Abstract
Purpose
There are no representative estimates of vitamin A deficiency (VAD) and risk of vitamin A (VA) dietary inadequacy in Indian children and adolescents. To evaluate, from national surveys, the prevalence of VAD measured by serum retinol concentrations (< 0.7 µmol/L or < 20 µg/dL), and the risk of VA dietary inadequacy and excess intake beyond the tolerable upper limit (TUL).
Methods
National and state-level VAD prevalence adjusted for inflammation was estimated in school-age children (5–9 years: 10,298) and adolescents (10–19 years: 9824) from the Comprehensive National Nutrition Survey (CNNS 2016-18). The risk of dietary inadequacy against age-specific average VA requirements, and excess intake against the TUL, was assessed from the National Sample Survey Office (NSSO 2014) data.
Results
Serum retinol concentrations increased with age (5–19 years) in both genders and were significantly lower in school-age children (1.02 µmol/L, CI: 1.01–1.03) compared to adolescents (1.13 µmol/L, CI 1.12–1.15). The inflammation-adjusted prevalence of VAD in school-age children and adolescents was 19.3% (CI 18.8–19.9) and 14.4% (CI 13.9–14.9) respectively, and this was > 20% in seven and four states for children and adolescents, respectively. The prevalence of VAD was significantly higher among children with lower socio-economic status. The risk of dietary VA inadequacy, from the NSSO survey, was 69 and 78% in children and adolescents, respectively. This risk reduced to 6 and 17% with VA fortified oil and milk intake, while the proportion of intakes exceeding the TUL became 6 and 0.5% in children and adolescents, respectively.
Conclusions
The national prevalence of VAD in school-age children and adolescents in India was just less than 20%. The risk of dietary VA deficiency is likely to decline substantially with VA fortified food intake, but a risk of excessive intake also begins to appear; therefore, a careful assessment of the risk of hypervitaminosis A is required at these ages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin A (VA) is vital for a plethora of biological activities ranging from the transduction of light in visual function to embryonic development to immune function and indeed the maintenance of overall health [1, 2]. Profound VA deficiency (VAD) can lead to a spectrum of eye pathologies ranging from a milder night blindness to more severe clinical conditions such as Bitot spots, corneal scars, keratomalacia and xerophthalmia, as well as an increased risk of morbidity and mortality, especially in younger children [1, 3, 4]. The prevalence of severe or clinical VAD is therefore measured through clinical signs, but milder deficiency or sub-clinical VAD is assessed by serum retinol concentrations that are lower than a diagnostic cutoff [5, 6]. It has been suggested that this sub-clinical VAD is a public health problem that requires VA supplementation when its prevalence in the population is > 20% [7, 8]. However, there are no recent representative or national estimates of the prevalence of sub-clinical VAD in Indian school-age children and adolescents.
A few isolated studies have reported 7–55% prevalence of sub-clinical VAD, based on serum retinol levels, in India among school-age children and adolescents (1–18 years) [9,10,11,12,13]. However, these studies were conducted between 15 and 30 years ago; dietary conditions in India have improved, and further, serum retinol concentrations were not adjusted for inflammation, which can lower their values and overestimate the prevalence of sub-clinical VAD. The recent Comprehensive National Nutrition Survey (CNNS, 2016–2018) [14] measured serum retinol and serum C-reactive protein (CRP), a marker of inflammation, in 1–19-year-old children and adolescents in a nationally representative sample, allowing for a broad evaluation of VAD in this group in India.
In 2020, the daily dietary VA requirement was published for Indians [15], providing both the estimated average requirement (EAR) which represents the population requirement, and the tolerable upper intake level (TUL). These two reference values, along with national VA intake data from the National Sample Survey Office (NSSO 2014) [16], allow for an evaluation of the population risk of dietary VA inadequacy as well as the risk of excess intake. Both VAD and the risk of dietary inadequacy can be compared, and recently, we have reported that the national prevalence of sub-clinical VAD among Indian preschool children (from the CNNS survey) was 15.7%, which was commensurate with the calculated risk of dietary VA inadequacy [17]. Finally, the fortification of milk and edible oil with VA has been operationalized in India from 2018 [18] and could offer a significant contribution to dietary preformed VA intake, which needs to be modelled.
In the present study, we evaluated the prevalence of inflammation adjusted sub-clinical VAD and the risk of dietary VA inadequacy in Indian school-age children and adolescents. The potential contribution of VA fortified milk and oil in either reducing the risk of dietary inadequacy or contributing to an excess intake, was also assessed.
Methodology
CNNS survey, serum retinol and CRP analysis
The Comprehensive National Nutrition Survey (CNNS 2016-18) was a community-based cross-sectional survey conducted among Indian children and adolescents in 29 states and union territory of Delhi of India, during February 2016 to October 2018, in collaboration with UNICEF-India and Population Council, under the supervision of the Ministry of Health and Family Welfare, Government of India. The detailed methodology of the sampling design is published elsewhere [14]. Briefly, a multi-stage, population proportional to size cluster sampling was done to enrol children (1–4 and 5–9 years) and adolescents (10–19 years), to adequately represent the national, state, male–female and urban–rural population. For biological sampling, 50% of all the children and adolescents who completed anthropometry were selected by systematic random sampling. Children/adolescents with physical deformity, cognitive disabilities, chronic illness, acute febrile/infectious illness, acute injury and pregnancy were excluded. Household socioeconomic and demographic characteristics and information on history of morbidity in the preceding two weeks, and anthropometric data of one child/adolescent per age group were collected from each household. The Wealth Index, based on possession of common household items and facilities, was computed as described in the National Family Health Survey-4 [19]. Access to facilities like drinking water, hand washing and sanitation was categorized based on the WHO/UNICEF Joint Monitoring Program guidelines [20]. Age-sex standardized height-for-age (HAZ), weight-for-height (WHZ), and BMI-for-age Z-scores were calculated using the WHO Growth Reference Standards [21].
The CNNS was conducted after obtaining due International Ethical approval from the Population Council’s International Review Board, New York, USA and National Ethical approval Post Graduate Institute of Medical Education and Research, Chandigarh, India [14]. Informed consent and assent procedures were described in detail in CNNS 2016-18 Report [14]. The details of biological sample collection, laboratory analysis, precision and limits of detection of serum retinol and CRP have been described previously [17]. A serum retinol cut-off of < 20 μg/dL (0.7 µmol/L) was taken to define sub-clinical VAD in both children and adolescents [7, 8]. The prevalence of VAD was adjusted for underlying inflammation based on serum CRP levels as described previously [17, 22]. Serum albumin concentration was measured using spectrophotometric Bromocresol purple (BCP) dye-binding method [14] and an albumin concentration < 3.5 g/dL was diagnosed as hypoalbuminemia [23].
A total of 38,355 school-age children (5–9 years) and 35,830 adolescents (10–19 years) were interviewed and anthropometric data collected. Out of this total sample, paired data on serum retinol and CRP measurements were available for 10,298 school-age children and 9824 adolescents (Fig. 1). The socio-demographic characteristics were similar among participants in whom anthropometric data were collected (total sample) and the study sample (with paired serum retinol and CRP), except that proportion of children included in the study sample was higher in 7–9-year-old compared to 5–6-year-old (61% vs 39%, Supplementary Table 1). Among school-age children, 20% were stunted, 24% were thin; about 10% had diarrhoea and 23% had fever two weeks prior to the survey. Among adolescents, 27 and 23% were either stunted or thin, respectively (Table 1). However, the prevalence of hypoalbuminemia (a biomarker for chronic dietary protein deficiency) was markedly low, 1.2 and 1.0%, respectively, in school-age children and adolescents (Table 1).
Dietary surveys (NSSO)
The National Sample Survey Office (NSSO) survey [16] was conducted in 29 states and 6 Union Territories of India during 2011–2012 across all socioeconomic strata. The survey covered 7469 villages and 5268 urban blocks, and data on monthly per-capita expenditure on household food purchases of 223 food items was collected. This survey had a wider coverage and used a food frequency for assessing the dietary intakes. Using the Indian Food Composition Tables [24], the quantities of different foods purchased by the household were converted to their VA as retinol activity equivalents (RAEs/d), and this was adjusted for the number of members in the household and the use of consumption units (CUs) for the individual quantity of intake [25]. To obtain the VA intake (RAE/CU/day), the CUs used were 0.8 for children aged 5–9 y and for adolescents aged 10–12 y (boys- 1.0; girls- 0.9) and 13–19 y (boys- 1.1; girls- 0.9), respectively. To clean the data, the lower 0.5% and the upper 99.5% of the energy intake data were censored.
Assessment of risk of dietary inadequacy of VA
The prevalence of risk of dietary inadequacy of VA (%) was assessed as described previously [17]. Briefly, the risk of dietary inadequacy of VA was assessed by comparing the distribution of VA intakes with the distribution of estimated average age-specific requirements of VA [15, 26]. The age-specific EARs of VA (RAE μg/day) were used for children and adolescent [15]. In a normal healthy population with adequate access to foods, the risk of inadequacy, with reference to the EAR, is expected to be 50% [17, 26].
Contribution of fortified milk and oil to VA intakes and risk of exceeding the TUL
Since the diet survey referred to above was performed before the gazetting of the Indian regulations on the mandatory VA fortification of oil (6.0–9.9 μg retinol/g oil) and voluntary fortification of milk (27–45 μg retinol/100 mL milk) in 2018 [27], this could contribute a significant present intake of preformed VA. Therefore, we evaluated the theoretical reduction in the prevalence of the risk of dietary inadequacy and the risk of the intake exceeding the TUL, considering the habitual (fortified) oil and milk consumption of children and adolescents, as described previously [17, 26]. For this purpose, the habitual intake of milk (128.1 ml/day; contributing an additional 46.0 RAE/day, if fortified) and oil (22.8 g/day; contributing an additional 184 RAE/day, if fortified) were derived from NSSO survey [16], to calculate a summed additional intake from these fortified foods. The reduction in the risk of dietary inadequacy was recalculated using the probability method described above [17, 26]. The age-specific TULs (5–8 y-900 RAE; 9–13 y-1700 RAE; 14–18 y-2800 RAE and 19 y-3000 RAE) were considered in computing the risk of the fortified intake exceeding the TUL [15, 26].
Statistical analyses
Statistical analyses were conducted using SPSS statistical package (version 23, SPSS Inc., Chicago, IL, USA) and R version 4.0.2 (R Core Team, 2020, Vienna, Austria). Appropriate sampling weights were used wherever indicated to ensure representativeness of the estimates at the national/ state level as well as at the local level, such as rural, urban and urban slum areas in metropolitan cities. The demographic characteristics of the study sample were compared in terms of proportion against the same characteristics in the total CNNS survey to rule out possible selection bias due to nested sampling. Serum retinol concentrations were adjusted for the CRP concentration by the probability method of correction for inflammation [17, 22]. The prevalence of sub-clinical VAD along with its 95% confidence intervals (CIs) was estimated for the country as well as for states. Sub-group analyses were also performed to understand urban–rural, age and sex, socio-demographic and WASH (water, sanitation and hygiene) differentials. Serum retinol concentrations and dietary intake of VA were presented as geometric mean (GM) and geometric SD (GSD), since their distributions were skewed. The relation of VAD prevalence with age group in different states and VAD prevalence with state or regional gross domestic product [28] was assessed using the Spearman rank-order correlation.
Results
Serum retinol concentration and prevalence of VAD by age and sex
The GM of serum retinol concentrations was significantly lower in school-age children (1.02 µmol/L, CI 1.01–1.03) compared to adolescents (1.13 µmol/L, CI 1.12–1.15) (Table 2). The GM of serum retinol concentration increased with age in both genders from 5 to 19 years, but this, or the inflammation-adjusted VAD prevalence, was independent of gender within each age group (Table 2). The overall (without consideration of age bands or gender) prevalence of inflammation-adjusted VAD in school-age children was significantly higher (19.3%, CI 18.8–19.9) than that in adolescents (14.4%, CI 13.9–14.9) (Table 2). The extent of increase in serum retinol concentrations in girls tended to plateau at 15 years, while it continued to increase in boys (Fig. 2).
The prevalence of sub-clinical VAD was significantly higher among rural (14.8%, CI 14.2–15.3) compared to urban (13.2%, CI 12.4–14.1) adolescents (Table 3). The prevalence of VAD was also significantly higher among school-age children and adolescents with poorest wealth index (26.7%, CI 25.1–28.3; 17.8%, CI 16.7–19.0) and unimproved sanitation facility (23.6%, CI 22.7–24.5; 18.9%, CI 18.0–19.7), and adolescents with lower maternal education (11.7%, CI 11.0–12.4) (Table 3). Severely stunted adolescents (18.1%, CI 15.9–20.5) had significantly higher VAD than non-stunted (14.7%, CI 14.2–15.3) or moderately stunted (13.8%, CI 12.8–14.9) adolescents (Table 3).
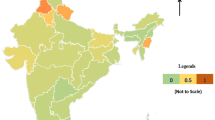
The point prevalence of VAD was > 20% in seven states for school-age children (Mizoram, Jharkhand, Telangana, Chhattisgarh, Uttar Pradesh, Bihar, New Delhi) and four states for adolescents (Jharkhand, Mizoram, Telangana, Uttar Pradesh) (Fig. 3). If the lower 95% CI was considered as the cutoff metric instead of the point prevalence value (meaning that the prevalence had to be significantly greater than 20%), the same seven states for school-age children, but only two states for adolescents (Mizoram, Telangana) had a VAD prevalence that needed some intervention (Fig. 3).
A significant positive correlation of prevalence of VAD was found between the age ranges of 5–9 years and 10–19 years (r = 0.858, p < 0.001) across all states (Supplementary Fig. 1). Further, though not significant, a decreasing trend in VAD prevalence with increasing state GDP (5–9 years: r = − 0.197, p = 0.297; 10–19 years: r = − 0.241, p = 0.199) was observed among both age groups (Supplementary Fig. 2).
Dietary inadequacy of VA in children and adolescents
The overall (regardless of urban or rural residence) average VA intake of 5–9 y and 10–19 y old children and adolescents from the NSSO survey was 179 and 221 μg RAEs/d (GM) with a GSD of 2.0 and 2.0 μg RAEs/d, respectively, yielding a prevalence of risk of inadequate intake of 69 and 78%, respectively (Table 4). Since the expected prevalence of risk of an inadequate dietary intake should be 50% in a healthy population, based on the overlapping distributions of requirement and intake in normalcy, the ‘real’ risk of dietary inadequacy in the population would be that proportion which is over 50%. This would be the prevalence of dietary VA inadequacy that should reasonably relate to the estimate of VAD based on a biomarker. The risk of dietary VA inadequacy, therefore, was 19 and 28%, respectively, for children and adolescents.
The average oil and milk consumption (GM ± GSD) was 19.0 ± 1.7 g/d and 108 ± 3.8 mL/d in children and 24.0 ± 1.7 g/d and 133 ± 3.8 mL/d in adolescents, respectively. The theoretical additional VA intake from these sources was calculated based on the individual intake of VA fortified oil alone, or fortified oil and milk together. The resultant total dietary VA intake was then evaluated for the risk of dietary inadequacy in children. With the additional retinol intake from oil fortification alone, the risk of dietary VA inadequacy dropped to 33% and 47% in children and adolescents, while with fortified oil and milk intake considered together, the risk of dietary VA inadequacy dropped to 6 and 17%, respectively. However, the risk of exceeding the TUL simultaneously increased to 4–8% in children while it was < 1.6% in adolescents (Table 4).
Discussion
The prevalence of sub-clinical VAD, adjusted for inflammation, in a nationally representative sample of 5–9-year-old children (19.3%) and adolescents (14.4%) was lower than 20%, indicating that VAD in these age groups is not a public health problem in India. The prevalence of VAD was, however, higher in children and adolescents with poor socioeconomic status. There was geographical variation, as seven (for school-age children) and two states (for adolescents) demonstrated a VAD prevalence that was significantly greater than > 20%. There are no contemporary prevalence estimates of VAD available for these age groups in India, except a few studies which reported VAD prevalence of 7–55% in Indian children [12, 13]. From the same CNNS survey data, the prevalence of VAD in under-five children, the most vulnerable group, was only 15.7% [17], which is in line with the VAD prevalence in older groups, where this might be slightly higher owing to higher requirements.
The risk of dietary inadequacy against age and gender-specific EAR was 70–80%. It is important to note that the assessment of the risk of dietary inadequacy is based on probability, where it is expected that in a healthy normal population, with a normal variability in requirement, this risk should be 50%. Therefore, direct comparisons of the proportion of the risk of dietary inadequacy with the prevalence of sub-clinical or biomarker-based deficiency should not be made. The theoretical frameworks that underlie these assessments of VA inadequacy are different. If comparisons between the risk of dietary inadequacy and the prevalence of sub-clinical deficiency must be made, then the comparable risk of dietary inadequacy is the proportion that is > 50%. Here, this translates to a risk of 20–30% which was somewhat comparable to the observed biomarker-based or sub-clinical VAD that was between 14 and 19%.
There are many variables in this comparison. First, it is important to note that VA is stored in the liver and utilized during periods of dietary deficits and that the dietary surveys cannot capture such seasonal variation in intakes (for example, mango, with a high carotene content, is widely consumed in India during summer). This could account for some of the expected difference between the risk of dietary inadequacy compared to biomarker-based deficiency. In agreement, a study in lactating Brazilian women found 5% VAD based on serum retinol deficiency when the risk of dietary inadequacy was 58% [29].
A second variable that could affect comparisons between the risk of dietary inadequacy and prevalence of sub-clinical VAD is that the EAR values represent a central value in a distribution of normal requirements, and it is possible that the distribution could get wider if cost-less adaptation to lower intakes occur. As an example, a recent report of 3 different clinical trials found that the VA intakes needed to maintain the liver VA stores were lower than the current EAR values, which could be due to physiological adaptations or incorrect factorial assessments of the requirement [30]. Interestingly, in the present study, the dietary inadequacy of VA was higher in adolescents (78%) compared to school-age children (70%), yet the former had a lower prevalence of biochemical VAD (14%), this could be a reflection of higher EARs (determined by a factorial method) recommended for this age group, against which dietary inadequacies are assessed.
A third variable affecting comparisons is the assumed cutoff for serum retinol (< 0.7 µmol/L or < 20 µg/dL) that is used in the determination of sub-clinical VAD. This cutoff is based on a small number of studies, and it has been suggested that the interpretation of population VAD status depends on the availability of reference data, preferably derived from ‘elite’ groups from within the population itself [31]. In addition, ethnic differences in serum retinol levels have been reported; Afro- and Asian-Americans have been shown to have lower serum retinol concentrations compared to Caucasians [32]. Hence, in the context of observed age, gender and ethnicity-related differences in serum retinol from multiple countries, a single serum retinol cutoff (0.7 µmol/L) to define VAD across all age groups may not be appropriate [7, 33]. This is particularly important, as the prevalence estimates based on these cutoffs guide national nutrition policies (like supplementation or fortification programs). Indeed, the higher prevalence of VAD in children compared to adolescents in the present study could be a reflection of poor specificity of serum retinol cutoffs, rather than the actual risk of VAD.
The wide variations in the VAD prevalence in Indian states are consistent with the regional dietary practices. Further, the higher VAD prevalence in children and adolescents with poor socioeconomic indicators is to be expected, considering the lower nutrient-quality of foods in their dietary intake and their higher morbidity. That the prevalence of VAD correlated with each of these age groups across the states as well as the negative correlation trend of VAD with the states’ GDP, suggests an important role of socioeconomic status. Therefore, holistic societal strategies are needed to improve the quality of life, including the reduction of morbidity which predisposes children to multiple nutrient deficiencies and affects their growth.
In the present study, serum retinol concentrations increased, and VAD prevalence decreased in either gender between 5 and 19 years, except in females where the serum retinol concentrations tended to decrease beyond the age of 11 years. This is similar to a study in Cambodian children and adolescents (6–18 y) which reported similar trends of increased serum retinol with age, the increase being proportionally higher in boys than in girls [34]. Further, in the National Health and Nutrition Examination Survey (NHANES) survey, serum retinol concentrations in young girls (4–11 y) were found to be 0.07 µmol/L lower compared to boys [35]. It has also been reported that serum retinol concentrations were lower in adult females compared to males [36,37,38].
The retinol fortification of oil and milk in India, which is being rapidly scaled up since 2018, will contribute to the intakes of preformed VA [18]. When the intake of preformed VA from either fortified oil and/or milk is factored in either individually or in combination, the risk of dietary inadequacy reduced substantially, more so in children (6%) compared to adolescents (17%), however, the risk of excessive intake beyond the TUL was increased to 3–6% in children, while it was 1% in adolescents. These results are consistent with our previous estimates of dietary inadequacy and excessive intakes in under 5-year-old children due to additional intake from fortified foods [17]. However, there could be a potential social inequity in exposure to risk with fortified foods, as children with high SES might be affected more due to higher intakes of milk and oil, in addition to their habitual intakes, including from other fortified health beverages.
The results of the present analyses have profound implications for VA food fortification in India, as there is a proportion of children with some risk of excess intake, at least in some sections of the society. Indeed, in countries with long-term successful VA intervention including that of fortification of multiple foods, the risk of hypervitaminosis has already been recognised [39, 40]. VA in high doses is also known to interfere with other nutrients like calcium, to cause bone resorption and inhibit bone formation. A high intake of VA (retinyl palmitate) has been associated with the development of osteoporosis by antagonizing the rapid intestinal calcium response to physiological levels of vitamin D [41]. Studies in many Western countries have suggested that the current levels of VA supplementation and food fortification may need to be reassessed [42].
The need and definition of regulatory levels for food fortification are also dependent on three important considerations. First is emerging evidence which suggests that the daily VA requirements may be lower than previously thought [30, 43]. Second, the ability of dietary carotene conversion to meet the daily requirement should be given more importance, since the regular consumption of vegetables and fruits have been shown to improve VA status [44, 45]. Therefore, concerted nutrition-sensitive agriculture and nutrition education efforts should be made toward sustainable, affordable and wholesome food-based interventions (e.g., green leafy vegetables, dietary diversification). Third, a careful consideration of the ethical dimensions of public health, particularly of autonomy and equity, relating to ‘mandatory’ fortification is required, as this has been passed into law in India for oil fortification. While fortification does indeed reduce disparities when applied appropriately, health risk and disparities could be generated when mandatory fortification results in the intake exceeding the TUL in some socioeconomic sections.
Strengths and limitations
The strength of the present study is its use of a large, quality controlled, recent and representative national sample, and triangulation of sub-clinical VAD with the risk of dietary VA inadequacy from large national surveys done in different time periods. The limitation is that the biochemical and dietary data were not paired and data on retinol-binding protein concentration are not available.
In conclusion, these results, together with our previous findings on preschool-children, suggest that VAD is not a public health problem in India at the national level. There is a real possibility of the risk of exceeding the TUL for VA intake in a proportion of children; this calls for a careful assessment of implications of VA food fortification with regard to deficiency and excess intake. Clearly, a dietary approach towards addressing VAD will be safer and ethically appropriate.
Abbreviations
- CNNS:
-
Comprehensive National Nutrition Survey
- CRP:
-
C-reactive protein
- EAR:
-
Estimated average requirement
- NSSO:
-
National Sample Survey Office
- RAE:
-
Retinol activity equivalents
- RDA:
-
Recommended dietary allowance
- SES:
-
Socioeconomic status
- TUL:
-
Tolerable upper limits
- VA:
-
Vitamin A
- VAD:
-
Vitamin A deficiency
- WASH:
-
Water, sanitation and hygiene
References
Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ (2016) Biomarkers of nutrition for development (BOND)-vitamin A review. J Nutr 146(9):1816S-1848S. https://doi.org/10.3945/jn.115.229708
Dowling JE (2020) Vitamin A: its many roles-from vision and synaptic plasticity to infant mortality. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 206(3):389–399. https://doi.org/10.1007/s00359-020-01403-z
Awasthi S, Peto R, Read S, Clark S, Pande V, Bundy D, team D, (2013) Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 381(9876):1469–1477. https://doi.org/10.1016/S0140-6736(12)62125-4
Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, Danaei G, Li G, White RA, Flaxman SR, Oehrle SP, Finucane MM, Guerrero R, Bhutta ZA, Then-Paulino A, Fawzi W, Black RE, Ezzati M (2015) Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health 3(9):e528-536. https://doi.org/10.1016/S2214-109X(15)00039-X
Laxmaiah A, Nair MK, Arlappa N, Raghu P, Balakrishna N, Rao KM, Galreddy C, Kumar S, Ravindranath M, Rao VV, Brahmam GN (2012) Prevalence of ocular signs and subclinical vitamin A deficiency and its determinants among rural pre-school children in India. Public Health Nutr 15(4):568–577. https://doi.org/10.1017/S136898001100214X
Vijayaraghavan K (2018) National control programme against nutritional blindness due to vitamin A deficiency: current status & future strategy. Indian J Med Res 148(5):496–502. https://doi.org/10.4103/ijmr.IJMR_1781_18
WHO (2009) Global prevalence of vitamin A deficiency in populations at risk 1995–2005. World Health Organization, Geneva, Switzerland
Reddy V, International Vitamin ACG (2002) History of the International Vitamin A Consultative Group 1975–2000. J Nutr 132(9 Suppl):2852S-2856S. https://doi.org/10.1093/jn/132.9.2852S
Pant I, Gopaldas T (1987) Effect of mega doses of vitamin A on the vitamin A status of underprivileged school-age boys (7–15 yr). Indian J Med Res 86:196–206
Pratinidhi AK, Shah U, Bapat VS (1987) Screening tests for vitamin A deficiency. Indian J Pediatr 54(4):563–569. https://doi.org/10.1007/BF02749054
Reddy V, Rao V, Arunjyothi RM (1989) Conjunctival impression cytology for assessment of vitamin A status. Am J Clin Nutr 50(4):814–817. https://doi.org/10.1093/ajcn/50.4.814
Sivakumar B, Vijayaraghavan K, Vazir S, Balakrishna N, Shatrugna V, Sarma KV, Nair KM, Raghuramulu N, Krishnaswamy K (2006) Effect of micronutrient supplement on health and nutritional status of schoolchildren: study design. Nutrition 22(1 Suppl):S1-7. https://doi.org/10.1016/j.nut.2005.07.009
Thankachan P, Rah JH, Thomas T, Selvam S, Amalrajan V, Srinivasan K, Steiger G, Kurpad AV (2012) Multiple micronutrient-fortified rice affects physical performance and plasma vitamin B-12 and homocysteine concentrations of Indian school children. J Nutr 142(5):846–852. https://doi.org/10.3945/jn.111.149021
CNNS (2019) Comprehensive National Nutrition Survey: National Report, Ministry of Health and Family Welfare (MoHFW). Government of India, New Delhi
ICMR-RDA (2020) Nutrient requirements and recommended dietary allowances for Indians—A report of the Expert Group of the Indian Council of Medical Research. NIN, New Delhi
NSSO (2014) National Sample Survey Office. Nutritional intake in India: 2011–12, NSS 68th round. National Statistical Organization, Government of India, New Delhi
Reddy GB, Pullakhandam R, Ghosh S, Boiroju NK, Tattari S, Laxmaiah A, Hemalatha R, Kapil U, Sachdev HS, Kurpad AV (2021) Vitamin A deficiency among children younger than 5 y in India: an analysis of national data sets to reflect on the need for vitamin A supplementation. Am J Clin Nutr 113(4):939–947. https://doi.org/10.1093/ajcn/nqaa314
FSSAI (2019) Food Safety and Standards Authority of India. Media upload—fortification touches 47% in packaged edible oil, 36.6% in milk [Internet]. Available from: https://fssai.gov.in/upload/media/FSSAI_News_Fortification_MoneyControl_28_08_2019.pdf.In. Accessed 3 Mar 2021
NFHS (2015–16) National Family Health Survey (NFHS-4), 2015–16: International Institute for Population Sciences (IIPS) and ICF, Mumbai: IIPS, India
WHO/UNICEF (2018) Joint Monitoring Programme (JMP) for Water Supply, Sanitation and Hygiene. JMP Methodology 2017 Update & SDG Baselines. https://washdata.org/sites/default/files/documents/reports/2018-04/JMP-2017-update-methodology.pdf. Accessed 3 Mar 2021
WHO (2006) World Health Organization child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. World Health Organization, Geneva, Switzerland
Ghosh S, Kurpad AV, Sachdev HS, Thomas T (2020) Inflammation correction in micronutrient deficiency with censored inflammatory biomarkers. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqaa285
Kim S, McClave SA, Martindale RG, Miller KR, Hurt RT (2017) Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg 83(11):1220–1227. https://doi.org/10.1177/000313481708301123
Longvah T, Ananthan R, Bhaskarachary T, Venkaiah K (2017) Indian food composition tables. National Institute of Nutrition, Indian Council of Medical Research, Hyderabad
Thimmayamma B, Rau P, Damayanti K (2016) Dietary assessment as part of nutritional status. In: Bamji MS, Krishnaswamy K, Brahmam GNV (eds) Textbook of human nutrition. Oxford & IBH Publishing Pvt Ltd, New Delhi (India), pp 121–151
IOM (2006) Institute of Medicine, National Academy of Sciences. Dietary reference intakes: the essential guide to nutrient requirements. National Academies Press, Washington (DC)
FSSAI (2018) Food Safety and Standards Authority of India. Gazette of India: notification, part-2: standards on fortification. Ministry of Health and Family Welfare, New Delhi (India)
GDP (2017–18) Gross Domestic Product. https://rbidocs.rbi.org.in/rdocs/Publications/PDFs/9T_HB15092019C2D5A67270EB4543949815919E8FE72F.PDF. Accessed 3 Mar 2021
da Silva A, de Sousa RA, Mendonca BMA, Silva D, Dimenstein R, Ribeiro K (2019) Relationship between the dietary intake, serum, and breast milk concentrations of vitamin A and vitamin E in a cohort of women over the course of lactation. Matern Child Nutr 15(3):e12772. https://doi.org/10.1111/mcn.12772
Sheftel J, Valentine AR, Hull AK, Fadjarwati T, Gannon BM, Davis CR, Tanumihardjo SA (2021) Findings in 3 clinical trials challenge the accuracy of the Institute of Medicine’s estimated average requirements for vitamin A in children and women. Am J Clin Nutr 113(5):1322–1331. https://doi.org/10.1093/ajcn/nqaa132
WHO (1996) World Health Organization. Indicators for assessing Vitamin A Deficiency and their application in monitoring and evaluating intervention programmes. WHO, Geneva (WHO/NUT/96.10)
Neuhouser ML, Rock CL, Eldridge AL, Kristal AR, Patterson RE, Cooper DA, Neumark-Sztainer D, Cheskin LJ, Thornquist MD (2001) Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J Nutr 131(8):2184–2191. https://doi.org/10.1093/jn/131.8.2184
McLaren DS, Frigg M (2001) Sight and Life guidebook on vitamin A in health and disease, Task Force Sight and Life Basel, Switzerland. ISBN 3-906412-07-5
Alvarez Uribe MC, Uscategui Penuela RM, Lopez Bedoya C, Baracaldo Barrera CM, Castro L, Noy Ballesteros V (2004) Plasma retinol concentration according to pubertal maturation in school children and adolescents of Medellin, Colombia. Eur J Clin Nutr 58(3):456–461. https://doi.org/10.1038/sj.ejcn.1601828
Lewis CJ, McDowell MA, Sempos CT, Lewis KC, Yetley EA (1990) Relationship between age and serum vitamin A in children aged 4–11 y. Am J Clin Nutr 52(2):353–360. https://doi.org/10.1093/ajcn/52.2.353
Olson JA (1984) Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst 73(6):1439–1444
Al-Saleh I, El-Doush I, Billedo G (2007) Age and gender-related reference values for serum dl-alpha-tocopherol and all-trans-retinol levels in Saudi population. Int J Vitam Nutr Res 77(5):326–335. https://doi.org/10.1024/0300-9831.77.5.326
Sivaprasad M, Shalini T, Reddy PY, Seshacharyulu M, Madhavi G, Kumar BN, Reddy GB (2019) Prevalence of vitamin deficiencies in an apparently healthy urban adult population: assessed by subclinical status and dietary intakes. Nutrition 63–64:106–113. https://doi.org/10.1016/j.nut.2019.01.017
Ford JL, Green JB, Haskell MJ, Ahmad SM, Mazariegos Cordero DI, Oxley A, Engle-Stone R, Lietz G, Green MH (2020) Use of model-based compartmental analysis and a super-child design to study whole-body retinol kinetics and vitamin A total body stores in children from 3 lower-income countries. J Nutr 150(2):411–418. https://doi.org/10.1093/jn/nxz225
Tanumihardjo SA, Gannon BM, Kaliwile C, Chileshe J (2015) Hypercarotenodermia in Zambia: which children turned orange during mango season? Eur J Clin Nutr 69(12):1346–1349. https://doi.org/10.1038/ejcn.2015.143
Johansson S, Melhus H (2001) Vitamin A antagonizes calcium response to vitamin D in man. J Bone Miner Res 16(10):1899–1905. https://doi.org/10.1359/jbmr.2001.16.10.1899
Michaelsson K, Lithell H, Vessby B, Melhus H (2003) Serum retinol levels and the risk of fracture. N Engl J Med 348(4):287–294. https://doi.org/10.1056/NEJMoa021171
Faye MH, Dieme MA, Idohou-Dossou N, Badiane A, Diouf A, Ndiaye Ndome NM, Tanumihardjo SA (2021) Adequate vitamin A liver stores estimated by the modified relative dose response test are positively associated with breastfeeding but not vitamin A supplementation in Senegalese urban children 9–23 months old: a comparative cross-sectional study. PLoS One 16(1):e0246246. https://doi.org/10.1371/journal.pone.0246246
Jayarajan P, Reddy V, Mohanram M (1980) Effect of dietary fat on absorption of beta carotene from green leafy vegetables in children. Indian J Med Res 71:53–56
Kalz F, Schafer A (1958) Vitamin A serum levels after ingestion of different vitamin A preparations. Can Med Assoc J 79(11):918–919
Acknowledgements
The CNNS study was conducted by the Ministry of Health and Family Welfare, Government of India, and the UNICEF, with support from the Mittal Foundation. The data were provided to Indian researchers after a data user workshop conducted by UNICEF, India. The views expressed here by the authors are in their individual capacity but not of the Institutions the authors belong to.
Funding
The Comprehensive National Nutrition Survey (CNNS 2016-18) was conducted by the Ministry of Health and Family Welfare, Government of India, and the UNICEF, with support from the Mittal Foundation.
Author information
Authors and Affiliations
Contributions
GBR, TS, SG, RP, NKB, BK, AL, UK, SD, RH, AVK and HSS performed initial statistical analyses on the CNNS data; further comments and iterations involved all authors. AVK and SG analysed the publicly available NSSO dietary intake data. GBR, TS, RP, and SG performed the initial iteration of the estimation of the distribution of requirements and analyses of dietary inadequacy. While GBR, TS, RP, AVK and HSS wrote and edited the manuscript, all authors were involved at every iteration of all analyses and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
HSS designed the draft protocol of the CNNS with consultancy support from the UNICEF, India. HSS, AL, UK and AVK were members of the Technical Advisory Committee of the CNNS, constituted by the Ministry of Health and Family Welfare of the Government of India, to oversee its conduct and analysis. HSS is a member of the World Health Organization Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health and member of Expert Groups of the Ministry of Health and Family Welfare on Nutrition and Child Health. AVK is a Nutrition Advisor to the Tata Trusts. SG has consultancy support for statistical analyses from UNICEF, India. There were no other conflicts to declare.
Ethical approval
The CNNS was conducted after obtaining due International Ethical approval from the Population Council’s International Review Board, New York, USA and National Ethical approval from Post Graduate Institute of Medical Education and Research, Chandigarh, India [14].
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reddy, G.B., Shalini, T., Ghosh, S. et al. Prevalence of vitamin A deficiency and dietary inadequacy in Indian school-age children and adolescents. Eur J Nutr 61, 197–209 (2022). https://doi.org/10.1007/s00394-021-02636-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02636-7