Abstract
Purpose
Few studies have described adherence to dietary patterns over time in women of childbearing age. This study aims to describe, examine the stability and changes in dietary patterns between pregnancy and 6 years post-pregnancy and the sociodemographic and lifestyle factors influencing the adherence over time.
Methods
During pregnancy and at 6 years post-pregnancy, 24-h recalls and food frequency questionnaires were collected, respectively, from 709 women. Data on sociodemographic and lifestyle factors were collected via questionnaires. Dietary patterns were identified using principal component analysis and stability assessed using Pearson’s correlation coefficients (r) and Cohen’s weighted kappa (κ). Associations with sociodemographic characteristics were assessed by multiple logistic regression.
Results
The ‘Fruits, Vegetables and Legumes’ (FVL) and ‘Seafood, Noodle, Soup’ (SNS) patterns were identified at both time points, with low correlation for the dietary pattern z scores (r 0.2 and 0.3, respectively) and modest agreement in tertile assignment, suggesting poor stability. An ‘unhealthy’ pattern was only observed at 6 years post-pregnancy. Women who showed increased adherence to FVL pattern had higher educational attainment and exhibited healthy lifestyle behaviours. Women who had gestational diabetes during pregnancy were less likely to decrease adherence to FVL pattern over time. Women who adhered more closely to the ‘unhealthy’ pattern at 6 years post-pregnancy tended to be younger, of Malay ethnicity, had lower socioeconomic status, were less physically active and had additional pregnancies.
Conclusions
Dietary habits of women became less healthy during the transition from pregnancy to 6 years post-pregnancy. However, results should be interpreted with caution due to the different dietary assessment tools used at the two time points.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing evidence suggests that maternal diet during pregnancy has substantial impact on pregnancy outcomes, as well as on long-term health consequences for both the mother and the child. Poor nutrition in utero has been implicated in increasing offspring susceptibility to chronic diseases, including cardiovascular disease, renal disease, obesity and type 2 diabetes (T2D) later in life [1,2,3,4,5]. Maintaining a balanced diet post-pregnancy is also crucial to ensure optimal maternal short-term and long-term health [6, 7]. A woman’s diet after delivery can influence postpartum weight retention, a significant contributor to obesity, which has been associated with an increased risk in morbidity and mortality [7, 8]. Furthermore, parents are role models for shaping children’s early experiences with food and for the establishment of healthy dietary behaviours [9,10,11]. Hence, it is important for mothers to maintain healthy eating behaviours both during and after pregnancy.
In epidemiology, tracking is defined as the stability of a given variable over a period of time [12]. Dietary tracking represents the maintenance of eating habits, nutrient intake or food intake throughout time [12]. There is an increasing interest in assessing dietary patterns when examining food intake, rather than single nutrient or food as food and nutrients are not eaten in isolation [13]. Examining dietary patterns has become a valuable approach in nutritional epidemiology studies as it evaluates the overall dietary habits of a specific population and takes into account the complex nutrient interactions.
Numerous studies have examined stability of dietary patterns over time in various population groups [14,15,16,17,18,19]. However, there remains a dearth of data on the stability of women’s dietary patterns in the continuum from pregnancy to the post-pregnancy period, especially among Asian populations. From our recent systematic review of observational studies [20], it appeared that mothers adhered less to a healthier dietary pattern post-pregnancy compared to during pregnancy. Of the 17 studies found to have examined changes in women's diet from pregnancy to the post-pregnancy period [20], only 3 have tracked maternal dietary patterns [21,22,23]. Of these three studies, only one examined the associations of sociodemographic and lifestyle factors to these patterns from preconception to 6 months postpartum [22]. In this study, smoking was positively associated with the ‘sweetened beverages and sugars’ pattern during preconception and in weeks 6 and 10 of pregnancy [22]. In weeks 10 and 38 of pregnancy, age was positively associated with the ‘vegetables and meat’ pattern [22]. None of the sociodemographic and lifestyle factors were associated with these dietary patterns at 6 months postpartum [22].
The aims of this longitudinal study were to (1) identify and describe women’s dietary patterns at pregnancy and at 6 years post-pregnancy in a multiethnic Asian cohort residing in Singapore, (2) investigate the stability and changes of these dietary patterns between pregnancy and 6 years post-pregnancy, and (3) identify the sociodemographic and lifestyle factors influencing the adherence to these identified dietary patterns over time. We hypothesised that there would be low stability of healthy dietary patterns from pregnancy to 6 years post-pregnancy. Additionally, we hypothesised that women’s socioeconomic status (SES) would influence adherence to healthy dietary patterns over time. Knowledge of all these can facilitate the design and implementation of more effective policies and interventions.
Methods
Study population
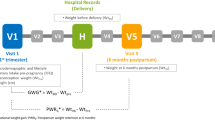
Data for this study were obtained from the Growing Up in Singapore Toward healthy Outcomes (GUSTO) Study, an on-going prospective birth cohort study to examine the early pregnancy influences on maternal and offspring health. Further details of the study population have been described previously [24, 25]. In brief, from June 2009 to October 2010, pregnant women were recruited at < 14 weeks of gestation from 2 major public maternity units of Kandang Kerbau Women's and Children's Hospital (KKH) and the National University Hospital (NUH) in Singapore. Eligibility criteria for the GUSTO study included: Singaporean citizens or permanent residents, of Chinese, Malay or Indian ethnicity with parents of homogeneous ethnic background, aged 18–50 years, intend to deliver in NUH or KKH and plan to reside in Singapore for the next 5 years, willing to donate cord, cord blood and placenta. Mothers receiving chemotherapy, psychotropic drugs or who had type 1 diabetes were excluded. This trial was registered at clinicaltrials.gov as NCT01174875. Ethical approval was granted by the Institutional Review Board of NUH and KKH. Written informed consent was obtained from all of the participants at recruitment.
Data collection
Maternal characteristics
Data on maternal sociodemographic and lifestyle characteristics (e.g., age, ethnicity, education and monthly household income) and obstetric and medical history were collected from participants at recruitment (< 14 weeks of gestation). Pre-pregnancy body mass index (BMI) (kg/m2) was derived using self-reported pre-pregnancy weight and measured height at the 26–28 weeks’ gestation follow-up visit. Standing heights were measured with a stadiometer (SECA model 213).
At the 26–28 weeks’ gestation follow-up visit, information on cigarette smoking and alcohol consumption during pregnancy was obtained and oral glucose tolerance test was conducted to identify women with gestational diabetes mellitus (GDM) according to WHO diagnostic criteria [26]. Hypertensive disorders of pregnancy, were obtained from hospital case notes.
Participants were classified into groups of inadequate, adequate and excessive gestational weight gain (GWG) based on the Institute of Medicine (IOM) recommended rate of weight gain (kg/week) in the second and third trimesters according to the pre-pregnancy BMI category [27]. To compute rate of weight gain during second and third trimesters, linear mixed-effects model with the best linear unbiased predictor was used to estimate linear trajectory of GWG per week [28]. Inadequate GWG was defined as a weight gain rate less than the IOM recommended lower limit, whereas excessive weight gain was defined a weight gain rate greater than the recommended upper limit. Those with weight gain rate within the recommended range were classified as having adequate GWG.
Postpartum weight retention (PPWR) was calculated by subtracting the self-reported pre-pregnancy weight from measured weight at 18 months follow-up visit. Maternal weights were measured using calibrated electronic weighing scales (SECA, Hamburg, Germany) to the nearest 0.1 kg. Women were categorised into non-substantial PPWR (< 5 kg) and substantial PPWR (≥ 5 kg) [29, 30].
Information on women’s sociodemographic and lifestyle characteristics, working status, BMI, parity, T2D and hypertension status post-pregnancy was collected at 4, 5 or 6-year follow-up visits. Mothers were asked to attend all 4, 5 and 6-year follow-up visits and different information were collected at each visit. Information regarding child feeding practices such as reading the Healthy Choice Symbol (HCS) when purchasing food for their child was obtained at year 5. The HCS is a front-of-pack nutrition labelling, which identifies food items within a specific category of foods as healthier choices [31]. The duration and frequency of physical activity were used to derive metabolic equivalent (MET-min/week) scores [32] and this information was obtained at year 6. Women completed the oral glucose tolerance test (OGTT) at either year 4 or year 5–6. Mothers were classified as having T2D if fasting glucose was equal to or more than 7 mmol/L or 2-h post challenge glucose was equal to or more than 11.1 mmol/L at either of these two time points [33]. Hypertension status was self-reported at year 4 visit.
Maternal dietary assessment and identification of dietary patterns
Maternal dietary intake at 26–28 weeks’ gestation was assessed using a single 24-h recall. The 24-h recall was administered by a trained clinical research staff on either a weekday or weekend using a five-step, multiple-pass interviewing method with standardised household measuring utensils and food pictures of various portion sizes to aid women in quantifying their dietary intake during the previous day. Details of the assessment of maternal dietary intake during pregnancy have been published elsewhere [34, 35].
At 6-year follow-up visit, women’s dietary intake for the previous month was assessed using a 133-food item semi-quantitative Food Frequency Questionnaire (FFQ), which was developed based on locally validated FFQ previously used in nationally representative samples of adults [36, 37] and it was administered by trained clinical research staff. Mothers had to indicate the frequency of consumption over the past month as ‘never’, ‘number of times per month’, ‘number of times per week’ or ‘number of times per day’. The average servings of food items consumed were also ascertained using photographs of standardised household measuring utensils and food pictures presented during the interview. Dietary intakes of food were standardised to daily frequencies, and multiplied by average amount per serving in grams (g), to obtain total intake in grams per day (g/day).
All food and drinks recorded were initially allocated into 68 food groups for the 24-h recalls at pregnancy and 57 food groups for the FFQ at 6 years post-pregnancy based on similarities in nutrient composition and culinary use. To avoid skewed distributions, foods that were rarely consumed (consumed by less than 10% of participants) and had little to no variance were either excluded or merged with food groups with food sharing a similar nutritional profile and culinary use [38]. A final standardised list of 30 non-overlapping food groups for both time points are reported in Supplementary Table 1.
Standardised intake (g/day) of food groups was used as the input value in the analysis. Principal component analysis (PCA) with varimax rotation was performed independently at each time point to empirically derive dietary patterns. The number of components best representing the data was chosen based on the break point of the scree plot, eigenvalues > 1 and the interpretability of the components [39]. Food groups with a loading coefficient > 0.25 or < − 0.25 were considered important for interpretability of the components (i.e. dietary patterns) [40, 41]. The components or dietary patterns were named according to the foods that loaded highly on the patterns [42].
The dietary pattern score for the derived patterns identified at both time points was calculated for each participant by summing the standardised intake of food groups (grams per day) weighted by their PCA loadings. Each participant also received a standardised z score (mean = 0; SD 1) indicating how closely their intake resembled the dietary pattern relative to the rest of the cohort.
Statistical analyses
Baseline maternal sociodemographic and lifestyle characteristics of respondents who provided complete dietary data at both time points were compared with those of non-respondents using Mantel–Haenszel chi-square test for categorical variables.
Tucker’s congruence coefficient quantifies the similarity of the PCA loadings across the two time points in each dietary pattern [43, 44], with absolute values ranging between 0 and 1. The coefficient may be interpreted as follows: > 0.8 “excellent”, 0.65 to 0.8 “good”, 0.5 to 0.65 “acceptable” and < 0.5 “poor” congruence [45]. Only dietary patterns that have quantitatively similar PCA loadings across the two time points can be tracked.
To evaluate the stability of dietary patterns, the dietary pattern z scores were used in the subsequent analyses as continuous variables or ranked into categorical variables (tertiles). Pearson’s correlation coefficients (r) between dietary pattern z scores (as continuous variables) at the two time points were calculated. An absolute coefficient value of less than 0.3 was indicative of ‘low’ correlation, 0.3–0.5 as ‘moderate’ correlation and more than 0.5 as ‘strong’ correlation [46].
Participants were then assigned into tertiles of dietary pattern z scores during pregnancy and at 6 years post-pregnancy, with low scores belonging to 1st tertile and high scores belonging to 3rd tertile for each dietary pattern (1st tertile (T1) = low adherence, 2nd tertile (T2) = moderate adherence, and 3rd tertile (T3) = high adherence). The proportion of participants (%) who remained in the same tertile for a dietary pattern from pregnancy to 6 years post-pregnancy was determined (cross-tabulations) and the level of agreement was estimated using Cohen’s weighted kappa (κ) [47]. Cohen’s weighted κ of one indicates perfect agreement and zero indicates agreement due to chance. In accordance to the scale of Landis and Koch [48], values of 0.01–0.20 are indicative of “slight” agreement; 0.21–0.40 as “fair”; 0.41 to 0.60 as “moderate”; 0.61 to 0.80 as “substantial” and 0.81 to 1.00 as “almost perfect” agreement between time points. The higher the agreement, the more stable is the dietary pattern across the two time points.
Participants who shifted from T1 at pregnancy to T2/T3 at post-pregnancy and T2 at pregnancy to T3 at post-pregnancy represent those with increased adherence to this particular dietary pattern. They were compared to participants in the consistently low adherence group (i.e. those who remained in T1 of a particular dietary pattern from pregnancy to 6 years post-pregnancy). Conversely, participants who shifted from T3 at pregnancy to T2/T1 at post-pregnancy and T2 at pregnancy to T1 at post-pregnancy represent those with decreased adherence to this particular dietary pattern. They were compared to those in the consistently high adherence group (participants who remained in T3 of a particular dietary pattern throughout the two time points).
Logistic regression models were used to assess the associations between sociodemographic and lifestyle characteristics and mother’s adherence to dietary patterns over time. In these models, we examined mother’s age at recruitment, ethnicity, maternal education, household income at recruitment and at 5 years post-pregnancy, pre-pregnancy BMI and BMI at 6 years post-pregnancy, parity at recruitment and at 4 years post-pregnancy, GDM, hypertensive disorders of pregnancy, hypertension at 4 years post-pregnancy, GWG, PPWR, mother’s working status at 5 years post-pregnancy, mother’s physical activity post-pregnancy, mother’s T2D status post-pregnancy and mother’s child feeding practices such as reading HCS when purchasing food for their child. The model was adjusted for sociodemographic and lifestyle variables that were significant at p < 0.05 in the univariate model. Only results from multiple adjusted models were reported.
To reduce potential bias associated with missing data in our study, missing data were imputed 20 times using multiple imputation technique with chained equations [49] for the following sociodemographic and lifestyle variables: n = 57 pre-pregnancy BMI, n = 6 maternal education, n = 48 household income at recruitment, n = 21 GDM, n = 81 GWG, n = 194 PPWR, n = 68 parity at 4 years post-pregnancy, n = 134 household income at 5 years post-pregnancy, n = 90 mother’s working status at 5 years post-pregnancy, n = 39 reading HCS label, n = 69 physical activity, n = 60 BMI at 6 years post-pregnancy, n = 72 hypertension at 4 years post-pregnancy and n = 120 mother’s T2D status post-pregnancy. The results of the 20 analyses were pooled using Rubin’s rule [50]. Complete-case analysis was performed as a sensitivity analysis. As similar effect estimates were found in analyses with imputed and unimputed data, pooled results after the multiple imputation were presented. All statistical analyses were conducted using STATA 14.2 (StataCorp, College Station, TX). Two-sided p < 0.05 was considered to be statistically significant.
Results
Study sample characteristics
Study sample selection is shown in Fig. 1. A total of 1450 pregnant women provided written consent and were recruited into the study. Pregnant women who were bearing twins were excluded (n = 10). Of the remaining 1440 pregnancies, 414 women were excluded from the analysis due to lost to follow-up. A final sample of 709 mothers had complete dietary data at both 26–28 weeks’ gestation and at 6 years post-pregnancy, and were thus included in the current analysis. A majority of the participants were non-smokers (87.5%) and did not consume alcohol during pregnancy (97.5%). Differences in baseline characteristics between women who had complete dietary data at both time points and those who did not are shown in Table 1. Maternal characteristics at baseline such as age, pre-pregnancy body mass index (BMI), ethnicity, educational attainment, household income, parity, hypertensive disorders and GDM status did not differ significantly between mothers who were included (n = 709) and those who were excluded from this analysis (n = 731).
Description of dietary patterns
Dietary patterns identified in pregnancy and at 6 years post-pregnancy are presented in Table 2. At 26–28 weeks’ gestation, two dietary patterns were identified. The first dietary pattern, labelled ‘Fruits, Vegetables and Legumes’ (FVL), was characterised by higher intakes (high positive PCA loadings) of vegetables, fruits, legumes, nuts, seeds, ethnic bread, whole milk, and non-refined grains. The second dietary pattern, labelled ‘Seafood, Noodle, Soup’ (SNS), was characterised by higher intakes of soup, noodles, seafood, healthy meat, and eggs and lower intakes of legumes, nuts and seeds and ethnic bread. These two dietary patterns were also identified at 6 years post-pregnancy. In addition, a third different dietary pattern, which was not observed in pregnancy, emerged only at 6 years post-pregnancy. This third dietary pattern, labelled ‘unhealthy’ dietary pattern, was characterised by higher intakes of processed meat, fast food, unhealthy local savoury snacks, flavoured rice, soft drinks, unhealthy meat and fried potatoes.
Stability of women’s dietary patterns over time
The congruence coefficient observed between the FVL dietary patterns at the two time points was 0.70 and considered “good”. This was similar for the SNS dietary pattern, at 0.71. These demonstrated high similarities in the PCA loadings of each dietary pattern between the two time points, suggesting that these two patterns were quantitatively similar across the two periods. The congruence coefficient observed between the third dietary pattern from the two time points was 0.27 and considered “poor”. This demonstrated low similarity in the PCA loadings of the third dietary pattern between the two time points. Thus, only the stability of FVL and SNS dietary patterns across the two time points was suitable to be assessed.
Pearson’s correlation coefficient between dietary pattern z scores at the two time points were 0.20 for the FVL dietary pattern and 0.30 for the SNS dietary pattern (both p < 0.05), indicative of low correlation. The proportion of participants who remained in the same tertile across time varied from 37 to 44% for FVL dietary pattern and 39% to 45% for SNS dietary pattern (Table 3). The weighted κ values for being in the same tertile at the two time points were 0.11 (95% CI 0.065–0.172) and 0.15 (95% CI 0.095–0.199) for FVL and SNS dietary patterns respectively, which indicates only a slight level of agreement between the categorised dietary pattern z scores for both dietary patterns in pregnancy and at 6 years post-pregnancy according to the identified cut-off criteria mentioned previously [48]. These results reflect poor stability of FVL and SNS dietary patterns across the two time points.
Associations between adherence to dietary patterns and women’s sociodemographic and lifestyle factors
‘Fruit, Vegetables and Legumes’ (FVL) dietary pattern
The associations between adherence to FVL dietary pattern over time and women’s sociodemographic and lifestyle factors are shown in Table 4. Mothers in the increased adherence group (n = 210; T1 in pregnancy to T2/T3 post-pregnancy and T2 in pregnancy to T3 post-pregnancy) were compared to those in the consistently low adherence group (n = 98; T1 in pregnancy and post-pregnancy), while those in the decreased adherence group (n = 209; T3 in pregnancy to T2/T1 post-pregnancy and T2 in pregnancy to T1 post-pregnancy) were compared to those in the consistently high adherence group (n = 105; T3 in pregnancy and post-pregnancy).
Mothers, with increased adherence to the FVL dietary pattern from pregnancy to 6 years post-pregnancy, tended to be of Indian ethnicity, higher educational attainment (post-secondary and beyond), more physically active (≥ 3000 MET-min/week) at year 6, read HCS when purchasing food for their child and were less likely to have substantial PPWR (≥ 5 kg) measured at 18 months post-pregnancy as compared to mothers with consistently low adherence to the FVL pattern. Maternal health status during pregnancy and post-pregnancy, such as GDM, T2D, hypertensive disorders during pregnancy and hypertension post-pregnancy, were not found to be associated with increased adherence to FVL pattern from pregnancy to post-pregnancy.
Compared to mothers with consistently high adherence to the FVL pattern, those with decreased adherence to the FVL dietary pattern from pregnancy to 6 years post-pregnancy, were less likely to have the following characteristics: of Indian ethnicity, higher educational attainment (post-secondary and beyond), had GDM during pregnancy and read HCS when purchasing food for their child. Other maternal sociodemographic, lifestyle factors and health status examined were not associated with the adherence to FVL dietary pattern over time.
‘Seafood, Noodle and Soup’ (SNS) dietary pattern
The associations between adherence to SNS dietary pattern over time and women’s sociodemographic and lifestyle factors are shown in Supplementary Table 2. Mothers in the increased adherence group (n = 197) were compared to those in the consistently low adherence group (n = 108), while those in the decreased adherence group (n = 206) were compared to those in the consistently high adherence group (n = 107).
Mothers, with increased adherence to the SNS dietary pattern from pregnancy to 6 years post-pregnancy, tended to have post-secondary education and less likely to be of Malay and Indian ethnicity as compared to mothers with consistently low adherence to the SNS dietary pattern.
Mothers, with decreased adherence to the SNS dietary pattern from pregnancy to 6 years post-pregnancy, tended to be of Malay and Indian ethnicity and less likely to be older (≥ 30 years old) and less likely to have had a university level education as compared to mothers with consistently high adherence to the SNS pattern. Other maternal sociodemographic, lifestyle factors and health status examined were not associated with the adherence to SNS dietary pattern over time.
‘Unhealthy’ dietary pattern
Adherence to the ‘unhealthy’ dietary pattern was examined at 6 years post-pregnancy only and its association with women’s sociodemographic and lifestyle factors was assessed using multiple ordinal logistic regression analyses as shown in Table 5. The fit of model and proportional odds assumption were checked and met.
Maternal age ≥ 30 years, university qualification, higher household income (≥ $2000/month) at 5 years post-pregnancy and more physically active (≥ 600 MET-min/week) at post-pregnancy were associated with a significantly decreased odds of moderate and high adherence to the ‘unhealthy’ dietary pattern at 6 years post-pregnancy. Women of Malay ethnicity and women with additional pregnancies between birth of child in the study and at 4 years post-pregnancy were more likely to have moderate and high adherence to the ‘unhealthy’ dietary pattern at 6 years post-pregnancy. Other maternal sociodemographic, lifestyle factors and health status examined were not associated with the adherence to ‘unhealthy’ dietary pattern at 6 years post-pregnancy.
Discussion
To the best of our knowledge, this is the first longitudinal study that assessed the stability and changes in women’s dietary patterns between pregnancy and 6 years post-pregnancy in a multiethnic Asian population. We identified two distinct dietary patterns, the ‘Fruits, Vegetables and Legumes’ (FVL) and ‘Seafood, Noodle, Soup’ (SNS) patterns, which tracked poorly. Furthermore, an ‘unhealthy’ dietary pattern, which was not observed during pregnancy, was identified at 6 years post-pregnancy. We also found that the most significant sociodemographic circumstances and lifestyle habits associated with adherence to dietary patterns over time in our cohort were age, ethnicity, educational attainment, post-pregnancy household income, parity, physical activity level and reading of food labels.
Two different dietary assessment methods were used at the two time points—24 h recall during pregnancy and FFQ at 6 years post-pregnancy, which is one of the limitations of our study. This may have affected the ability to track dietary patterns over time. However, we ran Tucker’s congruence coefficient tests which showed that the identified patterns were quantitatively similar over time and thus indicated that these patterns can be tracked longitudinally. Similarly, previous studies found few differences in the factor loadings based on a long-term dietary assessment method, such as the FFQ, when compared to the factor loadings based on a short-term dietary assessment method, such as a 7-day weighted diet record [51, 52] and the dietary patterns generated were similar [53]. Nevertheless, future longitudinal studies should ideally ensure similar dietary assessment tools are used throughout the study so that the dietary data collected over time are comparable.
Our findings suggest that the stability of the FVL pattern—a healthy eating pattern high in vegetables, fruits, legumes, nuts, seeds, and non-refined grains—from pregnancy to 6 years post-pregnancy is poor. Furthermore, an ‘unhealthy’ dietary pattern, which was not observed during pregnancy, was identified at 6 years post-pregnancy. Evidence from our recent systematic review of observational studies also suggests that the transition from pregnancy to motherhood is generally associated with a progressive increase in intake of energy-dense, nutrient-poor food and a decline in the consumption of healthy food [20]. These results reflected a shift towards unhealthy dietary habits in women during the transition from pregnancy to post-pregnancy. Similarly, Northstone et al. [21] reported a decrease in adherence to the ‘health conscious’ dietary pattern and greater adherence to the ‘processed’ and ‘vegetarian’ dietary pattern over the four years postpartum follow-up period. Sotre-Alvarez et al. [23] found that women were most likely to stay in “prudent” and “western” than “health conscious western” dietary pattern from the second trimester to 1 year postpartum. Women adopt healthier dietary patterns such as increasing their fruits and vegetables intake during pregnancy [54]; however, these changes in dietary habits may not be sustained after delivery and their dietary habits often become less healthy [55].
Our study found that health-promoting lifestyle behaviours post-pregnancy, such as being more physically active and reading food labels, were associated with increased adherence to the healthier FVL dietary pattern over time and lower adherence to the ‘unhealthy’ dietary pattern post-pregnancy. Women who read food labels when purchasing food were less likely to decrease adherence to the FVL dietary pattern over time. This is expected as previous studies have reported a co-occurrence of health-promoting lifestyle behaviours and consumption of healthy diet and their potential synergistic effect on the risk of chronic conditions and health outcomes [56, 57]. Our findings help to deepen the understanding of the clustering of these healthy lifestyle factors and form the basis for intervention development and prevention strategies to further target lifestyle behaviours simultaneously and to improve mother’s diet post-pregnancy. Additional support and strategies which account for barriers to behaviour changes faced by women in the post-pregnancy period, such as time and financial constraints, returning to work and lack of partner support [58], are required.
We found that those women who decreased adherence to the FVL pattern over time were less likely to be those diagnosed with GDM during pregnancy. In other words, women, who were diagnosed with GDM during pregnancy, tended to adhere closely to the FVL pattern over time. Women who are diagnosed with GDM are at high risk of developing T2D after pregnancy [30, 59]. Similarly, there are a few qualitative studies that suggest that women diagnosed with GDM have slightly changed their postpartum lifestyle behaviours that are consistent with guidelines for the prevention of T2D, such as increasing intake of fruits and vegetables and avoiding high-fat foods [60,61,62,63]. However, other mothers’ health conditions such as hypertensive disorders during pregnancy, post-pregnancy T2D and hypertension did not appear to influence changes in the women’s diet over time. One reason for this could be that there is a greater emphasis placed on GDM and how to manage it in public health messages in Singapore as compared to other health conditions such as hypertensive disorders during pregnancy. It is also important to note that our study was potentially underpowered for the case groups and that the small sample size in each tertile may have contributed to the failure in detecting any association between mothers’ health conditions and adherence to dietary patterns over time. Further studies with comparable groups are warranted to confirm this association.
We found that mothers of Malay decent, who were younger (< 30 years old), had below degree-level education and lower household income tended to have greater adherence to an ‘unhealthy’ dietary pattern post-pregnancy. Similarly, we found that mothers with below post-secondary education tended to decrease adherence to the FVL pattern over time. Our results are in line with numerous studies which have shown that consumption of unhealthy food is strongly patterned by SES and that socio-economically disadvantaged individuals have less healthy dietary habits as compared to people with high SES [64,65,66,67,68]. It is well understood that higher educational attainment is often associated with lower consumption of energy-dense food [69,70,71,72,73,74,75,76,77]. Higher education may indicate better nutritional knowledge and ability to understand the information communicated by healthcare professionals or on food labels to facilitate a person’s food choices [65, 78].
Consistent with other studies [74, 79], older women were more likely to have a healthier diet compared to younger women. This may be due to older women being more informed regarding lifestyle choices and they have been found to have better nutritional knowledge and better adherence to national dietary guidelines; thus, a healthier dietary intake [80, 81]. Our findings are important in identifying ‘at risk’ populations during pregnancy in terms of their demographic characteristics and SES, in whom future nutritional promotion initiatives and public health policies might be prioritised to prevent reduced diet quality during the transition to motherhood.
In addition, we found that women with one or more pregnancies between the GUSTO birth and the 4-year follow-up visit tended to adhere more to the ‘unhealthy’ dietary pattern at 6 years post-pregnancy. This is comparable to previous findings showing women who had more children had lower intentions to eat healthy and had poorer-quality diets [82,83,84]. Many women may be responsible for meal preparation within a household [85]. The increased time and effort needed to prepare healthy meals for multiple children may lead to subsequent neglect of their own dietary behaviours. Further research should examine the effect of multiple children on women’s dietary habits over time.
Another important finding was that post-pregnancy household income, not the household income during pregnancy, was associated with adherence to the ‘unhealthy’ dietary pattern at 6 years post-pregnancy. Women with higher post-pregnancy household income (≥ $2000/month) adhered less to the ‘unhealthy’ dietary pattern at 6 years post-pregnancy. Lack of resources and the need to prioritise finances are frequently cited as barriers to healthy eating behaviours amongst women in their early years of motherhood [58]. Healthy food is often perceived as more expensive than unhealthy food and people of lower SES generally exhibit less healthy eating behaviours [86]. Due to the increased demand related to time, finances, fatigue, and social support for mothers, this may impact mothers’ motivation for healthy eating and their health-related attitudes post-pregnancy [87,88,89]. Women’s needs and experiences post-pregnancy should be considered when designing strategies to support and promote healthier eating behaviours and lifestyles for this vulnerable population.
The SNS pattern, which is unique in our population, is characterised by intake of noodles, soup and seafood and is reflective of a traditional Chinese meal pattern. In this study we found that women of Malay and Indian ethnicity were less likely to increase adherence to the SNS pattern over time as compared to Chinese women. This is not unexpected as a similar trend was found in a previous study in Singapore, in which large ethnic differences in dietary habits were observed during the postpartum period [90]. Cultural beliefs, traditional dietary practices and social influences can directly affect the food habits of postpartum mothers, and these should be taken into account when implementing dietary guidelines and nutrition interventions.
This study also reflects the importance of adopting a life course approach to chronic disease [91], such that interventions targeting population’s needs at different life-stages are necessary to mitigate the development of chronic diseases.
The present study has several strengths. This is the first study examining the stability of population-specific PCA-derived dietary patterns from pregnancy to 6 years post-pregnancy in an Asian population. Additionally, we examined the associated-sociodemographic and lifestyle factors from both time points, at 26–28 weeks’ gestation and at 6 years post-pregnancy, instead of just at baseline. Our study had the longest follow-up period of 6 years post-pregnancy as compared to the previous three studies [21,22,23], in which follow-up periods ranged from 3 months to 4 years post-pregnancy. We used PCA to derive dietary patterns at each time point separately. An advantage of deriving dietary patterns at different time points is that new dietary patterns that may have emerged over time could be identified [17, 21].
We acknowledge some other limitations of our study. First, one limitation of the 24-h recall is that dietary information collected may not be a good representation of an individual’s usual intake because of the day to day variation in daily intakes. However, we have shown previously that the 24-h recalls had good reproducibility [34], which allowed the generation of dietary patterns. Third, it is important to note that in some steps of this analysis, including the classifications of food into food groups, numbers of food groups, numbers of factors extracted, and the names of the factors extracted, decisions made were subjective [92]. Steps were taken to reduce subjectivity and increase comparability between studies. For example, the food groups were grouped based on approaches used in previous literature and dietary patterns were determined based on established criteria. Forth, retaining cohort participants can be a challenge in longitudinal research. Even though our cohort of n = 709 may seem small, it is comparable to other cohorts in similar studies found in our systematic review [20], given that our present study has the longest follow-up period. Also, we are limited by the small sample sizes of the case groups at the follow-up years, precluding robust conclusions regarding the association between mothers’ health conditions during pregnancy and post-pregnancy and their adherence to dietary patterns over time. Finally, it is important to be aware of the inherent problems of systematic measurement error associated with self-reported dietary data. In addition, self-reported dietary data are prone to social desirability bias [93], where participants tend to answer questions in a way that will be viewed favourably by others; thus, leading to under-reporting of data such as unhealthy dietary habits. This may be more prevalent amongst women during pregnancy [94, 95] and needs to be considered when interpreting the results. Nevertheless, self-reports of maternal dietary intake remain the most-used tool, especially in large cohorts.
In conclusion, our study found poor stability in the FVL and SNS patterns from pregnancy to 6 years post-pregnancy. An ‘unhealthy’ pattern emerged only at 6 years post-pregnancy, indicating a tendency amongst mothers in our cohort to have poor dietary intake at post-pregnancy. Several sociodemographic factors associated with the adherence to these dietary patterns from pregnancy to post-pregnancy in this multiethnic cohort of women were identified, with younger age, lower educational attainment, lower income and less favourable healthy lifestyle behaviours tending to associate with unhealthy dietary patterns. Our results highlight particular groups of women for whom additional support could help them achieve and maintain healthy dietary behaviours from pregnancy to the post-pregnancy period to improve nutrition-related health outcomes for mothers and their children. Finally, future longitudinal studies should strive to use similar dietary assessment tools at baseline and at follow-up time points to facilitate comparison of dietary intake.
Abbreviations
- T2D:
-
Type 2 diabetes
- GUSTO:
-
Growing Up in Singapore Toward healthy Outcomes
- BMI:
-
Body mass index
- GDM:
-
Gestational diabetes mellitus
- GWG:
-
Gestational weight gain
- PPWR:
-
Postpartum weight retention
- OGTT:
-
Oral glucose tolerance test
- HCS:
-
Healthy choice symbol
- FFQ:
-
Food Frequency Questionnaire
References
Barker DJP, Godfrey KM, Gluckman PD, Harding JE, Owens JA, Robinson JS (1993) Fetal nutrition and cardiovascular disease in adult life. Lancet 341(8850):938–941
Hales CN, Barker DJ (2001) The thrifty phenotype hypothesis. Br Med Bull 60:5–20
Harding JE (2001) The nutritional basis of the fetal origins of adult disease. Int J Epidemiol 30(1):15–23
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73
Godfrey K (2006) The ‘developmental origins’ hypothesis: epidemiology. In: Hanson M, Gluckman P (eds) Developmental origins of health and disease. Cambridge University Press, Cambridge, pp 6–32
Boghossian NS, Yeung EH, Lipsky LM, Poon AK, Albert PS (2013) Dietary patterns in association with postpartum weight retention. Am J Clin Nutr 97(6):1338–1345
Rong K, Yu K, Han X, Szeto IM, Qin X, Wang J, Ning Y, Wang P, Ma D (2015) Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr 18(12):2172–2182
Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, Ramey S, Wang C, Hobel C, Raju T, Shalowitz MU, Community Child Health Network of the Eunice Kennedy Shriver National Institute of Child H, Human D (2015) Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstetrics Gynecol 125(1):144–152
Birch LL, Davison KK (2001) Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr Clin N Am 48(4):893–907
Wansink B (2006) Nutritional gatekeepers and the 72% solution. J Am Diet Assoc 106(9):1324–1327
Ventura AK, Birch LL (2008) Does parenting affect children’s eating and weight status? Int J Behav Nutr Phys Activity 5:15
Twisk JWR, Kemper HCG, Mellenbergh GJ (1994) Mathematical and analytical aspects of tracking. Epidemiol Rev 16(2):165–183
Tucker KL (2010) Dietary patterns, approaches, and multicultural perspective. Appl Physiol Nutr Metab 35(2):211–218
Mikkila V, Rasanen L, Raitakari OT, Pietinen P, Viikari J (2005) Consistent dietary patterns identified from childhood to adulthood: the cardiovascular risk in Young Finns Study. Br J Nutr 93(6):923–931
Borland SE, Robinson SM, Crozier SR, Inskip HM, the SWSSG (2008) Stability of dietary patterns in young women over a 2-year period. Eur J Clin Nutr 62(1):119–126
Togo P, Osler M, Sørensen TIA, Heitmann BL (2004) A longitudinal study of food intake patterns and obesity in adult Danish men and women. Int J Obes 28(4):583–593
Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM (2009) Women’s dietary patterns change little from before to during pregnancy. J Nutr 139(10):1956–1963
Movassagh EZ, Baxter-Jones ADG, Kontulainen S, Whiting SJ, Vatanparast H (2017) Tracking dietary patterns over 20 years from childhood through adolescence into young adulthood: the Saskatchewan pediatric bone mineral accrual study. Nutrients 9(9):990
Johns DJ, Lindroos AK, Jebb SA, Sjöström L, Carlsson LMS, Ambrosini GL (2014) Tracking of a dietary pattern and its components over 10-years in the severely obese. PLoS ONE 9(5):e97457–e97457
Lee YQ, Loh J, Ang RSE, Chong MF-F (2020) Tracking of maternal diet from pregnancy to postpregnancy: a systematic review of observational studies. Curr Dev Nutr. https://doi.org/10.1093/cdn/nzaa118
Northstone K, Emmett PM (2008) A comparison of methods to assess changes in dietary patterns from pregnancy to 4 years post-partum obtained using principal components analysis. Br J Nutr 99(5):1099–1106
Cucó G, Fernández-Ballart J, Sala J, Viladrich C, Iranzo R, Vila J, Arija V (2006) Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur J Clin Nutr 60(3):364–371
Sotres-Alvarez D, Herring AH, Siega-Riz AM (2013) Latent transition models to study women’s changing of dietary patterns from pregnancy to 1 year postpartum. Am J Epidemiol 177(8):852–861
Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stunkel W, Holbrook JD, Kwek K, Chong YS, Saw SM (2014) Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol 43(5):1401–1409
Soh SE, Chong YS, Kwek K, Saw SM, Meaney MJ, Gluckman PD, Holbrook JD, Godfrey KM (2014) Insights from the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) Cohort Study. Ann Nutr Metab 64(3–4):218–225
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15(7):539–553
Institute of Medicine (1990) Nutrition during pregnancy: part I, weight gain: part II, nutrient supplements. Natl Academy Pr.
Cheung YB (2013) Statistical analysis of human growth and development. CRC Press
Gunderson EP (2009) Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin N Am 36(2):317–332 (ix)
Chen L-W, Soh SE, Tint M-T, Loy SL, Yap F, Tan KH, Lee YS, Shek LP-C, Godfrey KM, Gluckman PD, Eriksson JG, Chong Y-S, Chan S-Y (2021) Combined analysis of gestational diabetes and maternal weight status from pre-pregnancy through post-delivery in future development of type 2 diabetes. Sci Rep 11(1):5021
Health Promotion Board (2021) Healthier Choice Symbol (HCS). https://www.hpb.gov.sg/food-beverage/healthier-choice-symbol. Accessed 11 Jan 2021
Padmapriya N, Shen L, Soh SE, Shen Z, Kwek K, Godfrey KM, Gluckman PD, Chong YS, Saw SM, Müller-Riemenschneider F (2015) Physical activity and sedentary behavior patterns before and during pregnancy in a multi-ethnic sample of Asian women in Singapore. Matern Child Health J 19(11):2523–2535
American Diabetes A (2009) Diagnosis and classification of diabetes mellitus. Diabetes Care 32(Suppl 1):S62–S67
Chong MF, Chia AR, Colega M, Tint MT, Aris IM, Chong YS, Gluckman P, Godfrey KM, Kwek K, Saw SM, Yap F, van Dam RM, Lee YS (2015) Maternal protein intake during pregnancy is not associated with offspring birth weight in a multiethnic Asian population. J Nutr 145(6):1303–1310
Chen LW, Tint MT, Fortier MV, Aris IM, Bernard JY, Colega M, Gluckman PD, Saw SM, Chong YS, Yap F, Godfrey KM, Kramer MS, van Dam RM, Chong MF, Lee YS (2016) Maternal Macronutrient Intake during Pregnancy Is Associated with Neonatal Abdominal Adiposity: The Growing Up in Singapore Towards healthy Outcomes (GUSTO) Study. J Nutr 146(8):1571–1579
Deurenberg-Yap M, Li T, Tan WL, van Staveren WA, Deurenberg P (2000) Validation of a semiquantitative food frequency questionnaire for estimation of intakes of energy, fats and cholesterol among Singaporeans. Asia Pac J Clin Nutr 9(4):282–288
Neelakantan N, Whitton C, Seah S, Koh H, Rebello SA, Lim JY, Chen S, Chan MF, Chew L, van Dam RM (2016) Development of a semi-quantitative food frequency questionnaire to assess the dietary intake of a multi-ethnic urban Asian population. Nutrients 8(9):528
Ocké MC (2013) Evaluation of methodologies for assessing the overall diet: dietary quality scores and dietary pattern analysis. Proc Nutr Soc 72(2):191–199
Kline P (1994) An easy guide to factor analysis. Routledge, London
Moeller SM, Reedy J, Millen AE, Dixon LB, Newby PK, Tucker KL, Krebs-Smith SM, Guenther PM (2007) Dietary patterns: challenges and opportunities in dietary patterns research: an experimental biology workshop, April 1, 2006. J Am Dietetic Assoc 107(7):1233–1239
Fransen HP, Fransen HP, May AM, Stricker MD, Boer JMA (2014) A posteriori dietary patterns: how many patterns to retain? J Nutr 144(8):1274–1282
Schulze MB, Hoffmann K, Kroke A, Boeing H (2003) An approach to construct simplified measures of dietary patterns from exploratory factor analysis. Br J Nutr 89(3):409–418
Wrigley CF, Neuhaus JO (1955) The matching of two sets of factors. Am Psychol 10:418–419
Tucker LR (1951) A Method for Synthesis of Factor Analysis Studies. Personal Research Section Report No 984, Department of the Army: Washington, DC
Lorenzo-Seva U, Berge JMFT (2006) Tucker’s congruence coefficient as a meaningful index of factor similarity. Methodology 2(2):57–64
Akoglu H (2018) User’s guide to correlation coefficients. Turk J Emerg Med 18(3):91–93
Cohen J (1968) Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 70(4):213–220
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Jakobsen JC, Gluud C, Wetterslev J, Winkel P (2017) When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol 17(1):162
Rubin DB, Schenker N (1991) Multiple imputation in health-care databases: an overview and some applications. Stat Med 10(4):585–598
Khani BR, Ye W, Terry P, Wolk A (2004) Reproducibility and validity of major dietary patterns among Swedish women assessed with a food-frequency questionnaire. J Nutr 134(6):1541–1545
Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC (1999) Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 69(2):243–249
Newby PK, Tucker KL (2004) Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 62(5):177–203
Hillier SE, Olander EK (2017) Women’s dietary changes before and during pregnancy: a systematic review. Midwifery 49:19–31
Faria-Schützer DB, Surita FG, Rodrigues L, Turato ER (2018) Eating behaviors in postpartum: a qualitative study of women with obesity. Nutrients 10(7):885
Ding D, Rogers K, van der Ploeg H, Stamatakis E, Bauman AE (2015) Traditional and emerging lifestyle risk behaviors and all-cause mortality in middle-aged and older adults: evidence from a large population-based Australian cohort. PLOS Med 12(12):e1001917
Loef M, Walach H (2012) The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med 55(3):163–170
Dennison RA, Ward RJ, Griffin SJ, Usher-Smith JA (2019) Women’s views on lifestyle changes to reduce the risk of developing Type 2 diabetes after gestational diabetes: a systematic review, qualitative synthesis and recommendations for practice. Diabet Med 36(6):702–717
Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL (2020) Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 369:m1361
Krompa K, Sebbah S, Baudry C, Cosson E, Bihan H (2020) Postpartum lifestyle modifications for women with gestational diabetes: a qualitative study. Eur J Obst Gynecol Reproduct Biol 252:105–111
Svensson L, Nielsen KK, Maindal HT (2018) What is the postpartum experience of Danish women following gestational diabetes? A qualitative exploration. Scand J Caring Sci 32(2):756–764
Zulfiqar T, Lithander FE, Banwell C, Young R, Boisseau L, Ingle M, Nolan CJ (2017) Barriers to a healthy lifestyle post gestational-diabetes: an Australian qualitative study. Women Birth 30(4):319–324
Lie MLS, Hayes L, Lewis-Barned NJ, May C, White M, Bell R (2013) Preventing Type 2 diabetes after gestational diabetes: women’s experiences and implications for diabetes prevention interventions. Diabet Med 30(8):986–993
Giskes K, Avendano M, Brug J, Kunst AE (2010) A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes Rev 11(6):413–429
Darmon N, Drewnowski A (2008) Does social class predict diet quality? Am J Clin Nutr 87(5):1107–1117
Appelhans BM, Milliron BJ, Woolf K, Johnson TJ, Pagoto SL, Schneider KL, Whited MC, Ventrelle JC (2012) Socioeconomic status, energy cost, and nutrient content of supermarket food purchases. Am J Prev Med 42(4):398–402
Pechey R, Monsivais P (2016) Socioeconomic inequalities in the healthiness of food choices: exploring the contributions of food expenditures. Prev Med 88:203–209
Pechey R, Jebb SA, Kelly MP, Almiron-Roig E, Conde S, Nakamura R, Shemilt I, Suhrcke M, Marteau TM (2013) Socioeconomic differences in purchases of more vs. less healthy foods and beverages: analysis of over 25,000 British households in 2010. Soc Sci Med (1982) 92(100):22–26
Arabshahi S, Lahmann PH, Williams GM, Marks GC, van der Pols JC (2011) Longitudinal change in diet quality in Australian adults varies by demographic, socio-economic, and lifestyle characteristics. J Nutr 141(10):1871–1879
Nguyen HH, Wu F, Oddy WH, Wills K, Brennan-Olsen SL, Jones G, Winzenberg T (2019) Dietary patterns and their associations with socio-demographic and lifestyle factors in Tasmanian older adults: a longitudinal cohort study. Eur J Clin Nutr 73(5):714–723
Prevost AT, Whichelow MJ, Cox BD (1997) Longitudinal dietary changes between 1984–5 and 1991–2 in British adults: association with socio-demographic, lifestyle and health factors. Br J Nutr 78(6):873–888
Kant AK (2004) Dietary patterns and health outcomes. J Am Diet Assoc 104(4):615–635
Thorpe MG, Milte CM, Crawford D, McNaughton SA (2019) Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J 18(1):67
Han CY, Colega M, Quah EPL, Chan YH, Godfrey KM, Kwek K, Saw S-M, Gluckman PD, Chong Y-S, Chong MF-F, on behalf of the Gsg (2015) A healthy eating index to measure diet quality in pregnant women in Singapore: a cross-sectional study. BMC Nutr 1(1):39
Finger JD, Tylleskär T, Lampert T, Mensink GBM (2013) Dietary Behaviour and Socioeconomic Position: The Role of Physical Activity Patterns. PLoS ONE 8(11):e78390
Inskip H, Baird J, Barker M, Briley AL, D’Angelo S, Grote V, Koletzko B, Lawrence W, Manios Y, Moschonis G, Chrousos GP, Poston L, Godfrey K (2014) Influences on adherence to diet and physical activity recommendations in women and children: insights from six European studies. Ann Nutr Metab 64(3–4):332–339
Konttinen H, Sarlio-Lähteenkorva S, Silventoinen K, Männistö S, Haukkala A (2013) Socio-economic disparities in the consumption of vegetables, fruit and energy-dense foods: the role of motive priorities. Public Health Nutr 16(5):873–882
Giskes K, Turrell G, Patterson C, Newman B (2002) Socio-economic differences in fruit and vegetable consumption among Australian adolescents and adults. Public Health Nutr 5(5):663–669
Chatzi L, Mendez M, Garcia R, Roumeliotaki T, Ibarluzea J, Tardón A, Amiano P, Lertxundi A, Iñiguez C, Vioque J, Kogevinas M, Sunyer J (2012) Mediterranean diet adherence during pregnancy and fetal growth: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr 107(1):135–145
Hillesund ER, Bere E, Haugen M, Øverby NC (2014) Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth—a study performed in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr 17(9):1909–1918
Fowles ER, Bryant M, Kim S, Walker LO, Ruiz RJ, Timmerman GM, Brown A (2011) Predictors of dietary quality in low-income pregnant women: a path analysis. Nurs Res 60(5):286–294
Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW (2009) Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 109(6):1004–1011
Bodnar LM, Siega-Riz AM (2002) A Diet Quality Index for Pregnancy detects variation in diet and differences by sociodemographic factors. Public Health Nutr 5(6):801–809
Bassett-Gunter RL, Levy-Milne R, Naylor PJ, Symons Downs D, Benoit C, Warburton DE, Blanchard CM, Rhodes RE (2013) Oh baby! Motivation for healthy eating during parenthood transitions: a longitudinal examination with a theory of planned behavior perspective. Int J Behav Nutr Phys Activity 10:88
Patrick H, Nicklas TA (2005) A review of family and social determinants of children’s eating patterns and diet quality. J Am Coll Nutr 24(2):83–92
van der Velde LA, Schuilenburg LA, Thrivikraman JK, Numans ME, Kiefte-de Jong JC (2019) Needs and perceptions regarding healthy eating among people at risk of food insecurity: a qualitative analysis. Int J Equity Health 18(1):184
Bellows-Riecken KH, Rhodes RE (2008) A birth of inactivity? A review of physical activity and parenthood. Prev Med 46(2):99–110
Olson CM (2005) Tracking of food choices across the transition to motherhood. J Nutr Educ Behav 37(3):129–136
Darvill R, Skirton H, Farrand P (2010) Psychological factors that impact on women’s experiences of first-time motherhood: a qualitative study of the transition. Midwifery 26(3):357–366
Chen LW, Low YL, Fok D, Han WM, Chong YS, Gluckman P, Godfrey K, Kwek K, Saw SM, Soh SE, Tan KH, Chong MF, van Dam RM (2014) Dietary changes during pregnancy and the postpartum period in Singaporean Chinese, Malay and Indian women: the GUSTO birth cohort study. Public Health Nutr 17(9):1930–1938
Lynch J, Smith GD (2005) A life course approach to chronic disease epidemiology. Annu Rev Public Health 26:1–35
Marchioni DML, Latorre MdRDdO, Eluf-Neto J, Wünsch-Filho V, Fisberg RM (2005) Identification of dietary patterns using factor analysis in an epidemiological study in São Paulo. Sao Paulo Med J 123(3):124–127
Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK (1995) Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol 24(2):389–398
Santiago SE, Park GH, Huffman KJ (2013) Consumption habits of pregnant women and implications for developmental biology: a survey of predominantly Hispanic women in California. Nutr J 12(1):91
McGowan CA, McAuliffe FM (2012) Maternal nutrient intakes and levels of energy underreporting during early pregnancy. Eur J Clin Nutr 66(8):906–913
Acknowledgements
The authors would like to thank the study participants and the GUSTO study group, which includes Airu Chia, Allan Sheppard, Amutha Chinnadurai, Anna Magdalena Fogel, Anne Eng Neo Goh, Anne Hin Yee Chu, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit Froukje Philipp Broekman , Bobby Kyungbeom Cheon, Boon Long Quah, Candida Vaz, Chai Kiat Chng, Cheryl Shufen Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Ciaran Gerard Forde, Claudia Chi, Daniel Yam Thiam Goh, Dawn Xin Ping Koh, Desiree Y. Phua, Doris Ngiuk Lan Loh, E Shyong Tai, Elaine Kwang Hsia Tham, Elaine Phaik Ling Quah, Elizabeth Huiwen Tham, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Fabian Kok Peng Yap, Faidon Magkos, Falk Müller-Riemenschneider, George Seow Heong Yeo, Hannah Ee Juen Yong, Helen Yu Chen, Heng Hao Tan, Hong Pan, Hugo P S van Bever, Hui Min Tan, Iliana Magiati, Inez Bik Yun Wong, Ives Yubin Lim, Ivy Yee-Man Lau, Izzuddin Bin Mohd Aris, Jeannie Tay, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Jia Xu, Joanna Dawn Holbrook, Joanne Su-Yin Yoong, Joao Nuno Andrade Requicha Ferreira, Johan Gunnar Eriksson, Jonathan Tze Liang Choo, Jonathan Y. Bernard, Jonathan Yinhao Huang, Joshua J. Gooley, Jun Shi Lai, Karen Mei Ling Tan, Keith M. Godfrey, Kenneth Yung Chiang Kwek, Keri McCrickerd, Kok Hian Tan, Kothandaraman Narasimhan, Krishnamoorthy Naiduvaje, Kuan Jin Lee, Leher Singh, Li Chen, Lieng Hsi Ling, Lin Lin Su, Ling-Wei Chen, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael J. Meaney, Michelle Zhi Ling Kee, Min Gong, Mya Thway Tint, Navin Michael, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Peter David Gluckman, Pratibha Keshav Agarwal, Priti Mishra, Queenie Ling Jun Li, Rob Martinus van Dam, Salome A. Rebello, Sambasivam Sendhil Velan, Seang Mei Saw, See Ling Loy, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shiao-Yng Chan, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Stephen Chin-Ying Hsu, Sue-Anne Ee Shiow Toh, Suresh Anand Sadananthan, Swee Chye Quek, Varsha Gupta, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Wei Wei Pang, Wen Lun Yuan, Yanan Zhu, Yap Seng Chong, Yin Bun Cheung, Yiong Huak Chan, Yung Seng Lee.
Funding
This research was supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding was provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042), NIHR Southampton 1000DaysPlus Global Nutrition Research Group (17/63/154) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus + Programme Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP and ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP) and the British Heart Foundation (RG/15/17/3174). None of the funding bodies identified had any role in the design of the study, in data collection, or in analysis, interpretation or writing the manuscript.
Author information
Authors and Affiliations
Contributions
KMG, KHT, LPCS, YSC and SYC: designed and led the GUSTO study; YQL and MF-FC designed the present study; MC, SLL and NP: collected and cleaned the data; YQL: performed the statistical analysis; RS, BCT, JSL and MF-FC: provided statistical input; YQL and MF-FC: wrote the manuscript and had primary responsibility for the final content; KMG, KHT, LPCS, FMR, YSC, JGE, SYC, MF-FC, BCT, RS, JSL, NP: reviewed the manuscript for intellectual content; and all authors: read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
KMG and Y-SC have received reimbursements for speaking at conferences sponsored by companies selling nutritional products and are part of an academic consortium that has received research funding from Abbott Nutrition, Nestle, and Danone. All other authors declare no competing interest.
Ethical standards
The GUSTO study has received ethical approval from the Institutional Review Board of KKH and NUH, and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all study participants prior to their inclusion in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, Y.Q., Colega, M., Sugianto, R. et al. Tracking of dietary patterns between pregnancy and 6 years post-pregnancy in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study. Eur J Nutr 61, 985–1001 (2022). https://doi.org/10.1007/s00394-021-02703-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02703-z