Abstract
Purpose
Data from in vitro and animal studies support the preventive effect of tea (Camellia sinensis) against colorectal cancer. Further, many epidemiologic studies evaluated the association between tea consumption and colorectal cancer risk, but the results were inconsistent. We conducted a meta-analysis of prospective cohort studies to systematically assess the association between tea consumption and colorectal cancer risk.
Methods
A comprehensive literature review was conducted to identify the related articles by searching PubMed and Embase up to June, 2019. Summary relative risks (RRs) and 95% confidence intervals (CIs) were calculated using a fixed effect model.
Results
Twenty cohort articles were included in the present meta-analysis involving 2,068,137 participants and 21,437 cases. The combined RR of colorectal cancer for the highest vs. lowest tea consumption was determined to 0.97 (95% CI 0.94–1.01) with marginal heterogeneity (I2 = 24.0%, P = 0.093) among all studies. This indicated that tea consumption had no significant association with colorectal cancer risk. Stratified analysis showed that no significant differences were found in all subgroups. We further conducted the gender-specific meta-analysis for deriving a more precise estimation. No significant association was observed between tea consumption and colorectal cancer risk in male (combined RR = 0.97; 95% CI 0.90–1.04). However, tea consumption had a marginal significant inverse impact on colorectal cancer risk in female (combined RR = 0.93; 95% CI 0.86–1.00). Further, we found a stronger inverse association between tea consumption and risk of colorectal cancer among the female studies with no adjustment of coffee intake (RR: 0.90; 95% CI 0.82–1.00, P < 0.05) compared to the female studies that adjusted for coffee intake (RR = 0.97; 95% CI 0.87–1.09, P > 0.05).
Conclusions
Our finding indicates that tea consumption has no significant impact on the colorectal cancer risk in both genders combined, but gender-specific meta-analysis shows that tea consumption has a marginal significant inverse impact on colorectal cancer risk in female.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is a major public health concern worldwide [1]. Despite various treatment strategies have been developed and used in clinic, the 5-year overall survival rate of metastatic colorectal cancer is only approximately 10% [2]. The morbidity and mortality rates of colorectal cancer present an upward trend in the world, especially in many medium-to-high human development index countries including China, Russia, and Brazil. It is estimated that over 1.8 million new colorectal cancer cases and 881,000 deaths occurred in 2018. Colorectal cancer has become the third most frequently diagnosed cancer but the second most common cause of cancer death [3]. The pathogenesis of colorectal cancer has not been clearly demonstrated until now. Nevertheless, limited evidence suggests that dietary patterns are important factors to influence the morbidity of colorectal cancer. Dietary intervention has become an important strategy for the colorectal cancer prevention [2].
As a crucial dietary factor, tea, which is originated from the dried leaves of plant Camellia sinensis, is gaining increasing attention due to its possible therapeutic effect on various cancers, especially on colorectal cancer [4]. Tea is one of the most widely consumed beverages, second only to water [5, 6]. In vitro and animal studies have shown that tea intake contributes to the prevention of colorectal cancer. This effect is mainly attributed to its main active ingredient, epigallocatechin-3-gallate (EGCG). Numerous studies have showed that EGCG prevents colorectal cancer by various mechanisms, such as antioxidation, growth inhibition, and apoptosis induction [7]. Furthermore, the bioavailability of EGCG in humans is estimated to be only 0.32% after oral administration, and most of EGCG is oxidized and decomposed in large intestine, leading to colorectum as the major target organ of EGCG [8, 9]. Thus, the anti-colorectal cancer effect of tea attracts more attention.
The preventive effect of tea against colorectal cancer is supported by some epidemiological studies [10, 11]. However, the results of epidemiological studies are not always consistent [12, 13]. Furthermore, the association between tea consumption and colorectal cancer risk also remains controversial based on meta-analysis [14,15,16,17]. Some meta-analysis showed no significant association between tea consumption and colorectal cancer risk [14,15,16], while the meta-analysis reported by Chen et al. [17] found an inverse association. This inconsistency may be caused by several factors, such as case–control design related biases and limited sample size. Further, the meta-analysis based on or included case–control studies may be influenced by recall bias and reverse causality, leading to biased results [15, 17]. Additional evidence is necessary to reveal the association between tea consumption and colorectal cancer risk. A prospective cohort study of 0.5 million Chinese adults (a follow-up of 10.1 years) and a prospective cohort study of 31,552 Japanese adults (a follow-up of 8.0 years) were reported recently. Participants recruited in the two prospective cohort studies are about one third of the total number of subjects in the previous meta-analysis [4, 17, 18]. Therefore, we aimed to provide an updated meta-analysis of prospective cohort studies to evaluate the association between tea consumption and colorectal cancer risk.
Materials and methods
Literature search
We conducted a literature search in PubMed and Embase up to June, 2019. The following search terms were used: (1) “colorectal” or “colonic” or “colon” or “rectal” or “large bowel”; (2) “neoplasm” or “cancer” or “carcinoma” or “tumor”; (3) “tea”; (4) “cohort studies” or “prospective studies”. These search themes were combined using “and” without restrictions. The articles satisfying the exposure, outcome, and study design criteria were pulled.
Study selection
Studies were selected for meta-analysis if they meet the following criteria: (1) published as an original article; (2) belonged to prospective cohort study; (3) evaluated the association between tea consumption and colorectal cancer risk; (4) provided the quantity of participants or person-years; (5) supplied the relative risk (RR) value with corresponding 95% confidence intervals (CIs) for highest vs. lowest level of tea consumption. Meanwhile, studies were excluded if they satisfy at least one of the following characteristics: (1) review article; (2) case–control study; (3) animal trials; (4) less than one year of follow-up; (5) no quantitative analysis on tea consumption, colorectal cancer risk, RR values or 95% CIs.
Data extraction and quality assessment
The search, data extraction, and quality assessment were completed independently by two reviewers (M.Z. and D.L.). Any discrepancies between the two reviewers were resolved by consultation with the third reviewer (F.Z.). Data were collected using a standardized extraction form. The following information was collected: (1) first author’s last name, (2) population of country, (3) case/participants, (4) follow-up period, (5) tea consumption (highest vs. lowest), (6) exposure level, (7) tea type, (8) gender, (9) cancer site, (10) adjusted RRs and corresponding 95% CIs for extreme categories of exposure, (11) adjustment confounding variables. Study quality was evaluated according to the Newcastle–Ottawa quality assessment scale [19]. Eight domains were evaluated in each included study as follows: representativeness of the exposed cohort; selection of the non-exposed cohort; ascertainment of exposure; interest of the outcome at start of study; comparability of cohorts on the basis of the design or analysis; assessment of outcome; follow-up duration; adequacy of follow up of cohorts. A possible score between 0 and 9 was acquired by each study. Score > 7 and ≤ 5 were defined as high quality and low quality, respectively.
Statistical analysis
Data were analyzed by Stata version 12.0 (State Corporation, College Station, TX, USA). The combined RR was calculated by pooling RRs for highest vs. lowest categories of tea consumption from each study. Heterogeneity of effect size across the studies was examined using the Cochran’s Q test and I2 statistics. I2 statistic from 0 to 30% was defined as no or marginal heterogeneity, 30–75% as mild heterogeneity, and over 75% as notable heterogeneity [20]. The random effect model was used only when there existed significant heterogeneity; otherwise, the fixed effect model was used for further analysis [21]. The causes of heterogeneity were further explored through stratified and meta-regression analysis. The potential confounders included geographic region, tea type, cancer site, quality score, and adjustment for age, smoking, and coffee. Sensitivity analysis was performed to test the robustness of main results. Publication bias was visually evaluated for any asymmetry of the funnel plots. The funnel plots were further checked with Egger’s regression asymmetry test and Begg’s adjusted rank correlation test, and the statistical significant was set to P < 0.05 [22].
Results
Search results, study characteristics, and quality assessment
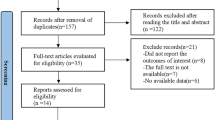
Detailed process of the relevant study selection was shown in Fig. 1. A total of 208 articles were initially screened from PubMed and Embase. 156 of 208 articles were excluded because they were obviously irrelevant to the current meta-analysis by a careful review of the title and abstract. Then we screened the remaining 52 full-text articles. 32 of 52 articles were excluded because of no available data on tea consumption, colorectal cancer risk, RR values or 95% CIs. Finally, 20 articles involving 2,068,137 participants and 21,437 cases of colorectal cancer were recruited for meta-analysis [4, 10,11,12,13, 18, 23,24,25,26,27,28,29,30,31,32,33,34,35,36]. The characteristics of the 20 studies were summarized in Table 1. Four studies were conducted in Europe (332,300 participants and 3778 cases), five in North America (731,273 participants and 10,015 cases), and eleven in Asia (1,004,564 participants and 7644 cases). As shown in Table 1, the quality scores of all studies ranged from 3 to 8. Nine studies were considered to have medium or low quality, and eleven studies had high quality.
Tea consumption and colorectal cancer risk
As shown in Fig. 2, the multivariable-adjusted RRs from twenty studies were extracted. A fixed effect model was used for the calculation of the combined RR due to the marginal heterogeneity (I2 = 24.0%, P = 0.093). The combined RR was determined to 0.97 (95% CI 0.94–1.01) by comparing highest vs. lowest tea consumption levels against colorectal cancer. This indicated that tea consumption had no statistically significant association with colorectal cancer risk.
Subsequently, we stratified the studies by geographic region, tea type, cancer site, quality score, and adjustment for age, smoking, and coffee (Table 2). No statistically significant differences were found in these subgroups. The one-out sensitivity analysis demonstrated that the RRs and CIs values were 0.97–0.99 and 0.93–1.03, respectively. This indicated that the main result was robustness. Besides, the factors including geographic region, tea type, cancer site, quality score, and adjustment for age, smoking, and coffee, were taken into consideration for meta-regression analysis. As shown in Table 3, the P values ranged from 0.566 to 0.903, which indicated that none of them were the potential source of heterogeneity.
Gender-specific meta-analyisis for the association between tea consumption and colorectal cancer risk
Thirteen studies were used for the meta-analysis on the association between tea consumption and colorectal cancer risk in female [11, 18, 24, 26, 28,29,30,31,32,33, 35,36,37]. The fixed effect model was used for calculating the combined RR due to the homogeneity (I2 = 0, P = 0.918). As shown in Fig. 3a, the combined RR was determined to 0.93 (95% CI 0.86–1.00) by comparing highest vs. lowest tea consumption levels against colorectal cancer in female. Thus, there was a marginal significant inverse association between tea consumption and colorectal cancer risk in female. Further, we found a stronger inverse association between tea consumption and risk of colorectal cancer among studies with no adjustment of coffee intake (RR: 0.90; 95% CI 0.82–1.00, P < 0.05) compared to studies that adjusted for coffee intake (RR: 0.97; 95% CI 0.87–1.09, P > 0.05). Nevertheless, no statistically significant differences were found in other subgroups, including geographic region, tea type, cancer site, quality score, and adjustment for age and smoking (Table 2).
Besides, eleven studies were used for the meta-analysis on the association between tea consumption and colorectal cancer risk in male [10, 11, 25, 28,29,30,31, 33, 35,36,37]. As shown in Fig. 3b, the combined RR was determined to 0.97 (95% CI 0.90–1.04) with mild heterogeneity (I2 = 45.0%, P = 0.007), indicating that no significant association was observed between tea consumption and colorectal cancer risk in male. Stratified analysis showed that no statistically significant differences were found by geographic region, tea type, cancer site, quality score, and adjustment for age, smoking, and coffee (Table 2). When we omitted one study in each turn, the RRs and CIs values were 0.97–1.02 and 0.89–1.10, respectively, indicating the main result was robustness. Meta-regression analysis showed that geographic region, quality score, and adjustment for age and coffee may be the causes of heterogeneity (Table 2). However, the stratified analysis in male suggested that the four factors were not heterogeneous sources.
Publication bias
The funnel plot was visually symmetrical, indicating no publication bias. This result was further confirmed by Begg's rank correlation test and Egger’s tests on whole groups (Begg's test P = 0.116; Egger's test P = 0.181), female (Begg's test P = 0.254; Egger's test P = 0.170), and male (Begg's test P = 0.895; Egger's test P = 0.517) (Fig. 4).
Discussion
Strong evidences from animal and cell experiments have demonstrated that tea could inhibit the formation and proliferation of colorectal cancer [38]. Some epidemiological studies have also sought to reveal the association between tea consumption and colorectal cancer risk in the last few decades, but there was no consensus. Recently, Chen et al. [17] reported a meta-analysis on the association between tea consumption and colorectal cancer risk, which enrolled both cohort studies and case–control studies. Their results showed that the summary odds ratio (OR) of colorectal cancer for the highest vs. lowest tea consumption was 0.93 (95% CI 0.87–1.00) among all studies, which indicated that tea consumption had an inverse impact on colorectal cancer risk. Stratified analysis showed that tea, especially green tea (OR = 0.87, 95% CI 0.76–0.98), had a protective effect for female (OR = 0.86, 95% CI 0.78–0.94) and rectal cancer patients (OR = 0.91, 95% CI 0.85–0.99). Nevertheless, all ORs and 95% CIs were close to 1, suggesting that tea consumption was just a slightly prevention strategy for colorectal cancer. Further, they recruited case–control studies for meta-analysis, which might be influenced by recall bias and reverse causality [16].
In the present study, we provided a meta-analysis based on prospective cohort studies to evaluate the association between tea consumption and colorectal cancer risk. Participants recruited in our meta-analysis was up to about 2 million, outpacing the previous meta-analysis by more than about 0.5 million [17]. Thus, our meta-analysis could offer more precise and credible risk estimate than the previous meta-analysis. We found that highest vs. lowest level of tea consumption was not associated with a decreased risk of colorectal, colon, or rectal cancer. It is also worth noting that the differences of morbidity and pathogenesis of colorectal cancer exist between men and women, which may lead to potential differences of tea consumption on the prevention of colorectal cancer [1,2,3]. So, we further conducted the gender-specific meta-analysis for deriving a more precise estimation. No significant association was observed between tea consumption and colorectal cancer risk in male. However, tea consumption had a marginal significant inverse impact on colorectal cancer risk in female.
Several limitations should be taken into consideration for our study. First, some studies included in our meta-analysis had certain weakness in experimental design, such as non-stratification of tea type and caner site. Additionally, although some important confounding factors including gender, age, and smoking were included in the most of studies, some other potentially important variables, such as coffee, alcohol, and fruits, were ignored in some studies. Besides, colorectal cancer is an extremely complicated and heterogeneous disease, which is well-known for remarkable global variations in etiology and morbidity [1,2,3]. Heterogeneity could not be fully eliminated in the present meta-analysis. The results obtained in the present study should be considered cautiously due to the existence of confounding factors. Second, measurement error in dietary assessment is an inherent problem [39]. The methods for measuring tea consumption in the included studies were different, which may result in the deviation of risk estimate values and confounding factors. In fact, we detected marginal to moderate heterogeneity among all studies. Third, the sample size of Asians in the present meta-analysis was relatively large due to the popularity of tea in Asia, especially in China and Japan, resulting in the potential selection bias [4, 18]. The results should be cautiously extrapolated to the populations in other countries.
Conclusions
Our finding suggests that tea consumption has no significant impact on the colorectal cancer risk in both genders combined, but gender-specific meta-analysis indicates that tea consumption has a marginal significant inverse impact on colorectal cancer risk in female. Large prospective cohort studies are warranted to reach a more definitive conclusion on the association between tea consumption and colorectal cancer risk.
References
Siegel RL, Miller KD, Fedewa SA et al (2017) (2017) Colorectal cancer statistics. Cancer J Clin 67(3):177–193
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 68(6):394–424
Li XY, Yu CQ, Guo Y et al (2019) Association between tea consumption and risk of cancer: a prospective cohort study of 0.5 million Chinese adults. Eur J Epidemiol 34(8):753–763
Zhu MZ, Wen BB, Wu H et al (2019) The quality control of tea by near-infrared reflectance (NIR) spectroscopy and chemometrics. J Spectrosc. https://doi.org/10.1155/2019/8129648
Zhu MZ, Li N, Zhao M, Yu WL, Wu JL (2017) Metabolomic profiling delineate taste qualities of tea leaf pubescence. Food Res Int 94:36–44
Yang CS, Maliakal P, Meng XF (2002) Inhibition of carcinogenesis by tea. Annu Rev Pharmacol 42:25–54
Nakagawa K, Miyazawa T (1997) Chemiluminescence high-performance liquid chromatographic determination of tea catechin, (-)-epigallocatechin 3-gallate, at picomole levels in rat and human plasma. Anal Biochem 248(1):41–49
Gan RY, Li HB, Sui ZQ, Corke H (2018) Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): an updated review. Crit Rev Food Sci 58(6):924–941
Yang G, Zheng W, Xiang YB et al (2011) Green tea consumption and colorectal cancer risk: a report from the Shanghai Men's Health Study. Carcinogenesis 32(11):1684–1688
Su LJ, Arab L (2002) Tea consumption and the reduced risk of colon cancer—results from a national prospective cohort study. Public Health Nutr 5(3):419–425
Dominianni C, Huang WY, Berndt S, Hayes RB, Ahn J (2013) Prospective study of the relationship between coffee and tea with colorectal cancer risk: the PLCO Cancer Screening Trial. Br J Cancer 109(5):1352–1359
Sinha R, Cross AJ, Daniel CR et al (2012) Caffeinated and decaffeinated coffee and tea intakes and risk of colorectal cancer in a large prospective study. Am J Clin Nutr 96(2):374–381
Sun CL, Yuan JM, Koh WP, Yu MC (2006) Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis 27(7):1301–1309
Wang XJ, Zeng XT, Duan XL, Zeng HC, Shen R, Zhou P (2012) Association between green tea and colorectal cancer risk: a meta-analysis of 13 case-control studies. Asian Pac J Cancer Prev 13(7):3123–3127
Wang ZH, Gao QY, Fang JY (2012) Green tea and incidence of colorectal cancer: evidence from prospective cohort studies. Nutr Cancer 64(8):1143–1152
Chen YT, Wu Y, Du ML et al (2017) An inverse association between tea consumption and colorectal cancer risk. Oncotarget 8(23):37367–37376
Wada K, Oba S, Tsuji M et al (2019) Green tea intake and colorectal cancer risk in Japan: the Takayama study. Jpn J Clin Oncol 49(6):515–520
Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Duan P, Hu CH, Quan C et al (2015) Body mass index and risk of lung cancer: systematic review and dose-response meta-analysis. Sci Rep Uk. https://doi.org/10.1038/srep16938
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Br Med J 327(7414):557–560
Haghighatdoost F, Nobakht BF, Hariri M (2018) Effect of green tea on plasma leptin and ghrelin levels: a systematic review and meta-analysis of randomized controlled clinical trials. Nutrition 45:17–23
Goldbohm RA, Hertog MGL, Brants HAM, vanPoppel G, vandenBrandt PA (1996) Consumption of black tea and cancer risk: a prospective cohort study. J Natl Cancer Inst 88(2):93–100
Zheng W, Doyle TJ, Kushi LH, Sellers TA, Hong CP, Folsom AR (1996) Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am J Epidemiol 144(2):175–182
Hartman TJ, Tangrea JA, Pietinen P et al (1998) Tea and coffee consumption and risk of colon and rectal cancer in middle-aged Finnish men. Nutr Cancer 31(1):41–48
Terry P, Wolk A (2001) Tea consumption and the risk of colorectal cancer in Sweden. Nutr Cancer 39(2):176–179
Nagano J, Kono S, Preston DL, Mabuchi K (2001) A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan). Cancer Cause Control 12(6):501–508
Michels KB, Willett WC, Fuchs CS, Giovannucci E (2005) Coffee, tea, and caffeine consumption and incidence of colon and rectal cancer. J Natl Cancer Inst 97(4):282–292
Suzuki Y, Tsubono Y, Nakaya N et al (2005) Green tea and the risk of colorectal cancer: pooled analysis of two prospective studies in Japan. J Epidemiol 15(4):118–124
Oba S, Shimizu N, Nagata C et al (2006) The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: a prospective study in Japan. Cancer Lett 244(2):260–267
Sun CL, Yuan JM, Koh WP, Lee HP, Yu MC (2007) Green tea and black tea consumption in relation to colorectal cancer risk: the Singapore Chinese Health Study. Carcinogenesis 28(10):2143–2148
Yang G, Shu XO, Li HL et al (2007) Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiol Biomark Prev 16(6):1219–1223
Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S (2007) Coffee consumption and risk of colorectal cancer in a population-based prospective cohort of Japanese men and women. Int J Cancer 121(6):1312–1318
Suzuki E, Yorifuji T, Takao S et al (2009) Green tea consumption and mortality among Japanese elderly people: the prospective Shizuoka Elderly Cohort. Ann Epidemiol 19(10):732–739
Simons CCJM, Leurs LJ, Weijenberg MP, Schouten LJ, Goldbohm RA, van den Brandt PA (2010) Fluid intake and colorectal cancer risk in the Netherlands Cohort Study. Nutr Cancer 62(3):307–321
Nechuta S, Shu XO, Li HL et al (2012) Prospective cohort study of tea consumption and risk of digestive system cancers: results from the Shanghai Women's Health Study. Am J Clin Nutr 96(5):1056–1063
Goldbohm RA, VandenBrandt PA (1996) Consumption of black tea and cancer risk: a prospective cohort study—response. J Natl Cancer Inst 88(11):768–769
Yang CS, Wang H (2016) Cancer preventive activities of tea catechins. Molecules. https://doi.org/10.3390/molecules21121679
Weng H, Zeng XT, Li S, Kwong JSW, Liu TZ, Wang XH (2017) Tea consumption and risk of bladder cancer: a dose–response meta-analysis. Front Physiol. https://doi.org/10.3389/fphys.2016.00693
Funding
This research was funded by National Key R&D Program of China (Grant no. 2018YFC1604405), China Postdoctoral Science Foundation (Grant no. 2018M632962), Hunan Provincial Natural Science Foundation of China (Grant no. 2019JJ50238), Open Foundation of Hunan Provincial Key Laboratory for Germplasm Innovation and Utilization of Crop (Grant no. 18KFXM10), Natural Science Foundation of China (Grant no. 31901672), Provincial Natural Science Foundation of Anhui (Grant no. 1908085MC57), and Macau Science and Technology Development Fund (Grant no. 009/2017/A1).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception and design of the study. MZ, DL, and FZ conducted the search and data extraction. JO, PH, BG, JT, FS, JL, XZ, YL, HL, SC, JH, JL, KW, and JW collected the data. MZ and ZL analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhu, Mz., Lu, Dm., Ouyang, J. et al. Tea consumption and colorectal cancer risk: a meta-analysis of prospective cohort studies. Eur J Nutr 59, 3603–3615 (2020). https://doi.org/10.1007/s00394-020-02195-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02195-3