SG, Endres JR (2010) GanedenBC30™ cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol 11:15. https://doi.org/10.1186/1471-2172-11-15
Kimmel M, Keller D, Farmer S, Warrino DE (2010) A controlled clinical trial to evaluate the effect of GanedenBC(30) on immunological markers. Methods Find Exp Clin Pharmacol 32:129–132. https://doi.org/10.1358/MF.2010.32.2.1423881
Fitzpatrick LR, Small JS, Greene WH et al (2012) Bacillus coagulans GBI-30, 6086 limits the recurrence of Clostridium difficile-induced colitis following vancomycin withdrawal in mice. Gut Pathogens. https://doi.org/10.1186/1757-4749-4-13
Stecker RA, Moon JM, Russo TJ et al (2020) Bacillus coagulans GBI-30, 6086 improves amino acid absorption from milk protein. Nutr Metab (Lond). https://doi.org/10.1186/S12986-020-00515-2
Courtois P, Sener A, Scott FW, Malaisse WJ (2004) Disaccharidase activity in the intestinal tract of Wistar-Furth, diabetes-resistant and diabetes-prone biobreeding rats. Br J Nutr 91:201–209. https://doi.org/10.1079/BJN20041026
Norton R, Leite J, Vieira E et al (2001) Use of nucleotides in weanling rats with diarrhea induced by a lactose overload: effect on the evolution of diarrhea and weight and on the histopathology of intestine, liver and spleen. Braz J Med Biol Res 34:195–202
Boakye PA, Brierley SM, Pasilis SP, Balemba OB (2012) Garcinia buchananii bark extract is an effective anti-diarrheal remedy for lactose-induced diarrhea. J Ethnopharmacol 142:539–547. https://doi.org/10.1016/j.jep.2012.05.034
Arciniegas EL, Cioccia AMHP (2000) Effect of the lactose induced diarrhea on macronutrients availability and immune function in well-nourished and undernourished rats. Arch Latinoam Nutr 50:48–54
Bueno J, Torres M, Almendros A et al (1994) Effect of dietary nucleotides on small intestinal repair after diarrhoea. Histological and ultrastructural changes. Gut 35:926–933. https://doi.org/10.1136/gut.35.7.926
Alexandre V, Even PC, Larue-Achagiotis C et al (2013) Lactose malabsorption and colonic fermentations alter host metabolism in rats. Br J Nutr 110:625–631. https://doi.org/10.1017/S0007114512005557
Alexandre V, Davila AM, Bouchoucha M et al (2013) Agreement between indirect calorimetry and traditional tests of lactose malabsorption. Dig Liver Dis 45:727–732. https://doi.org/10.1016/J.DLD.2013.03.015
Weir JBV (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109:1–9. https://doi.org/10.1113/JPHYSIOL.1949.SP004363
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634
Carraro F, Stuart CA, Hartl WH et al (1990) Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol 259:E470–E476
Bircher S, Knechtle B (2004) Relationship between fat oxidation and lactate threshold in athletes and obese women and men. J Sport Sci Med 3:174–181
Sung J, Morales W, Kim G et al (2013) Effect of repeated Campylobacter jejuni infection on gut flora and mucosal defense in a rat model of post infectious functional and microbial bowel changes. Neurogastroenterol Motil 25:529–537. https://doi.org/10.1111/nmo.12118
Abdin AA (2013) Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol 718:145–153. https://doi.org/10.1016/J.EJPHAR.2013.08.040
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209. https://doi.org/10.1111/1523-1747.ep12506462
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Nieto N, Lopez-Pedrosa JM, Mesa MD et al (2000) Chronic diarrhea impairs intestinal antioxidant defense system in rats at weaning. Dig Dis Sci 45:2044–2050. https://doi.org/10.1023/A:1005603019800
van de Heijning BJM, Kegler D, Schipper L et al (2015) Acute and chronic effects of dietary lactose in adult rats are not explained by residual intestinal lactase activity. Nutrients 7:5542–5555. https://doi.org/10.3390/NU7075237
Collares EF, Rossi MA, Macedo AS (1985) Experimental dilatation of the cecum and colon in rats. II. Reversion after induction by the continuous administration of lactose. Arq Gastroenterol 22:192–195
Kim KI, Benevenga NJ, Grummer RH (1979) In vitro measurement of the lactase activity and the fermentation products of lactose in the cecal and colonic contents of rats fed a control or 30% lactose diet. J Nutr 109:856–863. https://doi.org/10.1093/JN/109.5.856
Poulsen SB, Fenton RA, Rieg T (2015) Sodium-glucose cotransport. Curr Opin Nephrol Hypertens 24:463–469
Gisbert JP, McNicholl AG (2009) Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis 41:56–66. https://doi.org/10.1016/J.DLD.2008.05.008
Nanini HF, Bernardazzi C, Castro F, De Souza HSP (2018) Damage-associated molecular patterns in inflammatory bowel disease: from biomarkers to therapeutic targets. World J Gastroenterol 24:4622–4634. https://doi.org/10.3748/WJG.V24.I41.4622
Lehmann FS, Burri E, Beglinger C (2015) The role and utility of faecal markers in inflammatory bowel disease. Ther Adv Gastroenterol 8:23–36. https://doi.org/10.1177/1756283X14553384
Kalla R, Kennedy NA, Ventham NT et al (2016) Serum calprotectin: a novel diagnostic and prognostic marker in inflammatory bowel diseases. Am J Gastroenterol 111:1796–1805. https://doi.org/10.1038/AJG.2016.342
Khan AA, Alsahli MA, Rahmani AH (2018) Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med Sci (Basel, Switzerland). https://doi.org/10.3390/MEDSCI6020033
Celi P, Verlhac V, Pérez Calvo E et al (2019) Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim Feed Sci Technol 250:9–31. https://doi.org/10.1016/J.ANIFEEDSCI.2018.07.012
Günzel D, Yu ASL (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93:525–569. https://doi.org/10.1152/physrev.00019.2012
Lee SH (2015) Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 13:11. https://doi.org/10.5217/ir.2015.13.1.11
Venugopal S, Anwer S, Szászi K (2019) Claudin-2: roles beyond permeability functions. Int J Mol Sci 20:5655
Corfield AP (2015) Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim Biophys Acta 1850:236–252. https://doi.org/10.1016/J.BBAGEN.2014.05.003
Ma S, Yeom J, Lim YH (2020) Dairy Propionibacterium freudenreichii ameliorates acute colitis by stimulating MUC2 expression in intestinal goblet cell in a DSS-induced colitis rat model. Sci Rep. https://doi.org/10.1038/S41598-020-62497-8
Gao J, Li Y, Wan Y et al (2019) A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol. https://doi.org/10.3389/FMICB.2019.00477
Yu JY, He XL, Puthiyakunnon S et al (2015) Mucin2 is required for probiotic agents-mediated blocking effects on meningitic E. coli-induced pathogenicities. J Microbiol Biotechnol 25:1751–1760. https://doi.org/10.4014/JMB.1502.02010
Kuugbee ED, Shang X, Gamallat Y et al (2016) Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig Dis Sci 61:2908–2920. https://doi.org/10.1007/S10620-016-4238-7
Cervantes-Garciá D, Jiménez M, Rivas-Santiago CE et al (2021) Lactococcus lactis NZ9000 prevents asthmatic airway inflammation and remodelling in rats through the improvement of intestinal barrier function and systemic TGF-β production. Int Arch Allergy Immunol 182:277–291. https://doi.org/10.1159/000511146
Zhuge A, Li B, Yuan Y et al (2020) Lactobacillus salivarius LI01 encapsulated in alginate-pectin microgels ameliorates d-galactosamine-induced acute liver injury in rats. Appl Microbiol Biotechnol 104:7437–7455. https://doi.org/10.1007/S00253-020-10749-Y
Acknowledgements
We would like to express our thanks for their technical support to Yaiza Tobajas, Anna Antolín, Iris Triguero and Cristina Egea, who are laboratory technicians at the Technological Unit of Nutrition and Health.
Funding
This work was financially supported by the Centre for the Development of Industrial Technology (CDTI) of the Spanish Ministry of Science and Innovation under grant agreement of a project called TOLERA EXP 00101692.
Author information
Authors and Affiliations
Contributions
Conceptualization, JT, JMDB, AC, FP, AR-G, AH and VG; funding acquisition, JMDB, AC and FP; investigation, JT, UC, RM-C, CD-C, AM-C, JMA-H and GC; formal analysis, JT and UC; methodology, JT and RM-C; writing—original draft, JT; writing—review and editing, JT, UC, RM-C, CD-C, JMA-H, JMDB and AC; supervision, JMDB and AC.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teichenné, J., Catalán, Ú., Mariné-Casadó, R. et al. Bacillus coagulans GBI-30, 6086 (BC30) improves lactose digestion in rats exposed to a high-lactose meal. Eur J Nutr 62, 2649–2659 (2023). https://doi.org/10.1007/s00394-023-03183-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03183-z