Abstract
Purpose
Prenatal vitamin D (VitD) deficiency influences children’s health in later life. We aimed to test the associations between maternal VitD status in each of the three trimesters of pregnancy and cord blood 25(OH)D concentrations in newborns.
Methods
Participants were pregnant women recruited from the Shanghai Birth Cohort (SBC) (n = 1100). Of all the participants, 946 completed the collection of venous blood at early (< 16 weeks, T1), mid- (24–28 weeks, T2), and late (32–34 weeks, T3) pregnancy as well as the corresponding cord blood in the newborns. Maternal serum 25(OH)D concentrations were measured by LC–MS/MS, and the information on confounding factors was obtained through a standardized questionnaire.
Results
The mean 25(OH)D concentrations at time points T1, T2, T3 in maternal blood and cord blood of the newborns were 26.31 ng/mL, 31.92 ng/mL, 35.62 ng/mL, and 19.77 ng/mL, respectively. Neonatal 25(OH)D level in cord blood was positively correlated with maternal serum 25(OH)D levels at each trimester, and the strongest correlation was found at time point T3.
Conclusion
Maternal 25(OH)D concentrations at each trimester were positively associated with neonatal VitD status in cord blood, and the strongest correlation was found in the late stage of pregnancy, which could be considered as a sensitive time window. Attention should be paid to the nutritional status of VitD during pregnancy to better prevent the VitD deficiency in neonates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin D (VitD) is an important micronutrient essential for bone growth and regulation of calcium homeostasis [1]. Pregnancy is a unique and demanding life stage in terms of VitD and calcium metabolism, due to the increased need for fetal development of mineralized structures, while maintaining optimal maternal status [2]. VitD deficiency in pregnant women is associated with pregnancy complications, such as pre-eclampsia [3, 4], gestational diabetes [5], and preterm birth [6, 7]. Poor VitD status in pregnant women results in neonatal VitD insufficiency, because 25-hydroxyvitamin D [25(OH)D] readily passes through the placenta [8, 9]. Additionally, prenatal VitD deficiency may be associated with asthma, type I diabetes, impaired development, heart failure, and metabolic disease in later life [1, 10,11,12].
Over the past decade, numerous studies have reported the effects of VitD status on health in adults, the elderly, and increasingly, pregnant women and newborns. It has been demonstrated that 25(OH)D concentrations in cord blood of newborns are positively associated with maternal serum 25(OH)D levels in pregnancy [13, 14]. However, this association was not investigated in detail at each of the three pregnant trimesters (T1, T2, and T3).
Considering the long-term health problems led by VitD deficiency in early life, a better understanding of the longitudinal circulating VitD status throughout pregnancy in a “mother-infant blood set” is necessary. In this study, we aimed to test the associations between maternal VitD status in each of the three trimesters of pregnancy and cord blood 25(OH)D concentrations in the newborns, based on a large prospective Shanghai Birth Cohort. Our study will supplement the relevant results in Chinese population.
Materials and methods
Study design and participants
The Shanghai Birth Cohort (SBC) is a prospective cohort recruiting women in early pregnancy from six participating hospitals between 2013 and 2016 in Shanghai, China. These six hospitals were located in four clusters. Two clusters are in the urban setting; one cluster in a suburban area; and one in a semi-rural area. A detailed description of research recruitment has been published previously [15]. In brief, women were recruited at booking for prenatal care in the first trimester (< 16 weeks of gestation). Before recruitment, written informed consent was obtained from the mothers, and trained nurses conducted face-to-face interviews. They were asked to come to the study office for the second visit when they came for the oral glucose tolerance test at 24–28 weeks of gestation. And at 32–36 weeks of gestation, women came for the third visit in conjunction with their routine prenatal care. At the delivery, women were contacted by the coordinators. Medical records were reviewed and extracted. At each visit, maternal blood was collected, followed by cord blood at delivery, to form a “mother-infant blood set”. Ethics approval was obtained from the Ethics Committees of both Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine and the other participating hospitals.
Measurements of 25(OH)D and data collection
We randomly selected 1100 pregnant women who have a blood sample at their first visit. Of all the pregnant women, 1086 completed 25(OH)D measurements at T1, 983 at T2, 960 at T3 and 946 in the newborn cord blood, and submitted the questionnaire at the same time (Fig. 1). The blood samples were centrifuged and stored at − 20 °C in the dark, and then analyzed using the sensitive liquid chromatography–tandem mass spectrometry (LC–MS/MS) analytical method. Following the procedure reported in our current study [16], the serum samples (100 μL) were deproteinized and precipitated using methanol, acetonitrile, zinc sulfate, and standards that included 25(OH)D2 (100 μg/mL, purity 98%), 25(OH)D3 (100 μg/mL, purity 98%), 25(OH)D2-d3 (internal standard [IS] for 25(OH)D2, 100 μg/ mL, purity 98%), 25(OH)D3-d6 (IS for 25(OH)D3, 0.5 mg, purity 95%) (Sigma, St. Louis, MO, USA). Standard reference materials SRM 2972 and SRM 972a were purchased from the National Institute of Standards and Technology (NIST). Mixed calibration standards were prepared at concentrations of 6.25/6.25, 12.5/12.5, 25/25, 50/50, 125/125, 250/250, and 500/500 nmol/L [25(OH)D2/25(OH)D3] in methanol. The standards were certified according to NIST SRM2972. All calibrators were treated as samples in each batch. In this assay, the level of sensitivity for LC–MS/MS assay was 0.05 ng/mL for 25(OH)D2, and 0.1 ng/mL for 25(OH)D3. Chromatographic separations were obtained using an Agilent Poroshell 120 EC-C18 (50 × 2.1 mm, 2.7 μm) column with a gradient of water (containing 0.1% formic acid) and methanol as the mobile phase at a flow rate of 0.5 mL/min. Multiple reaction monitoring (MRM) of the analyses was performed under electrospray ionization (ESI) in the positive mode at m/z 401.3 → 383.2 and 401.3 → 159.1 for 25(OH)D3, m/z 413.3 → 395.3 and 413.3 → 355.2 for 25(OH)D2, and m/z 404.3 → 386.3 and 416.4.3 → 398.3 for d3-25(OH)D3 and d3-25(OH)D2, respectively. The standard curve equation was determined from the data of the instrument test standard substances (Sigma standard substances). The relative standard deviation was less than 15%. At least two quality control measurements were made in each batch of samples. The intra- and inter-batch variation coefficients were less than 5% for both 25(OH)D3 and 25(OH)D2.
There is no widely accepted definition of VitD deficiency or insufficiency during pregnancy and in newborns. Therefore, we referred to the two reference values provided by the Institution of Medicine and Endocrine Society as the reference for our grouping. Based on the criteria of the Institution of Medicine, VitD deficiency in adults is defined as a 25(OH)D below 20 ng/ml [17]. According to the Endocrine Society Clinical Practice Guideline in 2011, at least 1500–2000 IU/d of VitD may be needed to maintain a blood level of 25(OH)D above 30 ng/ml for pregnant and lactating women [18]. Therefore, we classified the participant VitD status as sufficient (≥ 30 ng/mL), insufficient (20–29.9 ng/mL) or deficient (< 20 ng/mL) in our analysis. As for cord blood, according to the well-accepted recommendations of the general population and existing studies in newborns [8, 18], 25(OH)D concentration ≥ 20 ng/ml was treated as the recommended level.
Statistical analysis
Normally distributed serum 25(OH)D concentrations were expressed as ng/mL. We first determined the percentiles of 25(OH)D and the prevalence of VitD deficiency and insufficiency in mothers during pregnancy and in cord blood of newborns (Table 2). The baseline descriptive characteristics were presented as means ± SDs for continuous variables and as numbers and percentages for categorical variables (Table 1). Associations between demographic characteristics among tertiles of 25(OH)D concentrations in cord blood were investigated using one-way ANOVA for quantitative parameters and chi-squared test (or Fisher exact test when it was appropriate) for qualitative factors. A bar chart was used to show the trend of VitD level throughout pregnancy (Fig. 2). Pearson’s correlation coefficients were used to assess associations between continuous variables (Fig. 3). To model the predictive potential of maternal 25(OH)D concentrations during pregnancy on 25(OH)D status in cord blood, we performed a multivariable analysis based on potential confounders of conception (Table 4). Receiver operating characteristic (ROC) curves were used to evaluate the performance of the prediction model (Fig. 4). P < 0.05 were considered statistical significance. For the missing data, we use the available data to produce efficient parameter estimates. All analyses were performed using Empower (R) (www.empowerstats.com, X&Y solutions, Inc., Boston, MA, USA) and R (http://www.R-project.org).
Results
Demographic characteristics of the participants
The flow chart of the study protocol is shown in Fig. 1. None of the participants were included twice during the study. General characteristics of pregnant women and their infants are present in Table 1. Maternal age, maternal education, VitD supplementation, and birth season were different among tertiles of 25(OH)D concentrations in cord blood (P < 0.05). Younger maternal age, lower maternal education, lower frequency of VitD supplementation, and birth in autumn or winter were related to lower neonatal VitD status (Table 1). No statistically significant differences were found in household income, gravidity, gestational weight gain, children’s gender, or birth size among tertiles of 25(OH)D concentrations.
VitD status in pregnant mothers and cord blood
Table 2 shows the tertile values of 25(OH)D concentration at T1, T2, T3 and in cord blood. Overall, the prevalence of maternal VitD deficiency was 30.57%, 16.68%, and 12.49% at T1, T2, and T3, respectively, while in cord blood, the prevalence of VitD deficiency was up to 57.98% (Table 2). From T1 to T3, maternal VitD status had a continuously increasing trend in both VitD supplemented group and non-supplemented group (Fig. 2). While at birth, average 25(OH)D concentration in neonates was significantly lower than maternal VitD status at any trimester (P < 0.05). The mean values of 2 (OH)D between the two groups were different in each period.
Association between maternal circulating VitD status during pregnancy and neonatal VitD concentrations in cord blood
Table 3 shows our results of the linear fitting. According to the linear regression equation Y = bX + a (the values of a and b were shown in Table 3), when 25(OH)D concentrations was 20 ng/ml at T1, T2, and T3, the corresponding 25(OH)D concentration in cord blood was 18.15 ng/ml, 15.44 ng/ml and 13.22 ng/ml, respectively. And when 25(OH)D concentrations were 30 ng/ml at T1, T2, and T3, the corresponding 25(OH)D concentration in cord blood were 20.71 ng/ml, 19.13 ng/ml, and 17.49 ng/ml, respectively. Figures 3a, b, c show that serum 25(OH)D concentration in cord blood positively correlated with those at T1 (r2 = 0.085, P < 0.05), T2 (r2 = 0.213, P < 0.05), and T3 (r2 = 0.358, P < 0.05), while the strongest positive correlation was found during the T3 stage.
Table 4 shows our results of multivariate regression analysis, which revealed the association between maternal 25(OH)D concentrations during pregnancy and neonatal 25(OH)D concentrations at birth. With a 1 ng/mL increase in the maternal 25(OH)D concentrations at T1, T2, and T3, neonatal 25(OH)D concentration increased by approximately 0.25 ng/mL, 0.37 ng/mL, and 0.41 ng/mL (Table 4). After adjusting for covariates including maternal VitD supplementation and outdoor activities during gestation, birth season, maternal age, and maternal education, maternal 25(OH)D concentrations at any trimester remained associated with neonatal 25(OH)D concentration (P < 0.05) (Table 4). Newborns that were given birth by mothers with VitD insufficiency [25(OH)D < 30 ng/mL] during pregnancy were at a higher risk of VitD deficiency [25(OH)D < 20 ng/mL]. After adjusting for the birth season, maternal age, maternal education, VitD supplementation, and outdoor activities, the relative risks were 2.74 (95% CI 1.80–4.17) at T1, 4.48 (95% CI 2.91–6.91) at T2, and 13.55 (95% CI 7.52–24.40) at T3 (Table 4).
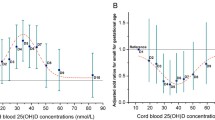
Figure 4 shows that the diagnostic efficiency of maternal 25(OH)D concentration on neonatal VitD deficiency reached the highest at T3 (AUC = 0.82, 95% CI 0.79–0.84), with sensitivity and specificity 0.81 and 0.72, respectively (Fig. 4).
Discussion
The prevalence of maternal or neonatal VitD deficiency and insufficiency has been extensively studied worldwide [3,4,5,6,7, 19,20,21]. The findings in our study contribute to the growing knowledge of VitD status during pregnancy in both mothers and neonates. Although it has been demonstrated that 25(OH)D concentrations in cord blood of newborns are positively associated with maternal serum 25(OH)D levels in pregnancy [13, 14, 22,23,24,25], most cohort studies included 25(OH)D concentrations in just one-time point in mid- or late pregnancy, only a French multicenter study studied VitD status during T1, T3 and in cord blood [13]. This French cohort study only presented 25(OH)D concentrations in each period and analyzed determinants of VitD deficiency. In contrast, our study focused on the correlation between maternal VitD status and neonatal 25 (OH) D concentration in cord blood, and their prediction effects on neonatal VitD deficiency, from which we could draw a sensitive time window. To the best of our knowledge, our study is the first longitudinal data including both maternal blood samples during three trimesters and neonatal blood samples at birth. Our main findings revealed that maternal 25(OH)D concentrations at each trimester were positively associated with neonatal VitD status in cord blood, and the strongest correlation was found in late pregnancy, which could be considered as a sensitive time window.
The data found in our study were agreed with other studies on VitD status in pregnant women and newborns in China [4, 7, 22,23,24,25], as well as our previous Shanghai Allergy Cohort Study on VitD status in newborns. Our findings have important implications for those living in China. First, the strongest correlation between maternal and cord blood 25(OH)D was found in late pregnancy. Besides, our results suggested that early pregnancy is the most vulnerable period for VitD deficiency. Consistent with our previous research [25], our study found a relatively low-frequency and small-dose VitD supplementation of pregnant women during their pregnancy. At the same time, our results showed that the frequency of VitD supplementation was different in different periods of pregnancy, and the increasing trend of VitD supplement frequency is consistent with that of maternal serum 25(OH)D concentrations, which could partly explain the evolution of VitD status during pregnancy beyond the known endogenous factors. Our results indicated that the positive correlation between maternal 25(OH)D and umbilical cord blood 25(OH)D increased from T1 to T3, with r value increased from 0.327 to 0.669, which may be explained by the fact that the late pregnancy is closest to delivery and the covariates are more consistent. We found a strong positive correlation between maternal serum 25(OH)D concentrations in the late pregnancy and neonatal 25(OH)D in cord blood (r = 0.669, P < 0.05), which basically consistent with other studies with the r value 0.64 in Bouillon et al. study [26] and 0.651 in Halicioglu et al. study [27]. In a Poland cohort [14], pre-delivery maternal venous blood and neonatal cord blood samples were collected, and the results indicated a strong correlation between maternal and neonatal 25(OH)D (r2 = 0.78, p < 0.0001), a r value much higher than our study, which suggested that the time of blood sample extraction was likely to affect the correlation results. Although our study showed that maternal 25(OH)D concentrations at T3 was more related to neonatal VitD status, it is also often suggested that the maternal VitD status in the early and mid- pregnancy are more likely to affect the children's health outcomes at birth and in the later life [28,29,30]. As a result, consideration should be given to make recommendations on VitD supplementation through pregnancy.
Meanwhile, in all mother-infant blood sets, 25(OH)D concentration in cord blood was lower by 9.2 ng/mL on average (approximately 50%), as compared to maternal levels, which is consistent with most studies worldwide, claiming that cord blood level was low than maternal venous blood level by 4.1–10.9 ng/mL [2, 7, 14, 27]. Physiological changes in pregnancy may account for this including variation in the vitamin D binding protein (VDBP) which has been shown to increase in pregnancy increase dramatically in pregnancy and thus influence the biologically and functionally active portion of VitD [31]. It is well documented that cord serum VDBP was half lower than that in mother [26], which may lead to an increase of free 25(OH)D. Therefore, although our measurement showed that the concentration of total 25(OH)D in cord blood was lower than that in maternal serum, a lower VDBP may lead to a higher free 25(OH)D in cord serum. It has been documented that free 25(OH)D could increase the activity of 1 α—hydroxyvitamin D in placenta and in fetal kidney [32], so that 1,25-dihydroxyvitamin D production can still be maintained at a high level in the fetus. Therefore, taking our present data into consideration, we should probably set the different thresholds for 25(OH)D concentration in pregnant women and cord blood. As for now, 20 ng/mL was still the recommended level and lower values signified VitD deficiency in cord blood [33]. Gould et al. [34] declared that cord blood 25(OH)D concentration which is necessary for early childhood growth and neurodevelopment ought to be > 10 ng/mL. There is still controversy about these thresholds, which needs to be confirmed by more large cohort studies and follow-up results.
A relatively large sample of mother-infant blood sets of maternal and neonatal 25(OH)D concentration throughout pregnancy are strengths of our study. Additionally, the longitudinal nature of the data collection, spanning multiple stages of pregnancy to birth is another strength. Due to the cohort design, only cord blood was drawn at delivery, which made it impossible to compare the maternal and neonatal 25(OH)D values at one time. Although this study was multi-center research with a representative population-based sample, it was only conducted in one city and not a national sample, which is one of the limitations of our study. Now, the results of this cohort can only represent the status in Shanghai. Shanghai is one of the first-tier cities in China, with a high-quality population, a developed economy, and better medical conditions. The participants in SBC are not the most vulnerable to VitD deficiency, thus general conclusions ought to be drawn with caution. In some remote or economically backward areas in China, pregnant women are more likely to suffer from VitD deficiency, which may occur throughout pregnancy. According to a study carried out in Guizhou [35], a comparatively remote region in China, the prevalence of VitD deficiency in pregnant women during early pregnancy (11–16 weeks of gestation) was up to 70%. For pregnant women with severe or long-term VitD deficiency, both cord blood and fetus may be affected. Although more data are needed to support this, it is suggested that targeted screening and proper supplementation are needed during early pregnancy. Currently, the latest Chinese dietary reference intakes recommend that the recommended nutrient intake (RNI) of VitD is 400 IU/d (10 µg/d) during pregnancy, with no difference among the three trimesters. RCTs in Canada [36], Ireland [37], and New Zealand [38] estimated that a minimum of 25 μg/d VitD/d might be needed to maintain newborn 25(OH)D at above 30 nmol/L, which may be related to better health outcomes. The daily intake of naturally VitD contained food, e.g., fatty fish, fish liver oil, and egg yolk, was insufficient in the Chinese diet. Given that VitD fortified food is rare used in China, VitD supplementation is the main source of VitD for pregnant women. Therefore, it is suggested that RNI of VitD for Chinese pregnant women should be increased, and an active supplementation plans should be implemented. More RCTs will help to determine the supplementation doses recommended.
In conclusion, neonatal 25(OH)D concentration in cord blood was positively correlated with maternal VitD status during pregnancy, especially in the late stage of pregnancy. It is suggested to strengthen the screening of VitD status in the third trimester of pregnancy. Considering that the level of VitD is influenced by regional factors and the degree of economic and cultural development. Attention should be paid to the nutritional status of VitD during pregnancy to better prevent the VitD deficiency in neonates.
References
Chang SW, Lee HC (2019) Vitamin D and health—the missing vitamin in humans. Pediatr Neonatol 60(3):237–244. https://doi.org/10.1016/j.pedneo.2019.04.007
Wheeler BJ, Taylor BJ, de Lange M et al (2018) A longitudinal study of 25-hydroxy vitamin D and parathyroid hormone status throughout pregnancy and exclusive lactation in New Zealand mothers and their infants at 45°S. Nutrients 10(1):E86. https://doi.org/10.3390/nu10010086
Magnus MC, Miliku K, Bauer A et al (2018) Vitamin D and risk of pregnancy related hypertensive disorders: mendelian randomisation study. BMJ 361:k2167. https://doi.org/10.1136/bmj.k2167
Zhao X, Fang R, Yu R et al (2017) Maternal Vitamin D Status in the late second trimester and the risk of severe Preeclampsia in Southeastern China. Nutrients 9(2):138. https://doi.org/10.3390/nu9020138
Xia J, Song Y, Rawal S et al (2019) Vitamin D status during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study in a multiracial cohort. Diabetes Obes Metab 21(8):1895–1905. https://doi.org/10.1111/dom.13748
Pérez-López FR, Pasupuleti V, Mezones-Holguin E et al (2015) Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 103(5):1278–1288. https://doi.org/10.1016/j.fertnstert.2015.02.019
Wang Y, Li H, Zheng M et al (2018) Maternal vitamin D deficiency increases the risk of adverse neonatal outcomes in the Chinese population: a prospective cohort study. PLoS ONE 13(4):e0195700. https://doi.org/10.1371/journal.pone.0195700
Kiely M, O’Donovan SM, Kenny LC et al (2017) Vitamin D metabolite concentrations in umbilical cord blood serum and associations with clinical characteristics in large prospective mother-infant cohort in Ireland. J Steroid Biochem Mol Biol 167:162–168. https://doi.org/10.1016/j.jsbmb.2016.12.006
Hollis BW, Wagner CL (2017) New insights into the vitamin D requirements during pregnancy. Bone Res 5:17030. https://doi.org/10.1038/boneres.2017.30
Shen SY, Xiao WQ, Lu JH et al (2018) Early life vitamin D status and asthma and wheeze: a systematic review and meta-analysis. BMC Pulm Med 18(1):120. https://doi.org/10.1186/s12890-018-0679-4
Miller KM, Hart PH, de Klerk NH et al (2017) Are low sun exposure and/or vitamin D risk factors for type 1 diabetes? Photochem Photobiol Sci 16(3):381–398. https://doi.org/10.1039/c6pp00294c
Larqué E, Morales E, Leis R et al (2018) Maternal and foetal health implications of vitamin D status during pregnancy. Ann Nutr Metab 72(3):179–192. https://doi.org/10.1159/000487370
Courbebaisse M, Souberbielle JC, Baptiste A et al (2019) Vitamin D status during pregnancy and in cord blood in a large prospective French cohort. Clin Nutr 38(5):2136–2144. https://doi.org/10.1016/j.clnu.2018.08.035
Wierzejska R, Jarosz M, Sawicki W et al (2017) Vitamin D concentration in maternal and umbilical cord blood by season. Int J Environ Res Public Health 14(10):E1121. https://doi.org/10.3390/ijerph14101121
Zhang J, Tian Y, Wang W et al (2019) Cohort profile: the Shanghai Birth Cohort. Int J Epidemiol 48(1):21–21g. https://doi.org/10.1093/ije/dyy277
Jiao X, Yuan Y, Wang X et al (2020) Development of a sensitive HPLC-MS/MS method for 25-hydroxyvitamin D(2) and D(3) measurement in capillary blood. J Clin Lab Anal 34(10):e23451. https://doi.org/10.1002/jcla.23451
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US) (2011)
Holick MF, Binkley NC, Bischoff-Ferrari HA et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930. https://doi.org/10.1210/jc.2011-0385
Baca KM, Simhan HN, Platt RW et al (2016) Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia. Ann Epidemiol 26(12):853–857. https://doi.org/10.1016/j.annepidem.2016.09.015
Andersen LB, Jørgensen JS, Jensen TK et al (2015) Vitamin D insufficiency is associated with increased risk of first-trimester miscarriage in the Odense Child Cohort. Am J Clin Nutr 102(3):633–638. https://doi.org/10.3945/ajcn.114.103655
Miliku K, Vinkhuyzen A, Blanken LM et al (2016) Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am J Clin Nutr 103(6):1514–1522. https://doi.org/10.3945/ajcn.115.123752
Zhu P, Tong SL, Hu WB et al (2015) Cord Blood 25-hydroxyvitamin D and Fetal Growth in the China-Anhui Birth Cohort Study. Sci Rep 5:14930. https://doi.org/10.1038/srep14930
Tao RX, Meng DH, Li JJ et al (2018) Current Recommended vitamin D prenatal supplementation and fetal growth: results from the china-anhui birth cohort study. J Clin Endocrinol Metab 103(1):244–252. https://doi.org/10.1210/jc.2017-00850
Tao M, Shao H, Gu J et al (2012) Vitamin D status of pregnant women in Shanghai, China. J Matern Fetal Neonatal Med 25:237–239. https://doi.org/10.3109/14767058.2011.569613
Yun C, Chen J, He Y et al (2017) Vitamin D deficiency prevalence and risk factors among pregnant Chinese women. Public Health Nutr 20(10):1746–1754. https://doi.org/10.1017/S1368980015002980
Bouillon R, Assche FAV, Baelen HV, et al (1981) Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest 67(3):589–596. https://doi.org/https://doi.org/10.1172/JCI110072.
Halicioglu O, Aksit S, Koc F, et al (2012) Vitamin D deficiency in pregnant women and their neonates in spring time in western Turkey. Paediatr Perinat Epidemiol 26(1):53–60. https://doi.org/10.1111/j.1365-3016.2011.01238.x
Roth DE, Morris SK, Zlotkin S et al (2018) Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N Engl J Med 379(6):535–546. https://doi.org/10.1056/NEJMoa1800927
von Websky K, Hasan AA, Reichetzeder C et al (2018) Impact of vitamin D on pregnancy-related disorders and on offspring outcome. J Steroid Biochem Mol Biol 180:51–64. https://doi.org/10.1016/j.jsbmb.2017.11.008
van der Pligt P, Willcox J, Szymlek-Gay EA et al (2018) Associations of maternal vitamin D deficiency with pregnancy and neonatal complications in developing countries: a systematic review. Nutrients 10(5):E640. https://doi.org/10.3390/nu10050640
Park H, Brannon PM, West AA, et al (2016) Vitamin D metabolism varies among women in different reproductive states consuming the same intakes of vitamin D and related nutrients. J Nutr 146: 1537–1545. https://doi.org/https://doi.org/10.3945/jn.116.229971
Karras SN, Wagner CL, Castracane VD (2018) Understanding vitamin D metabolism in pregnancy: from physiology to pathophysiology and clinical outcomes. Metabolism 86:112–123. https://doi.org/10.1016/j.metabol.2017.10.001
Chawla D, Daniels JL, Benjamin-Neelon SE et al (2019) Racial and ethnic differences in predictors of vitamin D among pregnant women in south-eastern USA. J Nutr Sci 8:e8. https://doi.org/10.1017/jns.2019.4
Gould JF, Anderson AJ, Yelland LN et al (2017) Association of cord blood vitamin D with early childhood growth and neurodevelopment. J Paediatr Child Health 53(1):75–83. https://doi.org/10.1111/jpc.13308
Song HB, Xu Y, Yang XW et al (2018) High prevalence of vitamin D deficiency in pregnant women and its relationship with adverse pregnancy outcomes in Guizhou. China J Int Med Res 46(11):4500–4505. https://doi.org/10.1177/0300060518781477
March KM, Chen NN, Karakochuk CD, et al (2015) Maternal vitamin D3 supplementation at 50 mug/d protects against low serum 25- hydroxyvitamin D in infants at 8 wk of age: a randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. Am J Clin Nutr 102:402–10. https://doi.org/10.3945/ajcn.114.106385.
O’Callaghan KM, Hennessy A, Hull GLJ et al (2018) Estimation of the maternal vitamin D intake that maintains circulating 25-hydroxyvitamin D in late gestation at a concentration sufficient to keep umbilical cord sera ≥ 25–30 nmol/L: a dose-response, double-blind, randomized placebo-controlled trial in pregnant women at northern latitude. Am J Clin Nutr 108(1):77–91. https://doi.org/10.1093/ajcn/nqy064
Grant CC, Stewart AW, Scragg R, et al (2014) Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics 133:e143–53. https://doi.org/10.1542/peds.2013-2602.
Acknowledgements
We are extremely grateful to all the families who took part in this study. And we would like to thank the participating doctors, midwives, and nurses for patient management.
Funding
National Natural Science Foundation of China (Grant No. 81773411), National Key Research and Development Project of China (Grant Nos. 2016YFC1305204, and 2020YFC2008002), Medical and Engineering Cooperation Project of Shanghai Jiao Tong University (Grant No. YG2017ZD15), Shanghai Children's Health Services Capacity Program (grant GDEK201708), the Science and Technology Commission Project of Shanghai Pudong New Area (Grant No. PKJ2017-Y05), the Shanghai Municipal Education Commission— Gaofeng Clinical Medicine Grant Support (Grant No. 20152220), the Shanghai Municipal Commission of Science and Technology Program (Grant No. 13JC1403700), “Eastern Scholar” project supported by Shanghai Municipal Education Commission (Grant No. ZXDF089002), and Shanghai Key Laboratory of Psychotic Disorders (Grant No. 13dz2260500, 14-K06). This study was partly funded by the Shanghai Municipal Health Commission and the Shanghai Jiao Tong University School of Medicine and supported by the National Human Genetic Resources Sharing Service Platform (Grant No.2005DKA21300).
Author information
Authors and Affiliations
Consortia
Contributions
The authors’ contributions were as follows—Xiaodan Yu, Mingqing Xu, Ying Tian, and Jun Zhang conceived of the research idea and designed the study; Xirui Wang, Xianting Jiao, Juan Li, and Fan Yang performed 25(OH)D measurements; Xirui Wang, Xianting Jiao, Mingqing Xu, and Yue Zhang analyzed the data and performed the statistical analysis; Xirui Wang and Xianting Jiao drafted the manuscript; Xiaodan Yu and Mingqing Xu revised the manuscript; Xiaodan Yu and Mingqing Xu supervised or mentored; Xiaodan Yu had primary responsibility for final content. Xiaodan Yu and Mingqing Xu jointly shared the senior authorship. All authors contributed important intellectual content during manuscript drafting or revision and are accountable for the overall work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, X., Jiao, X., Tian, Y. et al. Associations between maternal vitamin D status during three trimesters and cord blood 25(OH)D concentrations in newborns: a prospective Shanghai birth cohort study. Eur J Nutr 60, 3473–3483 (2021). https://doi.org/10.1007/s00394-021-02528-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02528-w