Abstract
Purpose
The aim of this study was to evaluate the prevalence of thyroid dysfunction in a cohort of healthy individuals in Mexico City, as well as to investigate the potential associations of these results with their estimated iodine intake (EII) as reflected by their 24-hour urinary iodine excretion (24-h UIE).
Methods
From the SALMEX cohort, 683 adults provided an appropriate 24-h urine sample. Thyroid function tests and thyroid antibody concentrations were determined in the participants’ sera. We analyzed discrepancies between the commonly used urinary parameters to determine the iodine intake status and the performance of thyroglobulin (Tg) as a biomarker of its status in the adult population.
Results
The prevalence of dysthyroidism was high, being similar to other studies. Subclinical hypothyroidism was detected in 5.0% of individuals, clinical hypothyroidism in 1.8% of individuals, and sub-clinical hyperthyroidism in 2.8% of individuals. The median EII was 285 μg/d (IQR 215.0–369.0); 94% of individuals had EII >150 µg/d recommended daily allowance (RDA) in adults. The urinary iodine concentration (UIC) and the UIE had relative biases in their averages of 34.4%. The Tg median was 7.21 ng/mL. The prevalence of increased Tg was 6.15%. There was no correlation between Tg and EII (r= 0.019, p= 0.606).
Conclusions
Thyroid dysfunction was highly prevalent in this population. Our cohort revealed a slight discrepancy between dysthyroidism manifestations and iodine intake markers; the latter represent a population with adequate iodine intake. Further studies are necessary to clearly define the prevalence of thyroid dysfunction as well as the iodine nutritional status in Mexico.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid dysfunction is one of the most frequent endocrine disorders. However, its prevalence and incidence reported by epidemiological studies conducted in various countries and regions differ significantly [1]. These variations depend on the selected population, the age and gender of the studied subjects, the definitions used for the various thyroid dysfunctions, and iodine intake [2]. Iodine intake is one of the most important environmental factors associated with thyroid dysfunction and autoimmunity, and salt consumption is the main dietary source of dietary iodine in countries in which salt is iodized [3, 4]. There is a close relationship between iodine intake and the risk of developing thyroid disease, which tends to appear in instances of extreme iodine intake, both excessive and deficient [3]. In recent years, several studies have demonstrated that excessive dietary iodine intake may lead to subsequent abnormalities of thyroid function—in particular, an increased incidence of autoimmune thyroiditis (AT) and hypothyroidism [5,6,7].
Urinary iodine concentration (UIC) is used as an indicator of recent iodine intake, as >90% is excreted in the urine [4]. UIC measurements can be performed either in spot samples or in 24-h urine samples. In population-based studies, the median urinary iodine concentration (UIC, expressed in µg/L) in spot urine samples are used, as 24-h collections are unfeasible. Nevertheless, 24-h urinary iodine excretion (24-h UIE) (expressed in µg/d) is considered to be the preferred tool for measuring iodine intake in an individual, as it provides a more precise estimate than spot urine samples do [8]. However, for a more accurate assessment of individual iodine status, multiple 24-h UIC samples would be required.
The most recent report of the Iodine Global Network (IGN) considered Mexico to be a country with adequate iodine intake, with a median UIC of 264 µg/L [9]. Salt iodination was approved in Mexico in 1942, but its implementation failed. It was not until 1963 that a new law was approved and implemented it, but it has been modified many times since then. It stipulates that salt must contain 30 ± 10 mg of iodine (KIO3/KI) per kg of salt. This normative law is also compulsory for the salt used in the food industry and for animal consumption [10]. Tg has also been considered to be a biomarker of iodine status in population studies, particularly in children. Its behavior in adults is not well known.
The aims of this study were: (1). To evaluate the prevalence of thyroid dysfunction in a cohort of healthy individuals in Mexico City; (2). investigate the potential associations of these results with their estimated iodine intake (EII) as reflected by their 24-hour urinary iodine excretion (24 h UIE); (3). examine the differences between the commonly used parameters to determine the iodine intake status in the population (UIC, UIE, I/Cr, EUIE); (4). examine the performance of Tg as a biomarker of the iodine intake status in the adult population.
Material and methods
The Salt and Mexico (SALMEX) cross-sectional, observational study was created to establish a cohort of urban workers to evaluate their salt consumption; average sodium, potassium, and iodine intake; and eating habits, along with their relation to the prevalence of chronic non-communicable diseases in the Valley of Mexico. The study included volunteers over 18 years of age with no prior diagnoses of congestive heart failure, advanced renal (eGFR < 60 ml/min/1.73 m2) or liver disease, intestinal resection, diuretic initiation within the previous 10 days, active infection, or previous personal history of thyroid disease, as well as those not currently pregnant or lactating. Subjects were recruited among our institute’s personnel (administrative, nursing, laboratory, and medical departments).
A fasting venous blood sample was obtained from each volunteer after a fast of no less than 8 h; the sample was collected between 0700 and 1000 h. After centrifugation, serum was aliquoted and frozen at − 20 °C until processing. Thyroid function tests were also performed in serum. 24 h urine samples for urinary iodine excretion determinations were obtained for all volunteers; they were transferred into 5-mL tubes and frozen at – 20 °C until processing.
The epidemiological criteria used to evaluate iodine nutritional status based on the median UIC were as follows: < 100 µg/L equaled deficient intake, 100–299 µg/L reflected adequate iodine intake, and > 300 µg/L was considered to be excessive iodine intake (World Health Organization [WHO]/United Nations International Children's Emergency Fund [UNICEF]/IGN) [9].
All volunteers answered a questionnaire on their families’ history of thyroid disease, their personal histories, and the presence of autoimmune comorbidities associated with thyroid dysfunction, as well as on the use of drugs or iodinated contrast agents for imaging studies. Volunteers who experienced exposure to iodinated products or substances within the previous 12 weeks of the blood sample collection were excluded, with a total of 44 volunteers being excluded for various reasons.
Diagnostic criteria on thyroid disease
Cohort-specific reference values for thyroid hormones, antithyroid antibodies, and thyroglobulin (Tg) were established according to the National Academy of Clinical Biochemistry (NACB) recommendations [11].
Samples for determining thyroid function reference values were obtained from individuals who answered the call for inclusion in the cohort but were unable to collect adequate 24 h urine samples. Samples were also obtained from individuals who refused to continue in the study for various reasons, but did accept participating in the determination of reference values. To establish the reference values for thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibodies (TgAb), some males in the study population were included due to the difficulty with fulfilling the necessary requirements.
Normal TPOAb and TgAb were determined in 120 healthy males, none of whom had a family or personal previous history of dysthyroidism or autoimmune disease, with TSH values between 0.5 and 2.0 mIU/L. They were non-smokers, and their ages ranged from 18 to 40 years.
Reference values for free thyroxine (FT4), free triiodothyronine (FT3), and thyroid stimulating hormone (TSH) were obtained in 402 healthy adults with no family or personal history of dysthyroidism, no TPOAb and/or TgAb antibodies, and no goiter or other comorbidities. The Tg reference value was established in 211 healthy, non-smoking volunteers between the ages of 18 and 40 years, with no personal or family history of dysthyroidism, TSH values between 0.5 and 2.0 mIU/L, and negative TgAb and/or TPOAb. Reference values were established between the empirical 2.5 and 97.5 percentiles; reference values and the characteristics of the equipment used are detailed below.
Dysthyroidism was defined according to the following criteria: clinical hypothyroidism if TSH was > 4.84 mIU/L and free T4 (FT4) was < 8.90 pmol/L; subclinical hypothyroidism if TSH was > 4.84 mIU/L and FT4 was between 8.90 and 14.81 pmol/L; clinical hyperthyroidism with a TSH < 0.63 mIU/L, FT4 > 14.81 pmol/L, and/or free T3 (FT3) > 5.49 pmol/L, and subclinical hyperthyroidism if TSH < 0.63 mIU/L, FT4 was between 8.90 and 14.81 pmol/L, and FT3 was between 3.30 and 5.49 pmol/L. AT was diagnosed if TPOAb was abnormally elevated (> 4.11 IU/mL), FT4 was between 8.90 and 14.81 pmol/L, and TSH was above the normal range (> 4.84 mIU/L); if these findings were isolated, they were not considered to be diagnostic of AT.
Laboratory evaluation
In serum, FT4, FT3, TSH, TPOAb, TgAb, and Tg concentrations were determined and analyzed using chemiluminescence. FT4 had an analytical sensitivity of 3.2 pmol/L, and the reference value in our population was 8.90–14.81 pmol/L. The intra-assay variation coefficient was < 4.40%, and the inter-assay VC was < 8.08% (Access free T4 assay; Beckman Coulter, Fullerton, CA).
The FT3 assay had an analytical sensitivity of 1.4 pmol/L, the reference value in our population was 3.30–5.49 pmol/L, the intra-assay VC was < 6.6%, and the inter-assay VC was < 8.0% (Access free T3 assay; Beckman Coulter, Fullerton, CA). TgAb had an analytical sensitivity of 0.17 IU/mL, and values below 1.71 IU/mL were considered to be non-significant in our population. The intra-assay VC was < 5.7%, and the inter-assay VC was < 5.2% (TgAb; Access thyroglobulin antibody assay, Beckman Coulter, Fullerton, CA). The assay used to determine the presence of TPOAb had an analytical sensitivity of 0.25 IU/mL; values below 4.11 IU/mL were considered to be non-significant in our population. The intra-assay VC was < 7.1%, and the inter-assay VC was < 4.4% (TPOAb; Access TPO antibody assay, Beckman Coulter, Fullerton, CA). In addition, the TSH assay with a functional sensitivity of 0.030 mIU/L had a reference value in our population of 0.63–4.84 mIU/L. The intra-assay VC was < 5.56%, and the inter-assay VC was < 5.59% (Access HYPERsensitive hTSH assay; Beckman Coulter, Fullerton, CA). Finally, the assay used to measure Tg had an analytical sensitivity of 0.1 ng/mL, a reference value in our population of 2.25–20.12 ng/mL, the intra-assay VC was < 2.2%, and the inter-assay VC was < 4.0% (Access Thyroglobulin Assay; Beckman Coulter, Fullerton, CA).
Urine collection and analysis
Urinary creatinine (UCr) was determined according to the modified Jaffé method and was quantified with a Synchron Cx5 PRO autoanalyzer (Beckman Coulter Fullerton, La Habra, CA, USA). The suitability of 24-h urine collections was determined using the UCr to weight ratio (mg/kg), considering that an adequate daily UCr excretion was 10–20 mg/kg in women and 15–25 mg/kg in men. Incomplete samples were excluded from the analysis.
The urinary iodine (UI) was determined with a modification of the Sandell–Kolthoff method using ammonium persulfate digestion and microplate reading (405 nm). The UIC was determined, and the results were expressed in µg/L. The intra- and inter-assay coefficients of variation for the UICs were 6.0% and 7.2% at 50.0 µg/L, respectively, and 3.3% and 4.0% at 200.0 µg/L, respectively. Iodine concentration in 24-h urine samples (µg/L) was multiplied by the collected urine volume to obtain the UIE (µg/24-h). The urinary iodine-to-creatinine ratio (I/Cr) (µg/g) was determined as follows: UIC (µg/L)/UCr (g/L). Estimated 24-h urinary iodine excretion (EUIE) was determined according to the following equation: I/Cr (µg/g) × expected creatinine excretion (g/day). The 24-h creatinine excretion in men decreased from 1.74g (25–49 y) to 1.63g (50–59 y), and from 1.47g (60–69 y) to 1.39g (min 70y). In women, the values were 1.23g (25–49y), 1.15g (50–59 y), 1.07g (60–69 y), and 1.0g (min 70y) [14]. No estimate of the predicted 24-h creatinine was available in individuals < 25 years in the study, so estimates for subjects from 25 to 49 years were used for those aged 18–24 years in the analysis.
Daily iodine intake was calculated based on the EII, as we considered that this value would be more suitable than the other UI determinations for estimating the nutritional iodine status. The following formula was used to calculate iodine intake (µg/day): UIC (µg / L) x 24h urine volume (L) /0.92. [4]. The 0.92 value is a constant, assuming that 92% of ingested iodine is excreted in the urine. We considered that the important cutoff value for evaluating estimated adult requirements (EAR) is 95 µg/day; the recommended daily allowance (RDA) in adults is 150 µg/day, and the highest tolerable levels (TUL) in adults are 600 µg/day for the European Union Scientific Committee on Food [15], and 1100 µg/day for the United States Institute of Medicine (IOM) [16].
All procedures were conducted according to the Declaration of Helsinki guidelines and approved by the Ethics Committee of the Institute (number 09/191).
Statistical analysis
Nominal categorical variables are presented as frequencies and proportions. Continuous numerical variables were analyzed to determine their distribution with the Kolmogorov–Smirnov test; those with a non-normal distribution were shown as medians and interquartile ranges (IQR). A two-group comparison was conducted with the Mann–Whitney U-test, and the Kruskal–Wallis test was used for the comparison of several groups. Proportion comparisons were made with the Chi-squared test (X2). Data with a non-normal distribution were logarithmically transformed for subsequent analysis. Additionally, multivariate analysis via linear regression was used to determine the association between Tg and age, gender, smoking status, TSH, EII, TgAb, TPOAb, and FT4. The geometric mean of TSH was calculated with its 95% CI in each age group and by gender. The association between the various UI parameters was established with Pearson´s correlation. Prior to the analysis of UI parameters, they were all compared: UIC (µg/L) with UIE (µg/24-h), and EII (µg/day) with EUIE (µg/day). Agreement between measurements was determined with the Bland–Altman analysis. Assuming that EII (µg/day) in the cohort was greater than the RDA of 150 µg/day, we doubled the RDA to 300 µg/day as the cutoff point of the ROC curve. We constructed a receiver operating characteristic (ROC) curve to determine the diagnostic performance of Tg as a biomarker of iodine status. All statistical analyses considered a p value < 0.05 to be statistically significant, and we used the MedCalc 19.1 statistical software (Ostend, Belgium) and SPSS 21.0 (IBM, Corp).
Results
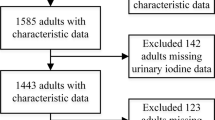
In a total population of 1009 participants in the SALMEX cohort, 727 had adequate 24-h urine collections for estimating creatinine and iodine 24-h excretions. Of these 727 individuals, 683 (94%) accepted participating in this sub-study, answering the thyroid disease-related questionnaire. Of these participants, 432 were female (63%) with a median age of 39 years (range, 19-73). Only 15.8% of the total cohort were over the age of 50 years. The general characteristics of the studied population are described in Table 1.
-
1.
Prevalence of thyroid dysfunction
The prevalence of thyroid abnormalities is shown in Table 2. The presence of dysthyroid states and all of its manifestations were more frequent in females than in males, but they were not statistically significant, except for AT (5% vs 0.8%, p = 0.002). TSH was found to be elevated above the upper reference value established for this cohort (4.84 mUI/L) in 6.73% of individuals, and among these, only 1.8% also had a FT4 below the lower limit of the reference value; 91.6% of these were females. The age ranges with clinical hypothyroidism in females were: 3.7% between 35 and 44 years, and 4.7% between 45 and 54 years. Although there is an increased prevalence of sub-clinical hypothyroidism in women, this has no statistical significance when compared with men (3.2% vs 2%, p = 0.470); the presence of sub-clinical hypothyroidism in females is greatest between the ages of 45 and 54 (9.4%) than in individuals < 25 years (3.4%). Sub-clinical hypothyroidism was also manifested more frequently in females than in males (p = 0.470), and it increased with age in both genders: in women > 55 years, it was 12.5%, and in men > 55 years, it was 4.3%. Clinical hyperthyroidism was detected only in females, mostly in young women < 44 years (80%). Among these, three had simultaneously increased anti-thyroid antibodies. TgAbs were not obtained in any hyperthyroid individuals. The presence of AT was an important manifestation of dysthyroidism in our cohort, with a significant proclivity for women in comparison with men (p = 0.002). It also became more frequent with age: 0% in individuals < 25 years, and 7.5% in females > 55 years.
Table 2 Dysthyroidism in the studied population. The cohort had a TSH geometric mean of 1.97 mIU/L (95% CI 1.85–2.10 mIU/L) with a range of 0.01–117.9 mIU/L, and it did not differ from the geometric mean obtained in the population used to determine reference values, which was 1.91 mIU/L (95% CI 1.84–2.04 mIU/L) (p = 0.1780). The geometric mean progressively increased with age (see Fig. 1). It was lowest in individuals < 25 years, 1.67 mIU/L (95% CI 1.37–2.03 mIU/L), and it reached its highest value in those between 45 and 54 years of age, 2.43 mIU/L (95% CI 2.15–2.74 mIU/L). The presence of increased TPOAb is significant in females compared with males (p = 0.035). In women, it reaches its highest percentage of positivity after the age of 45, with 22.5%, but there is no statistical significance in terms of age (p = 0.219). There is also a marked difference between sexes in TgAb positivity, with a female predominance (16.7% vs 9.6%, p = 0.012); however, there is no detectable difference in terms of age ranges (p = 0.912) (see Table 3).
Fig. 1 Geometric mean serum thyroid stimulating hormone (TSH) in different age groups (by sex) in the total population. The error bars represent 95% CIs of means, calculated using the SE calculated from log-transformed values. Filled square, males; unfilled square, females [MedCalc 19.1 statistical software (Ostend, Belgium) and SPSS 21.0 (IBM, Corp)]
Table 3 Thyroid parameters and thyroid antibodies (n=683) -
2.
Associations thyroid dysfunction with their estimated iodine intake
We did not detect differences in the biochemical manifestations of thyroid dysfunction among the volunteers from different groups of iodine intake (UIC, UIE), with a prevalence of 13% for any dysthyroidism in the volunteers with deficient intake, 9% in the volunteers with adequate iodine intake and 11% in the group with excessive iodine intake (p = 0.136). On the other hand, as most of the participants (94%) had an EII above the RDA (> 150 µg /d), we were unable to explore the differences in the prevalence of dysthyroidism between patients with normal and high EII.
-
3.
Comparison of the different parameters used to determine iodine intake in a population: UIC, UIE, I/Cr, EUIE, EII
The EII in the cohort was 285µg/d, and it was significantly greater in males than in females (p = 0.001); see Table 4. Only 0.9% of individuals had values < 95 µg/day, the EAR for adults; 94% of individuals had EII > 150 µg/d RDA in adults; 4.2% had EII above 600 µg/d; and 0.9% exceeded 1,100 µg/d, the tolerable upper limit (TUL) according to the European Union Scientific Committee on Food [14] and the United States Institute of Medicine [15], respectively. There were no significant differences by age range (p >0.05) in EII. The UIC (µg/L) and the UIE (µg/24-h) had medians of 180 µg/L and 262.2 µg/24-h, respectively, placing the cohort in the group with adequate iodine intake according to the WHO/UNICEF/IGN [9]. In general, males had greater UIC (µg/L) and UIE (µg/24-h) in comparison with females (p = 0.0001). There was a significant association between the UIC (µg/L) and the UIE (µg/24-h) (r= 0.570, p < 0.0001), but on the Bland–Altman graph, a relative bias in averages is shown, with a range of 34.4% (see Fig. 2). A significant association was also detected between the EII (µg/day) and the EUIE (µg/day) (r=0.457, p <0.0001), and on the Bland–Altman graph, the bias in averages was acceptably lower, − 4.2% (see Fig. 2).
-
4.
Evaluation of thyroid parameters as biomarkers of the iodine intake status in the adult population
a The Bland–Altman plots show the relative level of agreement between observed urinary iodine excretion in 24-hour urine samples, 24h-UIE (µg/24h); and urinary iodine concentration, UIC (µg/L). The dark solid line shows the mean relative difference. The dashed lines indicate the 95% limits of agreement of the relative difference. b The Bland–Altman plots show the relative level of agreement between observed estimated iodine intake, EII (µg/day); and estimated 24-hour urinary iodine excretion, EUIE (µg/day). The dark solid line shows the mean relative difference. The dashed lines indicate the 95% limits of agreement of the relative difference
In the multivariate analysis, Tg was associated only with TSH (p = 0.002) and the TgAb (p = 0.001); there was no association with gender, age, smoking status, FT4, EII, or TPOAb (p = 0.12, 0.14, 0.73, 0.26, 0.73 and 0.62, respectively). The Tg median was 6.74 ng/mL, IQR (4.74–10.9). The 96 individuals who were TgAb+ had a median Tg of 3.24 ng/mL, IQR (0.59–9.86) (p = < 0.0001). These were excluded from the subsequent analyses of Tg, but there were no differences between the group of Tg without TgAb and the entire cohort, without excluding the TgAb+ (p = 0.1415). There was no difference in Tg between genders. The prevalence of increased Tg (> 20.12 ng/mL, UL determined for the cohort) was 6.15%. There was also no correlation between Tg and EII (r= 0.019, p 0.606) or FT4 (r= 0.062, p 0.104), but there was a significant correlation with TSH (r=0.164, p < 0.0001). As previously mentioned, the cohort had EII > 150 µg/day, and in accordance with this finding, an ROC curve was constructed with a cutoff point of 300 µg/day, double the RDA, to determine the diagnostic performance of Tg. With this EII, the area under the ROC curve for Tg was 0.539 (95% CI 0.501–0.577) (p = 0.08) (see Fig. 3).
Discussion
We found a group of individuals in our study with similar characteristics to those reported in large epidemiological thyroid studies [17, 18]. However, the average age in our study was lower, which underscores some of our findings, as dysthyroidism tends to increase with age. The prevalence of elevated TSH was 6.73%, a value similar to that found in other studies [17], and it increased with age, particularly in females when compared with males [17, 19]. This was in spite of the fact that the elderly were underrepresented in our cohort.
Hypothyroidism was significantly prevalent (1.8%) when compared with the most recent epidemiological thyroid studies, and so was sub-clinical hypothyroidism (5.0%) [17, 19, 20]. This may result from the different TSH cutoff points established in the definition of each study. Our cutoff point was 4.84 mIU/L, a relatively high value, but the number of individuals with values above it was considerable for our group´s conditions.
No large epidemiological studies in Mexico report the prevalence of thyroid dysfunction in an open population, although recently, a group reported a prevalence of sub-clinical hypothyroidism of 8.3% in a relatively large population (n=3148) from the center area of the country. Their average TSH, after excluding detected thyroid patients, was 2.49 ± 1.42 mIU/L [21], and the prevalence of clinical hypothyroidism was 1.87% (personal communication). Their findings, in this aspect, are similar to ours.
Our cutoff point may overestimate the prevalence of sub-clinical hyperthyroidism; however, there is no consensus on the cutoff point. Studies have mentioned values from < 0.05 up to < 0.3 mIU/L in general. We used the lower value in our reference population (0.63 mIU/L) as other authors have [5]. The used value is important to discuss because in studies conducted in various populations with different iodine intakes, the population’s iodine status has been known to influence TSH reference values. Thus, in regions with adequate or a high iodine intake, both limits of the reference values are displaced upward, as seen in our population [22]. Clinical hyperthyroidism was of autoimmune origin as in other studies.
The paucity of data in Mexico, led us to compare our study with the National Health and Nutrition Examination Survey (NHANES III), which included Mexican Americans. Their prevalence of thyroid dysfunction was 0.2% for clinical hypothyroidism, 3.9% for sub-clinical hypothyroidism, 0.2% for clinical hyperthyroidism, and 0.5% for sub-clinical hyperthyroidism [19]. The median UIC in the NHANES III for Mexican Americans was 183.4 µg/L [23].
The presence of significant TPOAb and TgAb levels increased with age and were more prevalent in females than in males. This replicates the results of other studies [1,19].
In the definition of AT, we included only individuals with elevated TSH plus TPOAb+. We believe that our country has had adequate iodine intake for many years, so most cases of thyroid dysfunction are of autoimmune origin. Hence, in AT, we took into consideration only TSH increases associated with autoimmunity, which will be most relevant in the future. There are a few studies that have considered this in AT, and AT definitions are diverse, but our situation can be seen when comparing our study with other publications (see Table 5).
The behavior of TSH, with its age-related increasing geometric average, is similar to that in populations with iodine sufficiency [19]. In deficient populations [1] or in those with recent transitions from insufficiency to sufficiency, the opposite occurs [24].
In terms of the iodine urinary parameters, the cohort´s UIC (µg/L) reflects adequate iodine intake with a median of 180.0 µg/L (WHO/UNICEF/IGN), and the EII (µg/day) had a median of 285 µg/day, well above the RDA of 150 µg/day. Although we surpassed the TUL, this was not significant. Considering the definition of a prevalence of 2.5% below the EAR and 2.5% above the UL, it would be acceptable in a population with an adequate iodine intake [25]. Only 0.9% of individuals were below the EAR, 95 µg/day, and < 1% were above the TUL of 1100 µg/day (IOM). However, 4.2% surpassed the TUL´s 600 µg/day recommended by the European Union Scientific Committee on Food [15, 16]. According to these determinations based on urinary iodine excretion, we believe that our cohort reflects adequate iodine intake. Other studies and analyses that have been previously published were conducted with our cohort and can be associated with our current results. Twenty-four-hour urinary sodium (Na) excretion was measured, and we found that only 10.6% of individuals had sodium intakes within the recommended range (< 2 g/day), 45.4% had high values (2–3.6 g/day), and 44% had very high levels (> 3.6 g/day). We also detected a directly proportional relation between iodine and sodium intake after adjusting for the caloric intake (r = 0.57, p < 0.000) [12]. Moreover, we determined the food source that was providing the sodium intake in the cohort, and it was mainly the result of industry processed foods, such as bread, processed meats, cheeses, cereal and its byproducts, hot sauces, and street foods, such as “tamales” and “tacos” [12]. These previous studies conducted in the cohorts suggested that the main iodine source in our population comes from the iodinated salt in industrialized or processed foods eaten away from home.
Another important point of our study in terms of the UI parameters was their concordance between the UIC (µg/L) and the UIE (µg/24-h); the UIC (µg/L) underestimated the latter measurement by 34.4%. This was already known [26, 27], but rarely recorded in adults and in populations with adequate iodine intake. As expected, this could lead to the misclassification of the iodine status if the UIC (µg/L) is used in adults [28]. Concordance between the EII (µg/day) and the EUIE (µg/day) was superior, only overestimating the average differences by 4.2%, albeit with a tendency to overestimate the value within its limits. The formula used to predict 24-h UCr in the EUIE is the result of a study on a Caucasian population of Belgian origin [14]. Our population of Mexican “Mestizos “is a mixture of Caucasians of European origin and Native Americans. This has been recorded in other “mestizo” populations, reflecting that ethnicity was more relevant in creatinine excretion than weight was [29]. We believe that there is a similar phenomenon in our population.
Tg has been proposed as a proxy biomarker of individual iodine status and recently gained relevance, but studies have mainly focused on children [30]. We established our own reference values for our cohort, as commonly used reference values reflecting thyroid function do not apply to all populations [31]. We consider that transpolating the reference values established in the standard CRM-457 in adults (3–40 µg/L) [11] might be inappropriate. The upper Tg limit was much lower (20.12 ng/mL) in our reference population, following the criteria proposed by the NACB [11]: 6.15% of the cohort had values above the limit, above the < 3% recommended in populations with adequate iodine intake. However, the cohort had a median Tg of 7.21ng/mL (µg/L), a similar value to the old criterion established by the WHO that established iodine sufficiency with a median of Tg < 10 µg/L. It is also similar to the value recorded by Vejbjerg et al. [32], but it is far from the median of < 13 µg/L used as the new criterion for sufficiency determined in children [30]. We observed the described effect of the presence of TgAb on immunometric assays, such as those used in this study, because they tend to underestimate Tg values [33]; the exclusion of TGAb+ individuals does not affect the interpretation of Tg, at least at the population level, as described by others [32]. As in other studies in adults, we found no significant association between urinary iodine excretion and Tg [34, 35]. The diagnostic performance of Tg on the ROC curve showed an AUC of 0.539 that could not discriminate between individuals with iodine intake greater than 300µg/day, a similar finding to that in a study conducted on pregnant women in China, with a UI >500 µg/24-h , and a Tg AUC of 0.53 (p=0.74). [35]
This study has several strengths, such as the measurement of UI in 24-h samples, and it is definitely superior to spot urine measurements. Obtaining the EII and using the dietary reference intake (DRI) as cutoff points is desirable in epidemiological studies, especially when adults are included, which was the determination in this context. The Tg determination with ultrasensitive equipment that is commonly used in clinical practice, and the determination of reference values in iodine-sufficient populations are also strengths of this study.
One of the main limitations of this study was the single determination of UI in our cohort. Köning et al. [36] showed that to better evaluate iodine status, it is preferable to obtain multiple UIC measurements due to the known day-to-day variations in UI. However, although desirable, this is logistically difficult in a large cohort such as ours. Another limitation is that we did not use para-aminobenzoic acid (PABA) as a marker of adequate urine collection. Participants were, however, thoroughly instructed on the urine collection technique.
Conclusions
Our cohort reveals a slight discrepancy between dysthyroidism manifestations and iodine intake markers; the latter represent a population with an iodine intake within nutritional requirements (DRI). They also had a median Tg <13 ng/mL (µg/L) that suggests iodine sufficiency. However, thyroid autoimmune phenomena occurred more frequently than expected.
We attempted to use Tg as an individual biomarker of iodine status, but its performance was poor, perhaps as a result of the cutoff point—that is, within an adequate DRI range in populations with an appropriate iodine intake. The determination of the EII (µg/day) appears to be warranted instead of that of the UIC (µg/L) when establishing iodine status with UI. Further studies are necessary to clearly define the prevalence of thyroid dysfunction as well as the iodine nutritional status in Mexico.
References
Hoogendoorn EH, Hermus AR, de Vegt F, Ross HA, Verbeek AL, Kiemeney LA, Swinkels DW, Sweep FC, den Heijer M (2006) Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: influences of age and sex. Clin Chem 52(1):104–11. https://doi.org/10.1373/clinchem.2005.055194
Vanderpump MP (2011) The epidemiology of thyroid disease. Br Med Bull 99:39–51. https://doi.org/10.1093/bmb/ldr030
Burek CL, Talor MV (2009) Environmental triggers of autoimmune thyroiditis. J Autoimmun 33(3–4):183–9. https://doi.org/10.1016/j.jaut.2009.09.001
Zimmermann MB, Andersson M (2012) Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 70(10):553–70. https://doi.org/10.1111/j.1753-4887.2012.00528.x
Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C (2006) Effect of iodine intake on thyroid diseases in China. N Engl J Med 354(26):2783–93. https://doi.org/10.1056/NEJMoa054022
Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, Bai X, Li Y, Li N, Li Z, Wang S, Xing Q, Xue H, Zhu L, Hou X, Fan C, Teng W (2011) More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 164(6):943–50. https://doi.org/10.1530/EJE-10-1041
Camargo RY, Tomimori EK, Neves SC, G S Rubio I, Galrão AL, Knobel M, Medeiros-Neto G (2008) Thyroid and the environment: exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo. Brazil. Eur J Endocrinol 159(3):293–9. https://doi.org/10.1530/EJE-08-0192
Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Andersen S, Rasmussen LB, Ovesen L, Jørgensen T (2009) Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 19(11):1281–6. https://doi.org/10.1089/thy.2009.0094
The Iodine Global Network (2019) Global scorecard of iodine nutrition in 2019 in the general population based on school-age children (SAC) https://www.ign.org/cm_data/Global_Scorecard_MAP_2019_SAC.pdf. Iodine Global Network Webpage. Accessed 24 Oct 2019
Health Minister of Mexico (2003) Modificación a la Norma Oficial Mexicana NOM-040-SSA1-1993, Productos y servicios. Sal yodada y sal yodada fluorada. Especificaciones sanitarias. https://www.salud.gob.mx/unidades/cdi/nom/m040ssa193.html. Accesed 24 Oct 2019
Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR (2003) Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13(1):3–126. https://doi.org/10.1089/105072503321086962
Colin-Ramirez E, Espinosa-Cuevas A, Miranda-Alatriste PV, Tovar-Villegas VI, Arcand J, Correa-Rotter R (2017) Food Sources of Sodium Intake in an Adult Mexican Population: A Sub-Analysis of the SALMEX Study. Nutrients. https://doi.org/10.3390/nu9080810
Vega-Vega O, Fonseca-Correa JI, Mendoza-De la Garza A, Rincón-Pedrero R, Espinosa-Cuevas A, Baeza-Arias Y, Dary O, Herrero-Bervera B, Nieves-Anaya I, Correa-Rotter R (2018) Contemporary Dietary Intake: Too Much Sodium, Not Enough Potassium, yet Sufficient Iodine: The SALMEX Cohort Results. Nutrients. https://doi.org/10.3390/nu10070816
Kesteloot H, Joossens JV (1996) On the determinants of the creatinine clearance: a population study. J Hum Hypertens 10(4):245–9
European Food Safety Autority (2006) Tolerable upper intake levels for vitamins and minerals. https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf. Accesed 27 June 2019
Institute of Medicine (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. The National Academies Press, Washington https://doi.org/10.17226/10026
Canaris GJ, Manowitz NR, Mayor G, Ridgway EC (2000) The Colorado thyroid disease prevalence study. Arch Intern Med 160(4):526–34. https://doi.org/10.1001/archinte.160.4.526
O'Leary PC, Feddema PH, Michelangeli VP, Leedman PJ, Chew GT, Knuiman M, Kaye J, Walsh JP (2006) Investigations of thyroid hormones and antibodies based on a community health survey: the Busselton thyroid study. Clin Endocrinol (Oxf) 64(1):97–104. https://doi.org/10.1111/j.1365-2265.2005.02424.x
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE (2002) Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87(2):489–99. https://doi.org/10.1210/jcem.87.2.8182
Bjoro T, Holmen J, Krüger O, Midthjell K, Hunstad K, Schreiner T, Sandnes L, Brochmann H (2000) Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT). Eur J Endocrinol 143(5):639–47. https://doi.org/10.1530/eje.0.1430639
Garduño-Garcia Jde J, Alvirde-Garcia U, López-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, Choza R, Sauque L, Garay-Sevilla ME, Malacara JM, Gomez-Perez FJ, Aguilar-Salinas CA (2010) TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol 163(2):273–8. https://doi.org/10.1530/EJE-10-0312
Guan H, Shan Z, Teng X, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Chen W, Tong Y, Wang W, Gao T, Li C, Teng W (2008) Influence of iodine on the reference interval of TSH and the optimal interval of TSH: results of a follow-up study in areas with different iodine intakes. Clin Endocrinol (Oxf) 69(1):136–41. https://doi.org/10.1111/j.1365-2265.2007.03150.x
Caldwell KL, Jones R, Hollowell JG (2005) Urinary iodine concentration: United States National Health And Nutrition Examination Survey 2001–2002. Thyroid 15(7):692–9. https://doi.org/10.1089/thy.2005.15.692
Völzke H, Alte D, Kohlmann T, Lüdemann J, Nauck M, John U, Meng W (2005) Reference intervals of serum thyroid function tests in a previously iodine-deficient area. Thyroid 3:279–85. https://doi.org/10.1089/thy.2005.15.279
Institute of Medicine (2000) Dietary Reference Intakes: Applications in Dietary Assessment. Washington, DC: The National Academies Press. https://doi.org/10.17226/9956.
Johner SA, Shi L, Remer T (2010) Higher urine volume results in additional renal iodine loss. Thyroid 20(12):1391–7. https://doi.org/10.1089/thy.2010.0161
Johner SA, Thamm M, Schmitz R, Remer T (2016) Examination of iodine status in the German population: an example for methodological pitfalls of the current approach of iodine status assessment. Eur J Nutr 55(3):1275–82. https://doi.org/10.1007/s00394-015-0941-y
Andersen S, Waagepetersen R, Laurberg P (2015) Misclassification of iodine intake level from morning spot urine samples with high iodine excretion among Inuit and non-Inuit in Greenland. Br J Nutr 113(9):1433–40. https://doi.org/10.1017/S0007114515000653
Andersen S, Dehnfeld M, Laurberg P (2015) Ethnicity is import9ant for creatinine excretion among Inuit and Caucasians in Greenland. Scand J Clin Lab Invest 75(1):44–50. https://doi.org/10.3109/00365513.2014.965737
Zimmermann MB, Aeberli I, Andersson M, Assey V, Yorg JA, Jooste P, Jukić T, Kartono D, Kusić Z, Pretell E, San Luis TO, Jr Untoro J, Timmer A (2013) Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100–299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 98(3):1271–80. https://doi.org/10.1210/jc.2012-3952
Pearce EN, Caldwell KL (2016) Urinary iodine, thyroid function, and thyroglobulin as biomarkers of iodine status. Am J Clin Nutr 104(Suppl 3):898S–901S. https://doi.org/10.3945/ajcn.115.110395
Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Carlé A, Pedersen IB, Rasmussen LB, Ovesen L, Jørgensen T (2009) Thyroglobulin as a marker of iodine nutrition status in the general population. Eur J Endocrinol 161(3):475–81. https://doi.org/10.1530/EJE-09-0262
Spencer C, LoPresti J, Fatemi S (2014) How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes Obes 21(5):394–404. https://doi.org/10.1097/MED.0000000000000092
Ma ZF, Venn BJ, Manning PJ, Cameron CM, Skeaff SA (2018) The sensitivity and specificity of thyroglobulin concentration using repeated measures of urinary iodine excretion. Eur J Nutr 57(4):1313–20. https://doi.org/10.1007/s00394-017-1410-6
Li C, Peng S, Zhang X, Xie X, Wang D, Mao J, Teng X, Shan Z, Teng W (2016) The Urine Iodine to Creatinine as an Optimal Index of Iodine During Pregnancy in an Iodine Adequate Area in China. J Clin Endocrinol Metab 101(3):1290–8. https://doi.org/10.1210/jc.2015-3519
Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB (2011) Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 141(11):2049–54. https://doi.org/10.3945/jn.111.144071
Acknowledgements
We gratefully acknowledge the support of P. Chem. Bertha Herrero Bervera from Department of Nephrology and Mineral Metabolism. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical standards
his study was approved by the ethics committee and has been conducted in accordance with the ethical standards. All patients provided their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Flores-Rebollar, A., Pérez-Díaz, I., Vega-Vega, O. et al. Prevalence of thyroid dysfunction in healthy adults according to the estimated iodine intake in 24-hour urine samples: The SALMEX cohort. Eur J Nutr 60, 399–409 (2021). https://doi.org/10.1007/s00394-020-02254-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02254-9