Abstract
Purpose
Pu-erh tea can be classified into raw pu-erh tea and ripened pu-erh tea. Theabrownin (TB) is one of the major components of pu-erh tea. The difference of the anti-obesity activity between raw pu-erh tea TB (R-TB) and ripened pu-erh tea TB (F-TB) has not been comprehensively investigated yet. Therefore, this article aimed to systemically study the anti-obesity activity and the underlying mechanism of R-TB and F-TB.
Method
High-fat diet (HFD)-induced C57BL/6J mice with obesity were gavaged with R-TB or F-TB to assess the effect of R-TB and F-TB on the amelioration of obesity, the expression of lipid metabolism-related genes, and the regulation of gut flora imbalance.
Results
Administration of both R-TB and F-TB could suppress body weight gain, improve insulin sensitivity and glucose homeostasis, regulate the lipid level and reduce the chronic inflammation in obese mice. The underlying anti-obesity mechanism of R-TB and F-TB might involve the regulation of lipogenesis and lipolysis, amelioration of the gut microbiota disorder and promotion of microbial metabolism. Interestingly, R-TB was more efficient in the regulation of blood glucose, reduction of inflammation and suppression of partial adipogenesis-related genes and protein, while F-TB was more effective in the inhibition of lipolysis-related genes and protein. In addition, F-TB might be more effective in adjusting the dysbacteria caused by HFD back to normal by promoting the proliferation of the beneficial microbiota, such as Lactobacillus and Lachnospiraceae_NK4A136_group.
Conclusion
Taken together, both R-TB and F-TB had the potential to be developed as beneficial dietary supplements or functional foods for ameliorating obesity and obesity-related metabolic disorders, but their effects and the ability to regulate the intestinal flora varied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, a disorder of nutrient metabolism in the body, has been one of the common diseases in the twenty-first century. Excessive energy storage in the body caused by energy intake and metabolic imbalance leads to body weight gain. Over the past few decades, the development of obesity has been increased worldwide, with the survey showing that globally between 1980 and 2013, the prevalence of overweight increased by 28% in adults and 47% in children [1]. According to the report of world Health Organization (WHO), in 2014, there were 600 million people with obesity in the world, which has become an important challenge to the physical and mental health of people all over the world [2]. Obesity increases the risk of metabolic diseases [3], such as type 2 diabetes, cardiovascular diseases [4], and some types of cancer [5], which are difficult to be effectively cured, thus leading to a decline in people's quality of life and life span. Therefore, it is urgent to find effective strategies to prevent the development of obesity worldwide. Pu-erh tea is a famous traditional dark tea in Yunnan Province. With its excellent flavor, high nutritional value and unique health-care effect, pu-erh tea has become one of the most widely consumed beverages in the world and is deeply loved by the majority of consumers. A large number of studies have shown that pu-erh tea has a variety of biological activities, such as lowering blood lipids [6], anti-obesity [7], antibacterial, digestion [8] and detoxification [9]. According to the processing technology of pu-erh tea, it can be divided into two types: raw pu-erh tea and ripened pu-erh tea. The former is made of autoclaved and compressed sun-dried green tea and stored for “aging” for several years at room temperature, whereas the latter is “ripened” for several months which is the process of stacking fermentation using specific microbes under high humidity and temperature conditions. There are many active components in pu-erh tea, such as tea polysaccharide (TPS), tea polyphenol (TP) and theabrownin (TB). It has been reported that both TPS and TP have a good effect in ameliorating obesity [10,11,12,13,14]. However, so far, there are few studies on the anti-obesity activity of TB. Belonging to polyphenols, TB, is a general term for a class of products with very complex structures formed by the oxidative polymerization of catechins [15]. There is a significant positive correlation between the content of TB and the quality of pu-erh tea, and the content of TB is gradually accumulated with the prolongation of stacking time during processing [16]. In recent years, studies have shown that TB has antioxidant, antitumor, lipid-reducing and weight-reducing effects [11, 17]. Chen et al. verified the effect of TB on lipid reduction and weight loss through animal experiment. The results showed that TB of pu-erh tea could significantly reduce the levels of total cholesterol (TC), triglyceride (TG) and low-density lipoprotein-cholesterol (LDL-C) in high-fat rats, improve the level of high-density lipoprotein-cholesterol (HDL-C), reduce liver deposition and prevent the formation of fatty liver, thus exerting significant anti-obesity effect [18]. Huang et al. proved that TB in pu-erh tea could change the gut microbiota of mice and inhibit the gut FXR-FGF15 signaling pathway, which thereby increased the production of bile acids, reducing liver cholesterol and reducing fat production, suggesting that TB in pu-erh tea may be a potential anti-hypercholesterolemia and anti-hyperlipidemia drug [11]. The above-mentioned researches have proved that TB possesses the potential of lipid lowering; however, systematic studies on the anti-obesity activity of TB and the underlying mechanism have rarely been reported. These studies generally focus on the bioactivities of TB in ripened pu-erh tea, while little attention has been paid to TB in raw pu-er tea. The difference in TB activity between the two has not been reported, and there is no report on the difference in the activity between R-TB and F-TB.
Our previous study also verified that raw pu-erh tea and ripened pu-erh tea displayed similar anti-obesogenic effect in HFD-induced obesity mice and both of them could regulate the HFD-induced gut microbiota dysbiosis. Intriguingly, the treatment of ripened pu-erh tea extract had better effect on the increase of beneficial microbes and decrease of obesity-associated taxa. To explore the compounds contributing to the different regulatory effects on the compositions and abundance of gut microbiota in raw and ripened pu-erh tea, as part of a series of studies, we conducted a study of R-TB and F-TB on the HFD-induced obese mice and the underlying mechanism and the effect on the regulation of intestinal microbiota imbalance. Our study systematically demonstrated that both R-TB and F-TB could potentially serve as dietary supplements or functional foods for the treatment of obesity by regulating the dysbiosis of gut microbiota.
Materials and methods
Materials and chemicals
Raw pu-erh tea and ripened pu-erh tea were obtained from Tianxing Pu-erh Tea Co., Ltd, Pu er, China, in 2020. The diets including normal diet (10% fat diet, TP23302) and high-fat diet (60% fat diet, TP23300) were purchased from Trophic Animal Feed High-Tech Co. Ltd (Nantong, China). Anhydrous ethyl alcohol, ethyl acetate, chloroform and n-butyl alcohol of analytical grade were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All the other reagents used in this study were commercially available and of the needed grade.
Extraction and purification of F-TB and R-TB
100 g of raw pu-erh tea or ripened pu-erh tea powder was soaked in 1000 mL distilled water at 80 °C for 2 h. The mixture was concentrated by a rotary evaporator, and anhydrous ethyl alcohol was added and precipitated for 1 h. Then the anhydrous ethanol was removed by vacuum filtration and the precipitate was collected and dissolved in distilled water. Triploid ethyl acetate was then added to the solution to remove the tea polyphenols, followed by removing the proteins in the water layer using the Sevag method. Finally, the deproteinized water layer was mixed with triploid volume of anhydrous ethanol, and the precipitate was collected and freeze-dried to obtain R-TB and F-TB with a yield of 2.11% and 11.92%, respectively.
Animal experiments
All the animal procedures used in the present study followed the guidelines for the Care and Use of Laboratory Animals of Huaqiao University and the protocol was approved by the Committee of Experimental Animals of Huaqiao University (HQSY20200608). Fifty 6-week-old C57BL/6J male mice with average weight of 16 ± 0.85 g were purchased from Shanghai Slack Laboratory Animal Company (License No. SCXK (Shanghai) 2017-0005). The mice were housed in a specific semiconductor free (SPF) laboratory and kept at 22 ± 1 °C and 50 ± 5% humidity, with a 12 h light/dark cycle. After 1 week of adaptation, the mice were randomly divided into five groups with ten mice in each group: (1) normal chow diet control group fed with 10% fat diet and 0.2 mL normal saline orally every day (ND); (2) high-fat diet control group with 60% high-fat diet and 0.2 mL normal saline gavage daily (HFD); (3) positive control group treated with 60% high-fat diet and 60 mg/kg bw orlistat daily by gavage (AL); (4) raw pu-erh tea theabrownin group with 60% high-fat diet and 400 mg/kg bw daily raw pu-erh tea theabrownin (R-TB); (5) ripened pu-erh tea theabrownin group fed with 60% high-fat diet and intragastric administration of 400 mg/kg bw daily ripened pu-erh tea theabrownin (F-TB). During the 24 weeks long experiment, the corresponding components were given to each group at a fixed time every day, the food intake of mice in each group was recorded every day, and the weight of the mice was recorded every week.
At the end of the experiment, the blood samples were collected from the 12 h fasted mice by eyeball extirpating. Serum was obtained by centrifugation at 4 °C, 3000 rpm for 15 min and stored at − 80 °C until analysis. Tissues including liver and adipose tissue were harvested, rinsed, weighed and frozen in liquid nitrogen and kept at − 80 °C for further use. In addition, a portion of the liver, epididymal adipose tissue (eAT) and perirenal adipose tissue (pAT) and colon were fixed in 10% formalin and embedded in paraffin for histological studies. The cecum contents were collected and immediately stored at − 80 °C.
Histological analysis
The saline-washed liver and adipose tissue samples were fixed in 4% paraformaldehyde and embedded in paraffin. Then, the embedded tissues were sectioned into 3–6 μm and stained with hematoxylin and eosin for histological analysis. The histological appearance was observed by light microscope (Carl Zeiss AG, Jena, Germany).
Glucose tolerance tests
All the overnight-fasted mice had oral gavage with D-glucose (2 g/kg bw) and the blood glucose concentration in the tail vein was measured using an Accu-Chek glucometer (Abbott, USA) at 0, 15, 30, 60, 90, and 120 min. The area under the curve (AUC) for oral glucose tolerance test (OGTT) was calculated. Meanwhile, the fasting insulin levels of the mice were tested. Then, the homeostasis model assessment-insulin resistance (HOMA-IR) was calculated by the following formula: HOMA-IR = fasting blood glucose (mmol/L) × fasting insulin (mU/L)/22.5.
Serum biochemical analysis
The serum contents of TC, TG, HDL-C and LDL-C were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the manufacturer’s instructions. The serum contents of insulin, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) were detected by mouse ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Quantitative RT-PCR
Total RNA of the liver and adipose tissues was extracted using a column total tissue RNA extraction kit (Sangong Bioengineering (Shanghai) Co., LTD). Then, the total RNA was converted to cDNA by reverse transcription. Real-time quantitative PCR analysis was performed on the Roche PCR system using the Applied Biosystems Master Mix kit (New England Biolabs, Inc.). GAPDH was employed as an internal standard. Relative gene expression was determined by the 2−ΔΔCt method. The target genes and their primer sequences are shown in Table S1.
Western blot assay
60 mg liver tissue sample was homogenized and lysed in radio immunoprecipitation assay (RIPA) buffer (Sigma, St. Louis, MO, USA), then centrifuged to obtain the supernatant. Total protein content in the supernatant was determined by a BCA protein determination kit (Pierce, Rockford, IL, USA). Equal amount of protein (20 μg/lane) was separated by SDS-PAGE and blotted onto a polyvinylidene difluoride (PVDF) membrane. The membranes were sealed with 5% (w/v) skimmed milk in TBST. Then, the membranes were incubated with primary antibodies overnight at 4 °C. After washing with TBST five times, the membranes were incubated in horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Finally, the proteins were measured using enhanced chemiluminescence (ECL) and auto radiographed. GAPDH was used as internal control. Antibodies in the experiment were purchased from Proteintech Group, Inc. (Wuhan, Hubei, China), including the PPAR-α antibody, PPAR-γ antibody, SREBP-1c antibody and GAPDH antibody.
Gut microbiota analysis
Microbial DNA was extracted from feces samples of mice in each group using a DNA extraction kit, followed by the amplification and sequencing of the variable (V4) region in the 16S rRNA with PCR primer pair of 515F-907R with barcode (515F: 5′-GTGCCAGCMCCCGCGG-3′, 907R: 5′-CCGTCAATTCMTTTRAGTTT-3′). The PCR products were then purified and quantified using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and QuantiFluor® ST (Promega, Madison, WI, USA) according to the manufacturer’s instruction, respectively. The purified amplicons were then pooled at equimolar concentrations and paired-end sequenced on an Illumina MiSeq platform (Guangzhou Genedenovo Biotechnology Co., Ltd). Cutadapt software was used to screen out low-quality reads, and Uparse software was used to cluster the obtained effective data into operational taxonomic unit (OTU) with 97% similarity. QIIME software (Version1.9.1) was used to calculate the alpha diversity index and beta diversity, and R software (Version 2.15.3) was used to analyze the differences between groups by parametric and non-parametric tests.
Statistical analysis
All data are presented as the mean ± standard error of the mean (\(\overline{x}\) ± SEM), and all statistical analyses were performed using one-way analysis of variance by GraphPad Prism (version 9.0, San Diego, CA, USA). Results were considered to be statistically significant at p < 0.05.
Results
Effects of R-TB and F-TB on HFD-induced obesity
To study the influence of TB on the body weight of HFD-induced obese mice, in this study, their food intake was recorded every day and their body weight was recorded weekly for a total of 24 weeks. The weight changes and diet results are shown in Fig. 1a–c. As can be seen from Fig. 1a, ND mice fed a normal diet grew rapidly from the 1st week to the 10th week, and the weight went up slowly at the later part of the experiment. However, the weight of HFD group mice kept rising during the experiment and reached 42.54 ± 1.21 g at the 24th week, which was much higher than that of the ND group (p < 0.05). The total body weight gain of the AL group was significantly lower than that of the HFD group. Similar to the AL group, R-TB and F-TB intervention prevented the rapid increase of body weight caused by an HFD diet, and the body weight of mice in the R-TB and F-TB groups was 31.06 ± 0.55 and 32.82 ± 0.42 g, respectively, markedly lower than that of the HFD group.
As shown in Fig. 1b, c, after 24 weeks of feeding, the HFD group had significantly higher body weight gain than the other four groups (p < 0.05), while the R-TB and F-TB groups had even less body weight gain compared with the ND and AL groups, although there was no significance between them, suggesting that TB could reduce the HFD-induced high body weight gain to normal level. The liver index, eAT index and pAT index are shown in Fig. 1d–f. Compared with the ND group, the liver, pAT and eAT indexes in the HFD group were significantly increased (P < 0.05). After 24 weeks of TB treatment, the three indexes were significantly lower than those of the HFD group, and there was no significant difference between the TB groups and the ND group.
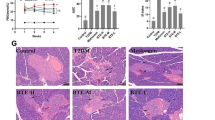
H&E staining was performed to examine the histopathological morphologies of the liver, pAT and eAT in each group. As can be seen from Fig. 2a, compared with the ND group, 24 weeks of HFD feeding led to lipid accumulation in hepatocytes and ballooning degeneration in the HFD group, while the R-TB, F-TB and orlistat treatment remarkably reduced the hepatic lipid droplets and improved lipid deposition. In the eAT of the HFD group mice, the average size of adipocytes increased nearly 200% (Fig. 2b, c) compared with that of mice in the ND group. The results indicated that R-TB and F-TB could inhibit the lipid accumulation in liver and fat tissues to a certain extent and ameliorate adipocytes hypertrophy to alleviate the lipid increases in the body.
Gut morphology is an important indicator reflecting the integrity of gut mucosa. The intestinal morphology of mice was investigated to explore whether R-TB and F-TB intervention could protect the gut of mice and the results are shown in Fig. 2d. Compared with the ND group, the intestinal integrity of mice in the HFD group was severely damaged and the intestinal villi were sparse and short due to long-term HFD feeding. Expectedly, the intestinal villi in the R-TB and F-TB groups were dense and high and the submucosa turned thicker, which was similar to that of the ND group. These results suggest that R-TB and F-TB administration has a protective effect on the intestinal integrity in mice fed HFD.
R-TB and F-TB reduced insulin resistance and improved glucose tolerance in HFD-fed mice
The OGTT results are shown in Fig. 3a, b. The HFD group had a significantly higher glucose level than the other groups at each detection time spot except the 0 min. AUC of glucose tolerance was significantly higher than that in the ND group; however, F-TB and R-TB treatment remarkably decreased the AUC. The AUC of F-TB and R-TB groups tended to be close to the AUC of ND group, showing that F-TB and R-TB could improve glucose tolerance. Notably, the AUC of the R-TB group was lower than that of F-TB group, suggesting that R-TB had better blood glucose regulation ability. The serum insulin level of mice in the HFD group was significantly higher than that in ND group (P < 0.05) (Fig. 3c), while the level in the F-TB and R-TB groups was profoundly lower than that in the HFD group (P < 0.05), indicating that TB administration could effectively improve the serum insulin level of HFD feeding mice and F-TB showed more efficiency than R-TB. Figure 3d indicates that HFD mice had significant insulin resistance, and TB could effectively improve the glycemic regulation ability of HFD diet mice. The above-mentioned results indicated that TB could significantly improve the glucose tolerance of mice fed HFD for 24 weeks, facilitate glucose metabolism, improve insulin sensitivity, reduce insulin resistance and play an effective role in regulating blood glucose level. On comparing F-TB with R-TB, F-TB had better blood glucose regulation ability.
Effects of R-TB and F-TB on serum lipid profile and inflammatory cytokines in obese mice
To systematically and comprehensively evaluate the effects of TB on the lipid metabolism of mice fed HFD, the contents of TC, TG, HDL-C, and LDL-C in the serum of mice in different treatment groups were detected in the experiment, and the results are shown in Fig. 4a. The contents of TC, TG and LDL-C in the serum of mice in the F-TB and R-TB groups were markedly decreased compared with the HFD group (P < 0.05), while the content of HDL-C was significantly increased (P < 0.05), indicating that long-term administration of both F-TB and R-TB could effectively regulate the dyslipidemia induced by HFD.
Effects of F-TB and R-TB on serum lipid profile and inflammatory cytokines in obese mice. a Serum contents of TC, TG, HDL-C, and LDL-C; b serum content of TNF-α; c serum content of IL-6 in serum; d serum content of IL-1β in serum. Different lowercase letters are significantly different at the level of p < 0.05
To study the effects of TB on systemic inflammation in HFD feeding mice, the contents of serum pro-inflammatory factors in each group of mice were detected. As shown in Fig. 4b–d, the contents of IL-1β, TNF-α, and IL-6 were significantly increased in the HFD group compared with the ND group. Notably, after periodic and quantitative administration of both F-TB and R-TB, the contents of the three inflammatory factors were reduced to nearly the same levels as those in the ND group, indicating that TB intervention significantly ameliorated the inflammatory state of the circulatory system and F-TB and R-TB showed similar effect on the production of the inflammatory factors.
Effects of R-TB and F-TB on the expression of genes and proteins related to lipid metabolism
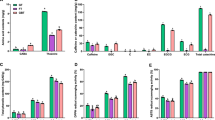
The mRNA expression levels of grape transporter 4 (Glut4) and insulin receptor 1 (IRS1) in the liver of each group were detected to further explore the mechanism of TB on the glucose metabolism. As shown in Fig. 5a, compared with the ND group, the mRNA levels of Glut4 and IRS1 in the HFD group were significantly down-regulated (P < 0.05), while the mRNA expression of Glut4 and IRS1 was prominently up-regulated in the F-TB and R-TB groups compared with the HFD group (P < 0.05), indicating that TB can affect glucose metabolism by regulating the expression of genes related to glucose metabolism.
Effects of F-TB and R-TB on the expression of genes and proteins related to lipid metabolism. a The mRNA expression levels of Glut4 and IRS1; b the mRNA levels of FAS, LXR-α, SREBP-1c and PPAR-α, PPAR-γ; c the mRNA expressions of IL-6, TNF-α and IL-1β; d protein expression of PPAR-α, PPAR-γ and SREBP-1c in mice and grayscale analysis of protein expression. Different lowercase letters are significantly different at the level of p < 0.05
In addition, the mRNA expression levels of FAS, LXR-α, SREBP-1c, PPAR-α and PPAR-γ in liver tissue were detected. As seen in Fig. 5b, the mRNA expression levels of lipid synthesis-related genes including FAS, LXR, SREBP-1c, and PPAR-γ in the HFD group were significantly increased, while the expression of lipid metabolism-related gene PPAR-α was dramatically decreased compared with the ND group. However, both F-TB and R-TB administrations could reverse the expression of the above-mentioned genes, indicating that both F-TB and R-TB could promote lipid metabolism to reduce body fat content. Interestingly, F-TB was more effective in reducing the expression of LXRα and FAS and in increasing the level of PPAR-α, while R-TB was more efficient in decreasing the expression of SREBP-1c and PPAR-γ. The effect of TB on the protein expression of lipid metabolism-related signaling pathways including PPAR-α, PPAR-γ and SREBP-1c in mice was also examined. The results are shown in Fig. 5d; compared with the ND group, the protein expression levels of PPAR-α and PPAR-γ in the HFD group were significantly decreased to 64.52% and 33.64% (P < 0.05), respectively. Although the protein expression of PPAR-α in the TB groups displayed no difference with that in the HFD group, the expression of PPAR-γ was increased by 1.32 times in the F-TB group and 1.61 times in the R-TB group compared with the HFD group, respectively. SREBP-1c is mainly expressed in the liver and adipocytes and plays an important role in regulating lipid synthesis in liver tissue cells. Compared with the ND group, liver SREBP-1c expression was prominently increased in the HFD group due to long-term HFD feeding. While both F-TB and R-TB supplementary showed remarkable decrease in the expression of SREBP-1c, which restored to normal level in F-TB and R-TB groups. These results suggest that R-TB and F-TB could reduce liver fat deposition by inhibiting fat synthesis and promoting fat metabolism in mice.
The mRNA expressions of inflammatory factors including IL-1β, IL-6 and TNF-α in liver tissues of mice in different treatment groups were also detected. As shown in Fig. 5c, compared with the ND group, the mRNA expression levels of IL-1β, IL-6, and TNF-α in liver tissues of the HFD group were significantly up-regulated. R-TB and F-TB administration dramatically decreased the expression of these three pro-inflammatory factors (P < 0.05), suggesting that R-TB and F-TB may exhibit anti-inflammatory effect by inhibiting the expression of inflammatory factor genes, which was in accord with previous results of reducing the serum content of pro-inflammatory factors. Noticeably, the expression of IL-1β, IL-6 and TNF-α in the R-TB group was much lower than that in the F-TB group (P < 0.05).
Effects of R-TB and F-TB on gut microbiota in obese mice
The dilution curve can directly reflect the rationality of sequencing data and indirectly reflect the species abundance in samples. As shown in Fig. 6a, the dilution curve gradually flattened out when the sequencing reached 60,000 reads, indicating that the high-throughput sequencing data is sufficient to cover almost all microbial species in the samples. Then, the richness and evenness of intestinal microorganisms in different groups were evaluated through the α diversity index, mainly including Sob, Chao1, Shannon and Simpson. The results shown in Fig. 6c–f indicate that the F-TB and R-TB supplement could significantly enhance the ACE, Chao1, and Shannon index, while reducing the Simpson index (P < 0.05), indicating that the number of gut microbiota species in obese mice was increased after TB intervention. Combining the results of α diversity indexes, it can be found that TB administration can improve the diversity and richness of gut microbiota in mice fed HFD.
Principal coordinate analysis (PCoA) is a dimension reduction analysis based on the distance matrix. In PCoA, if the samples are closer to each other, they would gather together in the figure, suggesting that the species composition and structure of these samples are similar; otherwise, the communities are different. As shown in Fig. 6b, the ND, AL, F-TB, and R-TB groups were clustered together and significantly separated from the HFD group, indicating that the microbiota of the HFD group was significantly different from that of the other four groups. The result suggested that TB intervention could restore the gut microbiota composition and structure of the HFD-induced obese mice.
To explore the differences in species composition of samples from different treatment groups, the present study mainly analyzed the distribution of species with higher abundance in the taxonomy of phylum, family and genus. Figure 7a shows the distribution of intestinal microbes at the phylum level. Firmicutes (Fig. 7b), Bacteroidetes (Fig. 7c) and Actinobacteria (Fig. 7d) are the dominant species at the phylum level in all the groups, accounting for more than 90% of the gut microbiota. Interestingly, HFD feeding significantly decreased the relative abundance of Firmicutes, Bacteroidetes and Verrucomicrobia (Fig. 7e) from 53.20 ± 19.45%, 34.11 ± 17.39% and 7.08 ± 10.49% to 50.34 ± 15.03%, 7.03 ± 5.61% and 1.67 ± 1.57%, respectively, but prominently increased the proportion of Actinobacteria and Proteobacteria (Fig. 7f) from 3.21 ± 0.75% and 0.82 ± 0.47% to 34.88 ± 17.08% and 4.71 ± 3.47%, respectively. After F-TB consumption, the levels of Firmicutes and Bacteroidetes were conspicuously enhanced to 61.35 ± 12.51% and 32.98 ± 11.76%, respectively, while F-TB consumption conspicuously enhanced the levels of Firmicutes and Bacteroidetes to 70.66 ± 12.90% and 21.57 ± 15.00%, respectively. Meanwhile, the abundance of Actinobacteria and Proteobacteria was dropped to 1.05 ± 0.42% and 0.68 ± 0.20% in the F-TB group, and 3.56 ± 2.85% and 0.75 ± 0.28% in the R-TB group, respectively. The Firmicutes/Bacteroidetes (F/B) ratio of the HFD group was significantly higher than that of the ND group (P < 0.01) (Fig. 7g); however, F-TB and R-TB supplements obviously reversed the F/B ratio in the HFD-induced obese mice (P < 0.05). Notably, the F/B ratio of the F-TB group was significantly lower than that of the R-TB group (P < 0.01) and much closer to that of the ND group, indicating that F-TB could be more effective in adjusting the dysbacteria back into normal.
Gut microbiota species distribution and differences among groups. a Gut microbiota distribution at the phylum level; b Firmicutes; c Bacteroidetes; d Actinobacteria; e Verrucomicrobia; f Proteobacteria; g ratio of Firmicutes to Bacteroidetes; h gut microbiota distribution at family level; i Lactobacillaceae; j Muribaculaceae; k Akkermansiaceae; l Atopobiaceae; m Erysipelotrichaceae, n Ruminococcaceae, o Desulfovibrionaceae; p Lachnospiraceae; q gut microbiota distribution at genus level; content of r Lactobacillu; s Coriobacteriaceae_UCG-002. Different lowercase letters are significantly different at the level of p < 0.05
The distribution of the top ten species in each group at the family level is shown in Fig. 7h. As can be seen, there is a remarkable difference in the composition and abundance of microbiota between the HFD group and ND group. The abundance of Lactobacillaceae (Fig. 7i), Muribaculaceae (Fig. 7j) and Akkermansiaceae (Fig. 7k) was remarkably reduced to 14.77 ± 11.04%, 4.50 ± 7.23%, and 1.67 ± 3.01% of that in the ND group, while the abundance of Atopobiaceae, Erysipelotrichaceae, Ruminococcaceae and Desulfovibrionaceae was 20.64-, 8.93-, 2.03- and 7.97-fold higher than that in the ND group (Fig. 7l–o). Expectedly, F-TB treatment markedly enhanced the proportion of Lactobacillaceae, Muribaculaceae and Lachnospiraceae (Fig. 7p) to 36.54 ± 14.75%, 24.57 ± 8.38%, and 18.75 ± 12.33%, respectively. After R-TB was administered, those taxa were enhanced to 43.19 ± 26.21%, 17.57 ± 11.59% and 20.25 ± 18.77%, respectively. In addition, the abundance of Erysipelotrichaceae was observably reduced in both the F-TB and R-TB group.
At the genus level shown in Fig. 7q, the distribution of species in the HFD group was significantly different from that in the other four groups. Lactobacillus (Fig. 7r) and Coriobacteriaceae_UCG-002(Fig. 7s) were the predominant genera, accounting for 40% of the total microorganism at the genus level. Compared with the ND group, the abundance of Lactobacillus was significantly decreased from 39.61 ± 20.72 to 14.77 ± 5.27%, while Coriobacteriaceae_UCG-002 was dramatically increased from 1.44 ± 0.77 to 29.79 ± 16.16%. After F-TB and R-TB administration, the proportion of Lactobacillus was enhanced to 36.54 ± 14.77% and 43.19 ± 26.21%, and the Lachnospiraceae_NK4A136_group was increased to 8.34 ± 5.98% and 5.64 ± 4.99%, while Coriobacteriaceae_UCG-002 was notably decreased to 0.23 ± 0.15% and 0.32 ± 0.26%, respectively.
Functional prediction analysis of the gut microbiota
To investigate the potential function of gut microbiota, PICRUSt was employed to generate the functional profiles of the samples based on the KEGG database. As shown in Fig. 8, metabolic profiles of gut microbiota in HFD-induced obese mice were markedly altered by R-TB and F-TB administration compared with the ND group. At the L2 level, as compared to the HFD group, F-TB and R-TB intervention both evidently up-regulated glycan biosynthesis and metabolism and cell motility, but down-regulated amino acid metabolism, biosynthesis of other secondary metabolites, energy metabolism and metabolism of terpenoids and polyketides. Notably, F-TB and R-TB treatment showed obvious difference in the metabolism of other amino acids, nucleotide metabolism, lipid metabolism, xenobiotics biodegradation and metabolism, and infectious diseases, which were reduced in F-TB group while enhanced in R-TB group. At the L3 level, multiple pathways including biosynthesis of ansamycins, valine leucine and isoleucine biosynthesis, pantothenate and CoA biosynthesis, lysine biosynthesis, pentose phosphate pathway and fatty acid biosynthesis were lowered; conversely, secondary bile acid biosynthesis was significantly enriched in both F-TB and R-TB. Overall, the level of the metabolic profiles of gut microbiota in the F-TB group was closer to the ND group than the R-TB group.
Gut microbiota function analysis at the L2 level and L3 level. Welch’s t test: the two groups were compared to determine whether there was a significant difference in the mean value of function abundance. a Comparison of L2 level functions between HFD and ND; b comparison of L2 level functions between HFD and F-TB; c comparison of the L2 level functions between HFD and R-TB; d gut microbiota function analysis at the L3 level
Specific bacteria induced by R-TB and F-TB intervention in HFD-induced obese mice
To further identify the specific bacterial taxa responding to the different treatments, LefSe assay was performed to compare the microbial community among the five groups. As shown in Fig. 9b, genera Ruminococcus_1, ASF356, Lachnospiraceae_UCG_001, Eubacterium_ventriosum_group, Ruminiclostridium_6, Rikenella, Anaeroplasma and Odoribacter displayed significant higher LDA scores in the ND group, suggesting that these were the enhanced genera in ND group, while genera Coriobacteriaceae_UCG_002, Faecalibaculum, Candidatus_Soleaferrea, Ruminococcaceae_UCG_004, Curvibacter, Gordonibacter and Allobaculum were considerably enriched in HFD group, indicating long-term HFD feeding changed the intestinal flora composition in mice. As shown in the result of the gut microbiota composition between the HFD and R-TB groups (Fig. 9c), similar to the ND group, the abundance of Ruminiclostridium_6, Anaeroplasma, and Odoribacter was markedly enhanced in the R-TB group, while F-TB treatment significantly enhanced the levels of genera Ruminococcus_1, ASF356, Rikenella, Ruminiclostridium_6, Anaeroplasma and Odoribacter, which was closer to the ND group (Fig. 9a). Therefore, these bacteria were suggested to serve as the biomarkers for the amelioration of R-TB and F-TB in the HFD-induced obesity mice.
Correlation between gut microbiota and obesity-related parameters in HFD-induced obese mice
To further reveal the correlation between gut microbiota and some key HFD-induced obesity-related parameters, Spearman correlation analysis was performed in the present study. The top 30 OUTs in abundance that responded to different dietary treatments were identified and are shown in Fig. 10. It can be seen that more than 80% of those top 30 taxa significantly affected by the different interventions strongly correlated with at least one obesity parameter. Genera Coriobacteriaceae_UCG-002 and Bifidobacterium belonging to phylum Actinobacteria, species Lactobacillus_reuteri belonging to genus Lactobacillus, genus Allobaculum, and family Muribaculaceae belonging to phylum Firmicutes and family Desulfovibrionaceae belonging to phylum Bacteroidia were positively correlated to body weight, liver weight, eAT, pAT, TC, LDL-c, fast insulin, blood glucose, hepatic SREBP-1c, FAS, LXR, TNF-α, IL-6 and IL-1β mRNA expression and negatively correlated with the HDL-c, glut4, Ins1, PPAR-α and PPAR-γ mRNA expression. Thus, by reducing the abundance of these bacteria, R-TB and F-TB intervention might exhibit effective effects on ameliorating obesity, promoting lipolysis, suppressing lipogenesis and inhibiting inflammation.
In contrast, a variety of microorganisms including OUT 000001 belonging to species Lactobacillus_murinus assigned to genus Lactobacillus, OUT 000009, 000006, 000015, 000011 and 000020 belonging to family Muribaculaceae, OUT 000008 belonging to genus Prevotellaceae_UCG-001 and OUT 000014 belonging to genus Muribaculum were strongly negatively correlated with body weight, liver weight, eAT, pAT, TC, fast insulin, blood glucose, hepatic SREBP-1c, FAS, LXR, TNF-α, IL-6 and IL-1β mRNA expression and positively correlated with glut4, Ins1, PPAR-α and PPAR-γ mRNA expression. Thus, the enhanced abundance of these bacteria caused by R-TB and F-TB consumption might contribute to the effective improvement of obesity, restriction of lipogenesis and alleviation of inflammation.
Discussion
Nowadays, obesity has become prevalent all over the world with the population of overweight and obese increasing every year. Various strategies have been developed to treat obesity and the associated complications including diet controls, exercises, surgeries and medicines. Since these approaches have their drawbacks, new treatment strategies are constantly being developed. With the deepening of understanding of the relationship between gut microbiota and obesity and the regulation of daily diet in the composition and abundance of gut microbiota, gut microbiota has emerged as a potentially important target for obesity treatment. There is a critical practical value to search foods and their chemical components with anti-obesity effect. Our previous study proved that both raw pu-erh tea and ripened pu-erh tea possess anti-obesity properties, but the underlying compositions in these two types of pu-erh tea contributing to the anti-obesogenic effect are still obscure. TB is one of the richest and most active pigments in pu-erh tea, and TB in ripened pu-erh tea has been proved to have cholesterol-lowering and anti-obesity properties. Yet so far, little attention has been paid to R-TB and the difference between R-TB and F-TB on the anti-obesity activity has not been studied. Our present study was conducted to evaluate the anti-obesogenic effect of R-TB and F-TB and revealed that both R-TB and F-TB could alleviate HFD-induced obesity via promotion of lipid metabolism, alleviation of inflammation and regulation of gut microbiota. R-TB was more efficient in the regulation of blood glucose, reduction of inflammation and suppression of partial adipogenesis-related genes and protein, while F-TB was more effective in the inhibition of lipolysis-related genes and protein.
Glut4 and IRS1 are important proteins in glucose metabolism. Glut4 works after being stimulated by insulin, and the utilization of exogenous glucose is mainly through Glut4, which carries glucose to the skeletal muscle cells for utilization. Glut4 plays an important role in maintaining glucose homeostasis [19]. IRS1, combined with insulin, stimulates a series of responses that lower blood glucose. In this study, both R-TB and F-TB could up-regulate the expression of Glut4 and IRS1, indicating that the insulin sensitivity of the obese mice was improved after TB feeding and the blood glucose level of mice was effectively regulated.
Increasing researches have indicated that excess lipid synthesis and accumulation would lead to metabolism syndrome, which eventually develops to obesity. Therefore, lipid metabolism has become a key target for the treatment of obesity. In general, the increased lipogenesis and reduced lipolysis are simultaneously emerged in HFD-induced obesity. Both adipogenesis and lipolysis are physiological processes mediated by a complex and tightly regulated transcriptional cascade [20]. Thereinto, PPAR-γ, SREBP-1c, FAS and LXRα are the four critical genes in regulating hepatic lipid synthesis [21]. As a major regulator of adipogenesis, PPAR-γ plays an essential role by maintaining and participating in the normal function of adipocytes [22]. SREBP-1c, a vital transcription factor, accompanies PPAR-γ to regulate the expression of genes related to fatty acids, TG and phospholipids synthesis [21]. FAS, a key downstream marker gene of SREBP-1c, can catalyze the de novo synthesis of fatty acid, and thus lead to liver TG accumulation [23]. LXRα, a transactivator of the SREBP1c promoter involved in the regulation of cholesterol and lipid metabolism, plays a vital role in hepatic lipogenesis [24]. Furthermore, PPAR-α, being an essential transcriptional regulator of lipid metabolism, triggers the expression of multiple target genes related to fatty acid uptake, transport and β-oxidation. PPARα also enhances fatty acid catabolism, which may prevent hepatic fat deposition [25, 26]. The present study showed that R-TB and F-TB conspicuously reduced the expression of SREBP-1c, PPAR-γ, FAS and LXRα, and ACC-1, while significantly increasing the mRNA expression of PPAR-α. Our study was in concordance with the study of Fan et al., which showed that TB in ripened pu-erh could regulate the activities of key enzymes in lipid metabolism, such as ACC and FAS, exhibiting a weight-losing effect. Wu et al. [27] also found that pu-erh tea could significantly inhibit the expression of FAS and SREBP-1c in a dose-dependent manner.
Patients with obesity are often in a state of chronic inflammation. Recent studies have also proved that the levels of IL-6, TNF-α and IL-1β in peripheral blood of patients with obesity are significantly higher than those of normal weight people. Therefore, chronic inflammation has become an important target for obesity [28]. In the present study, R-TB and F-TB supplement could restore the increased inflammatory factors to the normal level in obese mice. The mRNA expression of TNF-α, IL-6 and IL-1β was also significantly reduced after R-TB and F-TB administration, indicating that TB could obviously ameliorate the chronic inflammatory response in obese mice.
The gut microbiome is closely related to the health of the host. Intestinal microbiome disorders would inevitably affect host life activities and health [29]. The relative constant quantity and proportion of gut microbiota play an important role in maintaining the homeostasis of human internal and external environment [30].In recent years, a number of studies have proved that the imbalance of gut microbiota could promote obesity [31, 32]. Firmicutes and Bacteroidetes are the main microbiota in the intestinal tract of mice, their abundance changes and the increase of F/B ratio would lead to the occurrence of obesity [33]. Recent studies have revealed that the enhanced abundance of Proteobacteria and Actinobacteria could serve as a potential signal of dysbiosis of gut microbiota and is believed to be associated with the risk of obesity and related diseases [34, 35]. Interestingly, in the present study, instead of decreasing the relative abundance of Firmicutes, R-TB and F-TB treatment significantly increased the amount of Firmicutes. In addition, the propotion of Bacteroidetes was markedly enhanced, while the proportion of Actinobacteria and Proteobacteria and the F/B ratio were dramatically reduced in R-TB and F-TB treatment groups. At the genus level, F-TB and R-TB administration remarkably enriched the proportion of Lactobacillus and Lachnospiraceae_NK4A136_group belonging to phylum Firmicutes, which explained the reason for the increase in Firmicutes after F-TB and R-TB intervention. In addition, F-TB and R-TB intervention notably reduced the amount of Coriobacteriaceae_UCG-002. Lactobacillus, a typical type of probiotics, having the properties of maintaining the host’s health, regulating immune function and ameliorating intestinal oxidative stress, and thus prevent HFD-induced obesity and hepatic steatosis. The Lachnospiraceae_NK4A136_group, reported to be related to butyric acid-producing bacteria, takes a critical part in the regulation of the balance of the intestinal environment and reduction of the chronic inflammation induced by HFD [22]. Therefore, the enhancement of these bacteria is beneficial to the alleviation of obesity. Moreover, Coriobacteriaceae is generally associated with the occurrence and development of obesity and lipid metabolism disorder [36]. The alleviation of obesity by R-TB and F-TB administration might also be attributed to the reversion of the amount of Coriobacteriaceae_UCG-002. We found that the composition and abundance of gut microbiota in the F-TB intervention group were closer to those of the ND group compared with the R-TB group; therefore, F-TB might be more effective in adjusting the dysbacteria back to normal. Furthermore, Spearman’s correlation analysis indicated that Coriobacteriaceae_UCG-002, Bifidobacterium, species Lactobacillus_reuteri belonging to genus Lactobacillus, and Allobaculum were positively correlated to body weight, liver weight, eAT, pAT, TC, LDL-c, fast insulin, blood glucose, hepatic SREBP-1c, FAS, LXR, TNF-α, IL-6 and IL-1β mRNA expression and negatively correlated with the PPAR-α and PPAR-γ mRNA expression. In contrast, OUT 000009, 000006, 000015, 000011 and 000020 belonging to family Muribaculaceae, OUT 000008 belonging to genus Prevotellaceae_UCG-001 and OUT 000014 belonging to genus Muribaculum were strongly negatively correlated with body weight, liver weight, eAT, pAT, TC, fast insulin, blood glucose, hepatic SREBP-1c, FAS, LXR, TNF-α, IL-6 and IL-1β mRNA expression and positively correlated with glut4, Ins1, PPAR-α and PPAR-γ mRNA expression. It has been reported that Allobaculum may be closely related to obesity and could be used as an indicator of obesity [37]. It has been reported that L. murinus has anti-allergic activity through regulating enteric flora and can alleviate intestinal ischemia–reperfusion injury via promoting IL-10 released from M2 macrophages [38, 39]. Prevotella UCG-001 is reported to indirectly accelerate fiber degradation, which would produce short chain fatty acids [40]. In general, the genera Lactobacillus and Bifidobacterium belong to beneficial bacteria. In the present study, we found that genus Bifidobacterium and species Lactobacillus_reuteri are positively related to obesity parameters. However, the abundance of Bifidobacterium was 0.024% in the F-TB group and 1.60% in the R-TB group. The dominant genera in the F-TB and R-TB groups were Lactobacillus, Prevotellaceae_UCG-001 and Lachnospiraceae_NK4A136_group, and the sum of these three genera accounted for 49.71% and 50.18% of the total genera, respectively. Notably, Lactobacillus_murinus predominated in genera Lactobacillus, while Lactobacillus_reuteri only accounted for 0.60% and 0.54% of the total species. Based on those changes in HFD-induced obesity mice after F-TB and R-TB supplementation, it could be supposed that the F-TB and R-TB intervention-induced selective enhancement and reduction of bacteria contributed to the anti-obesity effects of F-TB and R-TB.
Conclusion
In conclusion, both R-TB and F-TB could suppress body weight gain, improve insulin sensitivity and glucose homeostasis, regulate lipid level and reduce chronic inflammation in obese mice. The underlying anti-obesity mechanism of R-TB and F-TB might involve the regulation of lipogenesis and lipolysis and the amelioration of the composition and abundance of the gut microbiota in obese mice. We found that R-TB was more efficient in the regulation of blood glucose, reduction of inflammation and suppression of partial adipogenesis-related genes and protein, while F-TB was more effective in the inhibition of lipolysis-related genes and protein. In addition, F-TB might be more effective in adjusting the dysbacteria back to normal than R-TB. Our results provide a reference for exploring the biological activities of in raw pu-erh tea theabrownin and ripened pu-erh tea theabrownin, and thus provide scientific basis for clarifying the difference between the activities of raw pu-erh tea and ripened pu-erh tea.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Räihä T, Tossavainen K, Turunen H, Enkenberg J, Kiviniemi V (2012) Effects of nutrition health intervention on pupils’ nutrition knowledge and eating habits. Scand J Educ Res 56(3):277–294. https://doi.org/10.1080/00313831.2011.581688
Quintero FS, Ariza AJ, García FB, Molano NCD, Chamorro JFV (2016) Overweight and obesity: review and update. Acta Gastroenterol Latinoam 46(2):131–159
Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, vander Hoorn S, Lawes CMM, Ali MK, Mozaffarian D, Ezzati M, Global Burden Metab Risk Factors C, Asia Pacific Cohort Studies C, Diabet Epidemiology C, Emerging Risk Factor C, Prospective Studies C (2013) The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0065174
Czernichow S, Kengne AP, Stamatakis E, Hamer M, Batty GD (2011) Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes reviews 12(9):680–687. https://doi.org/10.1111/j.1467-789X.2011.00879.x
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, Canc Int Agcy Res IARH (2016) Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med 375(8):794–798. https://doi.org/10.1056/NEJMsr1606602
Cao ZH, Gu DH, Lin QY, Xu ZQ, Huang QC, Rao H, Liu EW, Jia JJ, Ge CR (2011) Effect of pu-erh tea on body fat and lipid profiles in rats with diet-induced obesity. Phytother Research 25(2):234–238. https://doi.org/10.1002/ptr.3247
Yoo M, Kim J-W, Ye Q, Nam J, Tak DS, Kwon SI, SeungJu A, Kim S (2010) Dietary effect of puer tea extract on the body weight in rats. Biomedical Science Letters 16(1):68–70
Zhao X, Wang Q, Li GJ, Chen F, Qian Y, Wang R (2014) In vitro antioxidant, anti-mutagenic, anti-cancer and anti-angiogenic effects of Chinese Bowl tea. J Funct Foods 7:590–598. https://doi.org/10.1016/j.jff.2013.12.026
Yang L, Duan XH, Li SM, Li Q, Lin Q (2008) Effect of Pu'er tea on the carbonic particle clearances in the mononuclear phagocyte system of low immune mice. Yunnan J Trad Chin Med Materia Medica. https://doi.org/10.16254/j.cnki.53-1120/r.2008.10.034
Chen GJ, Wang MJ, Xie MH, Wan P, Chen D, Hu B, Ye H, Zeng XX, Liu ZH (2018) Evaluation of chemical property, cytotoxicity and antioxidant activity in vitro and in vivo of polysaccharides from Fuzhuan brick teas. Int J Biol Macromol 116:120–127. https://doi.org/10.1016/j.ijbiomac.2018.04.184
Huang FJ, Zheng XJ, Ma XH, Jiang RQ, Zhou WY, Zhou SP, Zhang YJ, Lei S, Wang SL, Kuang JL, Han XL, Wei ML, You YJ, Li MC, Li YT, Liang DD, Liu JJ, Chen TL, Yan C, Wei RM, Rajani C, Shen CX, Xie GX, Bian ZX, Li HK, Zhao AH, Jia W (2019) Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat Commun 10:4971. https://doi.org/10.1038/s41467-019-12896-x
Manna P, Jain SK (2015) Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metabol Syndr Relat Disord 13(10):423–444. https://doi.org/10.1089/met.2015.0095
Ren D, Hu Y, Luo Y, Yang X (2015) Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct 6(10):3342–3350. https://doi.org/10.1039/c5fo00557d
Yang F, Feng B, Niu YJ, Hu CY, Meng YH (2021) Fu instant tea ameliorates fatty liver by improving microbiota dysbiosis and elevating short-chain fatty acids in the intestine of mice fed a high-fat diet. Food Biosci 42:1–10. https://doi.org/10.1016/j.fbio.2021.101207
Wang WW, Jiang HY, Zhang JY, Yu LT, Yang LY (2015) Advances in extraction and purification technology and physiological efficacy of theabrownine. J Food Saf Qual 6:1187–1192. https://doi.org/10.19812/j.cnki.jfsq11-5956/ts.2015.04.018
Wang Q, Peng C, Gong J (2011) Effects of enzymatic action on the formation of theabrownin during solid state fermentation of Pu-erh tea. J Sci Food Agric 91(13):2412–2418. https://doi.org/10.1002/jsfa.4480
Zou XJ, Ding YH, Liang B (2012) The mechanisms of weight-cutting effect and bioactive components in Pu-erh tea. Zool Res 33(4):421–426. https://doi.org/10.3724/SP.J.1141.2012.04421
Chen T, Peng CX, Gong JS (2011) Effect of theabrownin extracted from Pu-erh tea on the metabolism of blood lipid in hyperlipidmia rats. J Chin Inst Food Sci Technol 11:20–27. https://doi.org/10.16429/j.1009-7848.2011.01.032
Zhang N, Zhao Y (2016) Mechanisms of glucose transporter GLUT4 expression regulation. Chin J Biochem Mol Biol 32(3):237–244. https://doi.org/10.13865/j.cnki.cjbmb.2016.03.01
Liu HM, Liu MH, Jin ZB, Yaqoob S, Zheng MZ, Cai D, Liu JS, Guo SD (2019) Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct 10(6):3603–3614. https://doi.org/10.1039/c9fo00027e
Yang C, Xu ZX, Deng QC, Huang QD, Wang X, Huang FH (2020) Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res Int. https://doi.org/10.1016/j.foodres.2020.108994
Song YJ, Shen HT, Liu TT, Pan BJ, De Alwis S, Zhang WY, Luo XG, Li ZY, Wang N, Ma WJ, Zhang TC (2021) Effects of three different mannans on obesity and gut microbiota in high-fat diet-fed C57BL/6J mice. Food Funct 12(10):4606–4620. https://doi.org/10.1039/d0fo03331f
Xing Y, Ren XX, Li X, Sui LP, Shi X, Sun Y, Quan CS, Xiu ZL, Dong YS (2021) Baicalein enhances the effect of acarbose on the improvement of nonalcoholic fatty liver disease associated with prediabetes via the inhibition of de novo lipogenesis. J Agric Food Chem 69(34):9822–9836. https://doi.org/10.1021/acs.jafc.1c04194
Hu HJ, Zhang SS, Pan SY (2021) Characterization of citrus pectin oligosaccharides and their microbial metabolites as modulators of immunometabolism on macrophages. J Agric Food Chem 69(30):8403–8414. https://doi.org/10.1021/acs.jafc.1c01445
Do MH, Lee HB, Lee E, Park HY (2020) The effects of gelatinized wheat starch and high salt diet on gut microbiota and metabolic disorder. Nutrients. https://doi.org/10.3390/nu12020301
Qu LL, Liu QQ, Zhang Q, Liu D, Zhang C, Fan DD, Deng JJ, Yang HX (2019) Kiwifruit seed oil ameliorates inflammation and hepatic fat metabolism in high-fat diet-induced obese mice. J Funct Foods 52:715–723. https://doi.org/10.1016/j.jff.2018.12.003
Wu YQ, Chen YL, Feng W, Guo ZP, Wang XQ, Song WJ, Bai XL, Li CW (2019) Effects of Pu-erh tea on the intervention of obesity and protection of liver in rats. Sci Technol Food Indus 40:281–285. https://doi.org/10.13386/j.issn1002-0306.2019.01.050
Hu SS, Li S, Liu Y, Sun K, Luo LY, Zeng L (2021) Aged Ripe Pu-erh tea reduced oxidative stress-mediated inflammation in dextran sulfate sodium-induced colitis mice by regulating intestinal microbes. J Agric Food Chem 69(36):10592–10605. https://doi.org/10.1021/acs.jafc.1c04032
Modi SR, Collins JJ, Relman DA (2014) Antibiotics and the gut microbiota. J Clin Investig 124(10):4212–4218. https://doi.org/10.1172/JCI72333
Jiang HM, Yuan T, Zhang MJ, Shang JQ, Bao Y (2020) Research progress of inflammatory response related to intestinal flora and obesity. Modern Prev Med 47(1):40–43
Rao WT, Luo SF, Jiang WH, Rao WT, Luo SF, Zhang YX, She KJ, He Y, Zeng P, Chen YH, Chen JH, Zhou FH (2020) Regulatory effects of ferulic acid on hepatic steatosis and intestinal flora in hyperlipidemic mice. Acta LaboRatoRium Animals Scientia Sinica 28:36–42. https://doi.org/10.3969/j.issn.1005-4847
Zhang CG, Li XT (2019) Research progress on the anti-obesity effect of functional oligosaccharides through improvement of gut microbiota. J Chin Inst Food Sci Technol 19(12):277–283. https://doi.org/10.16429/j.1009-7848.2019.12.034
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444(7122):1027–1031. https://doi.org/10.1038/nature05414
Li JJ, Jin H, Yan XM, Shao DY, Hu XZ, Shi JL (2021) The anti-obesity effects exerted by different fractions of Artemisia sphaerocephala Krasch polysaccharide in diet-induced obese mice. Int J Biol Macromol 182:825–837. https://doi.org/10.1016/j.ijbiomac.2021.04.070
Shin NR, Whon TW, Bae JW (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33(9):496–503. https://doi.org/10.1016/j.tibtech.2015.06.011
Yang C, Xu Z, Deng Q, Huang Q, Wang X, Huang F (2020) Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res Int 131:108994. https://doi.org/10.1016/j.foodres.2020.108994
Zhao RQ, Fang DL, Ji Y, Chen X, Ma GX, Su AX, Xie MH, Zhao LY, Hu QH (2020) In vitroandin vivofunctional characterization of an immune activationFlammulina velutipespolysaccharide based on gut microbiota regulation. Food Hydrocoll 31(1):667–686. https://doi.org/10.1080/09540105.2020.1754345
Huang CH, Shen CC, Liang YC, Jan TR (2016) The probiotic activity of Lactobacillus murinus against food allergy. J Funct Foods 25:231–241. https://doi.org/10.1016/j.jff.2016.06.006
Hu JJ, Deng F, Zhao BC, Lin ZB, Sun QS, Yang X, Wu M, Qiu SD, Chen Y, Yan ZZ, Luo SD, Zhao J, Liu WF, Li C, Liu KX (2022) Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome. https://doi.org/10.1186/s40168-022-01227-w
Xiao SW, Liu C, Chen MJ, Zou JF, Zhang ZM, Cui X, Jiang S, Shang EX, Qian DW, Duan JA (2020) Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl Microbiol Biotechnol 104(1):303–317. https://doi.org/10.1007/s00253-019-10174-w
Acknowledgements
The authors thank Instrumental Analysis Center of Huaqiao University for analysis support.
Funding
This work was supported by the National Nature Science Foundation of China (Grant Nos. 81703390, 22078120), the Major Special Topic of Science and Technology of Fujian Province (Grant No. 2020NZ012013) and the Foreign Cooperation Project of Fujian Province (Grant Nos. 2020I0018, 2021I0016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, X., Zhang, N., Wang, Q. et al. Theabrownin of raw and ripened pu-erh tea varies in the alleviation of HFD-induced obesity via the regulation of gut microbiota. Eur J Nutr 62, 2177–2194 (2023). https://doi.org/10.1007/s00394-023-03089-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03089-w