Abstract
Purpose
Compared with people without diabetes, people with type 2 diabetes (T2D) are at higher risk of both subnormal vitamin C status and increased oxidative stress. We aimed to investigate the associations of serum vitamin C concentrations with all-cause and cause-specific mortality among adults with and without T2D.
Methods
The current analysis included 20,045 adults (2691 people with T2D and 17,354 without T2D) from the Third National Health and Nutrition Examination Survey (NHANES III) and NHANES 2003–2006. Cox proportional hazards regression models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Restricted cubic spline analyses were used to examine the dose–response relationship.
Results
After a median follow-up of 17.3 years, 5211 deaths were documented. Individuals with T2D had a lower level of serum vitamin C concentrations compared with those without T2D (the median value: 40.1 vs. 44.9 μmol/L). Furthermore, the dose–response relationship between serum vitamin C and mortality showed different patterns between participants with and without T2D. In individuals without T2D, there was a nonlinear association of serum vitamin C concentrations with all-cause, cancer, and CVD mortality, with the lowest risk around a serum vitamin C concentration of 48.0 μmol/L (all Poverall < 0.05, Pnonlinearity < 0.05). In contrast, among those with T2D in the similar concentration range, higher serum vitamin C levels (ranged from 0.46 to 116.26 μmol/L) were linearly associated with lower all-cause and cancer mortality (both Poverall < 0.05, Pnonlinearity > 0.05). Significant additive interaction was observed between diabetes status and serum vitamin C levels with regard to all-cause and cancer mortality (P < 0.001). In addition, C-reactive protein, gamma-glutamyl transpeptidase, and HbA1c explained 14.08, 8.96, and 5.60% of the association between serum vitamin C and all-cause mortality among individuals with T2D, respectively.
Conclusions
Higher serum vitamin C concentrations were significantly associated with lower risk of mortality in participants with T2D in a linear dose–response manner, while a nonlinear association was observed in participants without T2D, with an apparent threshold around 48.0 μmol/L. These findings suggest that the optimal vitamin C requirement may differ in individuals with and without T2D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin C, also known as ascorbic acid, is a kind of essential micronutrient for metabolism in the human body [1], with the strong capacity for scavenging free radicals, attenuating oxidative stress and confronting inflammation [2]. However, epidemiological evidence pertaining to the relationships between vitamin C and long-term health outcomes remained inconsistent in general populations [3,4,5,6,7]. Some prospective studies suggested that blood concentrations of vitamin C was one of objective indicators of fruit and vegetable consumption and higher vitamin C levels were linearly associated with lower risk of incident diabetes, cardiovascular disease, cancer and mortality [3, 4, 8, 9], while others did not find any associations [7]. Furthermore, accumulating studies suggested that there was a threshold effect between serum vitamin C levels and mortality—when exceeding a threshold level, vitamin C was not inversely associated with mortality [5, 6, 10, 11].
In patients with diabetes, it was reported that there was an increment in oxidative stress which might impair insulin secretion and interfere with glucose disposal, while vitamin C, known as a common antioxidant, was more likely to be inadequate in blood in comparison with people without diabetes [12,13,14]. However, evidence regarding the health effects of vitamin C among patients with T2D is scarce and inconsistent. Several [15,16,17], but not all [18], clinical trials have found that vitamin C supplementation might be beneficial to some metabolic biomarkers (e.g., HbA1c, postprandial glycaemia, blood pressure) in diabetic patients. Nevertheless, those existing trials were predominantly short term with a relatively small number of participants, and they did not investigate the long-term health effects of vitamin C in patients with T2D. To our best knowledge, no study has investigated the dose–response relationship between serum vitamin C levels, a much more reliable indicator of body vitamin C status [19], and mortality in people with diabetes. In addition, whether the association of vitamin C status with the risk of mortality differs between people with and without T2D is unknown.
To fill these knowledge gaps, based on a nationally representative sample of participants from the National Health and Nutrition Examination Survey (NHANES), we compared the dose–response relationship between serum vitamin C and all-cause and cause-specific mortality among adults with and without T2D, and then further explored whether cardiometabolic biomarkers mediated the association between serum vitamin C and mortality.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a survey with a multi-stage, complex, probability sampling design performed by the Centers for Disease Control and Prevention (CDC). NHANES is committed to evaluating health and nutritional status of the noninstitutionalized U.S. population through a nationally representative sample. The study design and baseline characteristics have been described in detail previously [20]. NHANES was approved by the ethics review board of the National Center for Health Statistics Research and written informed consent was obtained from all participants. In the present study, we used data from NHANES III (1988–1994) and two cycles of NHANES from 2003 to 2006 in which serum vitamin C data were available.
T2D was defined as self-reported doctor diagnosis of diabetes, use of insulin or oral hypoglycemic medication, fasting glucose ≥ 7.0 mmol/L (≥ 126 mg/dL), postprandial 2-h plasma glucose ≥ 11.1 mmol/L (≥ 200 mg/dL) from an oral glucose tolerance test (OGTT), or glycated hemoglobin A1c (HbA1c) ≥ 6.5% (≥ 48 mmol/mol).
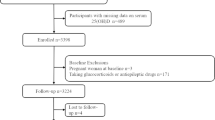
In total, there were 24,077 participants (aged 20 years and older) with data of serum vitamin C concentrations. Of these, we excluded participants who were pregnant (n = 704), or had CVD (n = 1744) or cancer (n = 1564) at baseline, and those without follow-up information (n = 20). Finally, a total of 20,045 adults (2691 with T2D and 17,354 without T2D) were included in our analyses (Supplementary Fig. 1).
Measurement of serum vitamin C
Serum vitamin C was the primary exposure of interest. Blood was drawn from participants at baseline in mobile examination centers. Serum specimens were processed, stored, and shipped to the Centers for Disease Control and Prevention for analysis. In NHANES 2003–2006, an isocratic reverse-phase high performance liquid chromatography method with electrochemical detection was used for vitamin C (ascorbic acid), and a similar method was used in NHANES III [21]. According to the NHANES official analytic notes, data from NHANES III were then converted by a Deming regression equation for comparison with data from NHANES 2003–2006. Serum vitamin C concentrations were categorized into quartiles. The vitamin C in mg/dL was converted to μmol/L by multiplying by 56.78. The details of analytical procedures for serum vitamins have been described elsewhere [21].
Ascertainment of mortality
Mortality status in NHANES was ascertained via linkage to the National Death Index through December 31, 2015. Mortality from all causes, CVD, and cancer was determined using the International Classification of Diseases (ICD), 10th revision (ICD-10). CVD mortality was defined as ICD-10 codes I00–I09, I11, I13, I20–I51, or I60–I69. Cancer mortality was defined as ICD-10 codes C00–C97. All-cause mortality was comprised of all specified and unknown causes.
Assessment of covariates
Information on age, sex, race/ethnicity, education level, family income, physical activity, smoking status, medication use and disease status were collected using standardized questionnaires in household interviews. Bodyweight, height and alcohol intake were examined at a mobile examination center. BMI was calculated as weight in kilograms divided by height in meters squared. Race/ethnicity was classified as non-Hispanic white or other. Educational attainment was categorized into less than high school, high school or equivalent, or college or above. The family income-poverty ratio level was defined as the total family income divided by the poverty threshold. Leisure-time physical activity was classified as inactive group (no leisure-time physical activity), insufficiently active group (leisure-time moderate activity 1–5 times per week with metabolic equivalents ranging from 3 to 6 or leisure-time vigorous activity 1–3 times per week with metabolic equivalents > 6), or active group (those who had more leisure-time activity than above) [22]. Smoking status was grouped into never smokers, former smokers, or current smokers. Drinking status was categorized into non-drinkers or drinkers.
Furthermore, dietary intake of vitamin A, vitamin E, selenium, beta-carotene, fiber, total fatty acids and total energy was obtained from USDA Food & Nutrient Database for Dietary Studies where nutrient intake was calculated by using dietary intake data from 24-h dietary recall interviews of participants [23].
In addition, metabolic biomarkers including C-reactive protein (CRP), gamma-glutamyl transpeptidase (GGT), insulin, plasma glucose, HbA1c, LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), total cholesterol, and triglyceride were measured at baseline among participants who provided blood samples. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated in accordance with the method reported before [24].
Statistical analyses
Considering the complex sampling design of the NHANES, sample weights, clustering, and stratification were incorporated in all analyses. Sample characteristics were reported as mean ± standard deviations for normally distributed continuous variables, medians (interquartile ranges) for non-normally distributed continuous variables and numbers with percentages for categorical variables. The generalized linear model was employed to examine the associations of serum vitamin C concentrations with serum concentrations of GGT, CRP, serum glucose, HOMA-IR, HbA1c, and insulin.
To examine the dose–response relationship between serum vitamin C and all-cause, CVD and cancer mortality among people with and without T2D, a restricted cubic spline regression model with three knots (5th, 50th, and 95th percentiles) was employed. The model excluded the most extreme 1% values to reduce the potential influence of outliers. Tests for nonlinearity were performed using the likelihood ratio test.
Person-years of follow-up were calculated as the interval between the date of the examination of serum vitamin C to the date of death, or the end of follow-up (31 December 2015), whichever occurred first. Cox proportional hazards regression models were used to estimate the hazard ratios and 95% CIs of mortality according to the quartiles of serum vitamin C concentrations among people with and without T2D. We fitted three Models. Model 1 was adjusted for age (continuous), sex (male, or female), and race/ethnicity (white, or non-white). Model 2 was further adjusted for BMI (< 25.0, 25.0–29.9, or ≥ 30.0 kg/m2), education level (less than high school, high school or equivalent, or college or above), family income-poverty ratio (≤1.0, 1.1–3.0, or > 3.0), drinking status (nondrinkers, or drinkers), smoking status (never smokers, former smokers, or current smokers), leisure-time moderate-to-vigorous physical activity (inactive group, insufficiently active group, or active group), HEI (in quartiles), supplement use (yes, or no), and total energy intake (in quintiles). In model 3, we further adjusted for self-reported hypertension, and hypercholesterolemia (yes, or no). The models for individuals with T2D were additionally adjusted for diabetes medication use (none, insulin, oral medicine, or other), and diabetes duration ( < 3, or ≥ 3 years). To minimize sample size reduction due to missing covariates, we imputed the missing values of covariates (≤ 7%) using multiple imputations by chained equations with 5 imputations (SAS PROC MI with a fully conditional specification method and PROC MIANALYZE). Tests for linear trend were performed using the median value in each category. Data were square root-transformed or logarithmically transformed to approximate a normal distribution, when appropriate. To reflect the biological plausibility of interaction between diabetes status (yes, and no with no as the reference level) and serum vitamin C (per 1-SD higher as the reference level), we evaluated additive interaction by two indexes: the relative excess risk because of the interaction (RERI) and the attributable proportion because of the interaction (AP). In the absence of additive interaction, the CIs of the RERI and AP would include 0.
We performed mediation analysis (SAS PROC CAUSALMED) [25] to investigate whether the biomarkers of inflammation, oxidative stress, and glucose metabolism, could mediate the associations of serum vitamin C with all-cause mortality. The 95% CIs of causal mediation effects were obtained using bootstrapping.
Stratified analyses were also conducted according to age (≤ 60, > 60 years), sex (male, female), smoking status (ever, never), BMI (< 30, ≥ 30 kg/m2) in participants with and without T2D. In participants with T2D, we further stratified by diabetes duration (< 3, ≥ 3 years), and HbA1c (< 7, ≥ 7%). Potential modifying effects were examined by testing the corresponding multiplicative interaction terms.
Furthermore, several sensitivity analyses were conducted to test the robustness of the results. First, to minimize the influence of reverse causation, we performed a sensitivity analysis by excluding participants who died during the first 2 years of follow-up. Second, instead of HEI, we further adjusted for individual nutrients, including dietary intakes of total fatty acids, vitamin A, vitamin E, selenium, beta-carotene, and fiber (all in quartiles). In addition, we further adjusted for other dietary biomarkers, including serum vitamin A, vitamin E, beta-carotene, and 25-hydroxyvitamin D (all in quartiles). Third, we repeated main analyses according to quartiles of serum vitamin C levels that were determined based upon the distribution in the total population rather than based upon participants with and without T2D.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics of 20,045 participants (mean age, 43.6 years; 48.8% male) are provided in Table 1. The median (interquartile range) serum vitamin C concentration was 40.1 (20.9, 56.9) μmol/L among diabetics and 44.9 (23.3, 62.2) μmol/L among non-diabetics. Compared to those without diabetes, those with diabetes were older, had higher levels of BMI while lower levels of education and family income, and were more likely to be female, former smokers and non-drinkers, physically inactive, have hypercholesterolemia and hypertension. Supplementary Table 1 shows the participants characteristics according to quartiles of serum vitamin C concentrations. Participants with higher serum vitamin C concentrations were more likely to be older, female, non-smokers, non-drinkers, and supplement users, and tended to have higher educational attainment, hypercholesterolemia and HEI.
The dose–response relationship between serum vitamin C and mortality showed different patterns between participants with and without T2D (Fig. 1). Among individuals with T2D, significant linear relationships were demonstrated between higher serum vitamin C concentrations (ranged from 0.46 to 116.26 μmol/L) and lower all-cause mortality (Poverall = 0.02, Pnonlinearity = 0.87, Fig. 1 panel A) and cancer mortality (Poverall = 0.03, Pnonlinearity = 0.80, Fig. 1 panel B), but not CVD mortality (Poverall = 0.32, Pnonlinearity = 0.44, Fig. 1 panel C). In contrast, among those without T2D, there was a nonlinear association between serum vitamin C concentrations and all-cause, cancer, and CVD mortality, with lowest risk around the serum vitamin C concentration of 48.0 μmol/L (all Poverall < 0.05, Pnonlinearity < 0.05, Fig. 1 panels D–F).
Association between serum vitamin C concentrations with all-cause, cancer and CVD mortality among individuals with and without type 2 diabetes in NHANES III and NHANES 2003–2006*. *Serum vitamin C was square root transformed in restricted cubic splines model and then converted. Hazard ratios were adjusted for age (continuous), sex (male, or female), and race/ethnicity (non-Hispanic white, or other), BMI (< 25.0, 25.0–29.9, or ≥ 30.0 kg/m2), education level (less than high school, high school or equivalent, or college or above), family income-poverty ratio (≤1.0, 1.1–3.0, or > 3.0), drinking status (nondrinker, or drinker), smoking status (never smoker, former smoker, or current smoker), leisure-time moderate-to-vigorous physical activity (inactive group, insufficiently active group, or active group), HEI (in quartiles), supplement use (yes, or no), total energy intake (in quintiles), self-reported hypertension (yes, or no), and hypercholesterolemia (yes, or no). The models for individuals with T2D were additionally adjusted for diabetes medication use (none, insulin, oral medicine, or other), and diabetes duration (< 3, or ≥ 3 years)
Table 2 shows the HRs and 95% confidence intervals (CI) for mortality by quartiles of serum vitamin C levels. During 38,659.58 person-years of follow-up (a median follow-up time of 12.33 years), there were totally 1394 deaths among individuals with T2D. During 298,230.75 person-years of follow-up (a median follow-up time of 19.96 years), there were totally 3817 deaths among individuals without T2D. In patients with T2D, higher serum vitamin C levels were significantly associated with lower risk of all-cause and cancer mortality, but not CVD mortality, after multivariable adjustment. Participants with T2D in the highest (> 56.9 μmol/L) compared with the lowest (< 20.9 μmol/L) quartile of serum vitamin C had a 24% lower all-cause mortality risk (HR: 0.76; 95% CI: 0.59, 0.97, Ptrend = 0.005) and a 53% lower cancer mortality risk (HR: 0.47; 95% CI: 0.27, 0.82, Ptrend = 0.001). In participants without T2D, compared with the first quartile (< 23.3 μmol/L), higher serum vitamin C was related to lower all-cause, cancer, and CVD mortality, with the lowest mortality risk in the third quartile (45.0–62.2 μmol/L) (all-cause mortality: HR 0.76 [95% CI 0.67, 0.87]; cancer mortality: HR 0.64 [95% CI 0.49, 0.84]; CVD mortality: HR 0.70 [95% CI 0.51, 0.95]), and no further decrease in HR in the highest quartile (> 62.2 μmol/L) (all-cause mortality: HR 0.87 [95% CI 0.75, 1.00]; cancer mortality: HR 0.70 [95% CI 0.56, 0.89]; CVD mortality: HR 0.90 [95% CI 0.66, 1.22]) (Table 2).
RERI and AP were calculated as measures of additive interaction and presented in Table 3. There was significant positive additive interaction between diabetes and low serum vitamin C levels (all P < 0.001). According to the two measures of additive interaction, there was 0.09 and 0.15 relative excess risk of all-cause and cancer mortality due to the additive interaction, and 6 and 13% of all-cause and cancer mortality in individuals exposed to both risk factors was attributable to the additive interaction.
The least-square means of metabolic biomarkers based on serum vitamin C concentrations are presented in Supplementary Table 2. Among participants with T2D, higher serum vitamin C concentrations were significantly associated with lower serum levels of CRP, GGT, and HbA1c levels (all Ptrend < 0.05). When exploring whether several metabolic biomarkers mediated the associations of serum vitamin C levels with all-cause mortality, we found that CRP, GGT and HbA1c explained 14.08, 8.96 and 5.60% of the association between serum vitamin C and all-cause mortality among individuals with T2D, respectively (Supplementary Table 3).
Consistent results were observed among patients with T2D when analyses were stratified by age, sex, race/ethnicity, BMI, smoking status, diabetes duration, and HbA1c levels, and no significant interactions were detected between serum vitamin C levels and these stratifying variables (all Pinteraction > 0.05) (Supplementary Table 4). In individuals without T2D, consistent results were observed in most subgroups, while inverse associations of serum vitamin C with all-cause mortality seemed to be somewhat stronger in smokers and participants except non-Hispanic white (all Pinteraction < 0.05) (Supplementary Table 4).
In the sensitivity analyses, consistent results were found after excluding participants who died within two years of follow-up in the analysis (Supplementary Table 5). The results did not significantly change when we further adjusted for dietary intakes of total fatty acids, vitamin A, vitamin E, selenium, beta-carotene and fiber, or serum levels of serum vitamin A, vitamin E, beta-carotene and 25-hydroxyvitamin D (Supplementary Table 6). Similar results were observed when analysis was conducted by quartiles of serum vitamin C concentrations among all individuals (Supplementary Table 7).
Discussion
In this large prospective study, we found that higher serum vitamin C levels (ranged from 0.46 to 116.26 μmol/L) were significantly associated with lower all-cause and cancer mortality in a linear dose–response manner among individuals with T2D, which was partly mediated by CRP, GGT and HbA1c. In contrast, among individuals without T2D, there was a nonlinear association between serum vitamin C concentrations and all-cause, cancer, and CVD mortality, with the lowest risk around a serum vitamin C concentration of 48.0 μmol/L.
Vitamin C, a water-soluble vitamin, was suggested to play an important role in the disease process [2], while epidemiological evidence regarding the association between vitamin C and mortality was inconsistent in general populations, with some studies showing inverse or null associations [3, 7, 26, 27]. Furthermore, accumulating studies suggested that there was a threshold effect between serum vitamin C levels and mortality [5, 6, 10, 11]. For example, data from NHANES 2003–2006 revealed a significant U-shaped relationship between serum vitamin C levels and all-cause or CVD mortality, with lowest risk at concentrations of 1.06 mg/dL (60.19 μmol/L) [6]. In addition, Gey reported that plasma vitamin C concentration at 50 μmol/L provided the optimal benefits with regard to CVD and cancer risk [11]. Notably, most aforementioned studies were conducted in general populations.
Previous studies have suggested that patients with diabetes are at high risk of both subnormal plasma vitamin C concentrations and increased oxidative stress, and whether relatively high vitamin C status may prevent against the development of T2D and associated complications remains unclear. To date, only a few small randomized controlled trials (RCTs) have investigated the association of vitamin C supplementation with short-term outcomes in patients with diabetes, with inconclusive findings. Several studies found that vitamin C supplementation could significantly improve glucose metabolism, alleviate inflammatory status, lower blood pressure, and arterial stiffness [15,16,17, 28], while others reported nonsignificant effect [18, 29]. Inconsistent findings among these trials might be due to the small sample size (n = 30–72) and differences in intervention durations (from 4 weeks to 4 months), supplementation dose (from 500 to 1000 mg/day), and characteristics of the study populations (with varying diabetes complications or different treatments before). In addition, less is known about the long-term health effects of vitamin C among people with T2D. In the only one existing cohort study among 2881 postmenopausal women with diabetes, vitamin C intake from supplements of > 300 mg/day was associated with an increased risk of CVD mortality but there was no clear association for vitamin C intake from food [30]. However, this study is subject to some limitations. For example, dietary measurements are prone to measurement error and cannot reflect bioavailability [31, 32]. Furthermore, the study ascertained diabetes based on self-report and mainly focused on postmenopausal women. In addition, the possibility of unknown or residual confounding cannot be ruled out. To our knowledge, no study has examined the dose–response relationship between serum vitamin C concentrations, a much more reliable indicator of body vitamin C status [19], and mortality in individuals with diabetes.
In our study, based on a nationally representative sample of U.S. adults, diabetes patients had a lower level of serum vitamin C compared with non-diabetics, which was consistent with previous studies [13]. While individuals with T2D had lower vitamin C levels, we found the optimal vitamin C requirement may be higher in patients with T2D than in subjects without T2D. Specifically, there was a linear inverse association between serum vitamin C and mortality with baseline serum vitamin C concentration ranging from 0.46 to 116.26 μmol/L in patients with T2D. However, the lowest risk of mortality was around the serum vitamin C level of 48.0 μmol/L in participants without T2D, and further elevation of vitamin C level was not associated with lower mortality risk. Of note, the threshold level was slightly lower from that in previous studies [6]. A possible explanation for the discrepancy is that previous study investigated the total population including diabetic patients. As we observed different patterns in the relationship between vitamin C and mortality among people with and without diabetes, it is important to take into account T2D status in the evaluation of health effects of vitamin C in future studies.
Additionally, we further quantified the additive interactions between diabetes status and vitamin C levels to reflect the biological plausibility of interaction. The results revealed the interactive effect of risk of diabetes and low vitamin C levels was greater than the sum of the two individual effects. Specifically, 6 and 13% of all-cause and cancer mortality risk could be attributed to the additive interactions. These results suggested that patients with T2D may benefit more from maintaining adequate vitamin C status. Furthermore, we found the protective associations of serum vitamin C with all-cause mortality seemed to be somewhat stronger in smokers and other races (i.e., non-Hispanic black, Mexican America and others), which may be partly explained by the subnormal serum vitamin C levels in these participants [33]. In addition, we cannot rule out the possibility of residual confounding by smoking.
Several possible mechanisms may be accounted for the association between vitamin C and mortality among individuals with and without T2D. The benefits of maintaining appropriate levels of vitamin C might be related to its function as a water-soluble antioxidant, which can inhibit lipid peroxidation, prevent protein or amino acid oxidation, as well as diminish DNA damage, closely related to the development of chronic disease [34]. However, it was reported that vitamin C at a higher concentration might serve as a pro-oxidant but not an antioxidant [35, 36]. Nevertheless, patients with diabetes are at higher risk of increased oxidative stress, hyperglycemia and microalbuminuria, all of which may cause lower concentrations of blood vitamin C and higher demand for vitamin C [13]. To provide more evidence regarding the mechanism to support the benefit of vitamin C in patients with T2D, we performed mediation analyses and found that CRP, GGT and HbA1c partly mediated the association between serum vitamin C concentration and all-cause mortality among patients with T2D, suggesting the inverse association between vitamin C and mortality may be partly attributed to its potential capability of reducing inflammatory response, reducing oxidative stress, and regulating glucose metabolism [16, 37]. More mechanistic studies are warranted to further investigate the potential mechanisms underlying the association between vitamin C and mortality risk.
The strengths of our study included using a nationally representative sample, relatively large sample size, as well as the careful adjustment of potential confounding factors. Furthermore, we used mediation analyses to quantify the relative contributions of cardiometabolic biomarkers on the outcomes in order to further explain the association between serum vitamin C and mortality. Several limitations should be taken into account as well. First, we cannot determine causality because of the observational study design. Second, the serum vitamin C concentration was based on a single measurement at baseline and we did not capture the time-varying association of serum vitamin C status with outcomes. However, some RCTs suggested that changes in circulating vitamin C concentrations were incredibly slight after four weeks in the placebo group [18] or after five years in the dropout group without vitamin C supplementation [38]. Individuals who have subnormal vitamin C concentrations at baseline are likely to remain subnormal throughout follow-up. Third, the present study did not provide detailed information on the severity of diabetes, although the results were still significant after further adjustment of diabetes medication use, diabetes duration, and self-reported comorbidities. Finally, unknown and residual confounding could not be entirely excluded.
Conclusions
In this nationally representative cohort study, among participants with T2D, higher serum vitamin C concentration (ranged from 0.46 to 116.26 μmol/L) was linearly associated with lower all-cause and cancer mortality in a dose–response manner. In contrast, a nonlinear association was observed in participants without T2D, with an apparent threshold around a serum vitamin C concentration of 48.0 μmol/L. These findings suggest that the optimal vitamin C requirement may be higher in individuals with T2D than in those without. Further well-designed RCTs are needed to examine the benefit of vitamin C in patients with T2D.
Data availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Code availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval from the corresponding author.
References
Carr AC, Frei B (1999) Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69(6):1086–1107. https://doi.org/10.1093/ajcn/69.6.1086
Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC (2014) Vitamin C revisited. Crit Care 18(4):460. https://doi.org/10.1186/s13054-014-0460-x
Khaw KT, Bingham S, Welch A et al (2001) Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 357(9257):657–663. https://doi.org/10.1016/s0140-6736(00)04128-3
Martín-Calvo N, Martínez-González M (2017) Vitamin C intake is inversely associated with cardiovascular mortality in a cohort of Spanish graduates: the SUN Project. Nutrients. https://doi.org/10.3390/nu9090954
Goyal A, Terry MB, Siegel AB (2013) Serum antioxidant nutrients, vitamin A, and mortality in US adults. Cancer Epidemiol Biomark Prev 22(12):2202–2211. https://doi.org/10.1158/1055-9965.EPI-13-0381
Tian T, Shao J, Shen Z et al (2022) Association of serum vitamin C with all-cause and cause-specific death: Data from National Health and Nutrition Examination Survey (NHANES 2003–2006). Nutrition 101:111696. https://doi.org/10.1016/j.nut.2022.111696
Buijsse B, Feskens EJ, Kwape L et al (2008) Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr 138(2):344–350. https://doi.org/10.1093/jn/138.2.344
Zheng JS, Sharp SJ, Imamura F et al (2020) Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ 370:m2194. https://doi.org/10.1136/bmj.m2194
Aune D, Giovannucci E, Boffetta P et al (2017) Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol 46(3):1029–1056. https://doi.org/10.1093/ije/dyw319
Stepaniak U, Micek A, Grosso G et al (2016) Antioxidant vitamin intake and mortality in three Central and Eastern European urban populations: the HAPIEE study. Eur J Nutr 55(2):547–560. https://doi.org/10.1007/s00394-015-0871-8
Gey KF (1995) Ten-year retrospective on the antioxidant hypothesis of arteriosclerosis: threshold plasma levels of antioxidant micronutrients related to minimum cardiovascular risk. J Nutr Biochem 6(4):206–236
Urakawa H, Katsuki A, Sumida Y et al (2003) Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab 88(10):4673–4676. https://doi.org/10.1210/jc.2003-030202
Wilson R, Willis J, Gearry R et al (2017) Inadequate vitamin C status in prediabetes and type 2 diabetes mellitus: associations with glycaemic control, obesity, and smoking. Nutrients 9(9):997. https://doi.org/10.3390/nu9090997
Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A (2020) Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med Cell Longev 2020:8609213. https://doi.org/10.1155/2020/8609213
Mullan BA, Young IS, Fee H, McCance DR (2002) Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 40(6):804–809. https://doi.org/10.1161/01.hyp.0000039961
Mason SA, Rasmussen B, van Loon LJC et al (2019) Ascorbic acid supplementation improves postprandial glycaemic control and blood pressure in individuals with type 2 diabetes: findings of a randomized cross-over trial. Diabetes Obes Metab 21(3):674–682. https://doi.org/10.1111/dom.13571
Mason SA, Keske MA, Wadley GD (2021) Effects of vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care 44(2):618–630. https://doi.org/10.2337/dc20-1893
Chen H, Karne RJ, Hall G et al (2006) High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol 290(1):H137-145. https://doi.org/10.1152/ajpheart.00768.2005
Dehghan M, Akhtar-Danesh N, McMillan CR, Thabane L (2007) Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J 6:41. https://doi.org/10.1186/1475-2891-6-41
Centers for Disease Control and Prevention (2006) The National Health and Nutritional Examination Survey (NHANES) analytic and reporting guidelines. CDC, Atlanta. https://wwwn.cdc.gov/Nchs/Nhanes/AnalyticGuidelines.aspx. Accessed 30 Oct 2022
Margolis SA, Duewer DL (1996) Measurement of ascorbic acid in human plasma and serum: stability, intralaboratory repeatability, and interlaboratory reproducibility. Clin Chem 42(8 Pt 1):1257–1262
Beddhu S, Baird BC, Zitterkoph J et al (2009) Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 4(12):1901–1906. https://doi.org/10.2215/CJN.01970309
Haytowitz DB, Ahuja JK, Wu X et al (2019) USDA National Nutrient Database for standard reference, legacy release. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA. https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release. Accessed 30 Oct 2022
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
Yung YF, Lamm M, Zhang W (2018) Causal mediation analysis with the CAUSALMED procedure. SAS Institute Inc, USA. https://www.sas.com/content/dam/SAS/support/en/sas-global-forum-proceedings/2018/1991-2018.pdf. Accessed 30 Oct 2022
Agudo A, Cabrera L, Amiano P et al (2007) Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Am J Clin Nutr 85(6):1634–1642. https://doi.org/10.1093/ajcn/85.6.1634
Loria CM, Klag MJ, Caulfield LE, Whelton PK (2000) Vitamin C status and mortality in US adults. Am J Clin Nutr 72(1):139–145. https://doi.org/10.1093/ajcn/72.1.139
Mason SA, Della Gatta PA, Snow RJ et al (2016) Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with type 2 diabetes: findings of a randomized controlled study. Free Radic Biol Med 93:227–238. https://doi.org/10.1016/j.freeradbiomed.2016.01.006
Upritchard JE, Sutherland WH, Mann JI (2000) Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 23(6):733–738. https://doi.org/10.2337/diacare.23.6.733
Lee DH, Folsom AR, Harnack L et al (2004) Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr 80(5):1194–1200. https://doi.org/10.1093/ajcn/80.5.1194
Aune D, Keum N, Giovannucci E et al (2018) Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose–response meta-analysis of prospective studies. Am J Clin Nutr 108(5):1069–1091. https://doi.org/10.1093/ajcn/nqy097
Fletcher AE, Breeze E, Shetty PS (2003) Antioxidant vitamins and mortality in older persons: findings from the nutrition add-on study to the Medical Research Council Trial of assessment and management of older people in the community. Am J Clin Nutr 78(5):999–1010. https://doi.org/10.1093/ajcn/78.5.999
Schleicher RL, Carroll MD, Ford ES, Lacher DA (2009) Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 90(5):1252–1263. https://doi.org/10.3945/ajcn.2008.27016
Padayatty SJ, Katz A, Wang Y et al (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22(1):18–35. https://doi.org/10.1080/07315724.2003
Chen Q, Espey MG, Sun AY et al (2007) Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 104(21):8749–8754. https://doi.org/10.1073/pnas.0702854104
Bouayed J, Bohn T (2010) Exogenous antioxidants–double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 3(4):228–237. https://doi.org/10.4161/oxim.3.4.12858
Kanagasabai T, Riddell MC, Ardern CI (2022) Inflammation, oxidative stress, and antioxidant micronutrients as mediators of the relationship between sleep, insulin sensitivity, and glycosylated hemoglobin. Front Public Health 10:888331. https://doi.org/10.3389/fpubh.2022.888331
Kim MK, Sasazuki S, Sasaki S et al (2003) Effect of five-year supplementation of vitamin C on serum vitamin C concentration and consumption of vegetables and fruits in middle-aged Japanese: a randomized controlled trial. J Am Coll Nutr 22(3):208–216. https://doi.org/10.1080/07315724.2003.10719295
Funding
Gang Liu was funded by Grants from National Nature Science Foundation of China (82073554 and 82273623), the Hubei Province Science Fund for Distinguished Young Scholars (2021CFA048), and the Fundamental Research Funds for the Central Universities (2021GCRC076). An Pan was supported by Grants from National Nature Science Foundation of China (81930124 and 82021005), and the Fundamental Research Funds for the Central Universities (2021GCRC075). The funders had no role in the study design, data acquisition, analysis, or interpretation of results.
Author information
Authors and Affiliations
Contributions
GL designed the study. ZQ performed statistical analysis. YO drafted the manuscript and checked the accuracy of statistical analysis. All of the authors participated in the interpretation of the results and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ou, Y., Qiu, Z., Geng, T. et al. Associations of serum vitamin C concentrations with risk of all-cause and cause-specific mortality among individuals with and without type 2 diabetes. Eur J Nutr 62, 2555–2565 (2023). https://doi.org/10.1007/s00394-023-03173-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03173-1