Abstract
Purpose
In the present study, we investigated whether intra-islet GLP-1 production and its modulation have a role in apoptosis, proliferation or neogenesis that is compromised by protein restriction during the foetal and suckling periods.
Methods
Exendin-4, a GLP-1 receptor agonist (treated groups), or saline (non-treated groups) was intraperitoneally administered for 15 days from 75 to 90 days of age in female adult rats consisting of offspring born to and suckled by mothers fed a control diet (control groups) and who had the same diet until 90 days of age or offspring born to and suckled by mothers fed a low-protein diet and who were fed the control diet after weaning until 90 days of age (protein-restricted group).
Results
The β-cell mass was lower in the protein-restricted groups than in the control groups. Exendin-4 increased β-cell mass, regardless of the mother’s protein intake. The colocalization of GLP-1/glucagon was higher in the protein-restricted rats than in control rats in both the exendin-4-treated and non-treated groups. The frequency of cleaved caspase-3-labelled cells was higher in the non-treated protein-restricted group than in the non-treated control group and was similar in the treated protein-restricted and treated control groups. Regardless of treatment with exendin-4, Ki67-labelled cell frequency and β-catenin/DAPI colocalization were elevated in the protein-restricted groups. Exendin-4 increased the area of endocrine cell clusters and β-catenin/DAPI and FoxO1/DAPI colocalization regardless of the mother’s protein intake.
Conclusions
Protein restriction in early life increased intra-islet GLP-1 production and β-cell proliferation, possibly mediated by the β-catenin pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein restriction at critical developmental phases, such as during intrauterine life and lactation, causes morphological adaptations that continue to be expressed even with the recovery of nutritional status and results in a predisposition to the development of type-2 diabetes mellitus (T2DM) in adult life [1]. β-Cell mass is maintained by the balance between production (replication, neogenesis and transdifferentiation) and death (apoptosis, necrosis and autophagy), as well as by the number and size of β-cells [2, 3]. Replication refers to the expansion of preexisting adult β-cells through cell division, whereas neogenesis comprises β-cell differentiation from progenitors within the pancreas [4]. Proteins expressed at certain times in the cell cycle, such as Ki67, have been used as cell replication markers [2]. For neogenesis identification, the criterion most commonly used is islet hormone-positive cells that seem to be budding from the pancreatic duct epithelium or tiny clusters (one to three cells) of scattered insulin-positive cells [5]. Apoptosis is a cellular process characterized by membrane blebbing, cell shrinkage, condensation of chromatin, and fragmentation of DNA followed by rapid engulfment of the corpse by neighbouring cells [6]. The identification of highly condensed and/or fragmented nuclei (apoptotic bodies) with DNA dyes, such as propidium iodide, is a classic morphological method for apoptosis detection [2]. Another marker for apoptosis is cleaved caspase-3, the main effector caspase of the apoptotic cascade within cells [7]. The two mechanisms of β-cell formation from the embryo, neogenesis or differentiation from ductal precursor cells, and replication of a differentiated β-cell, are maintained postnatally and even in adult life [4]. In humans with T2DM and in a mouse model of T2DM, apoptosis is the major cause of β-cell death [8]. In adult rats that recover from protein restriction in the early stages of life, a reduction in β-cell mass is attributed to low proliferative capacity and elevated apoptosis [9].
Glucagon-like peptide-1 (GLP-1) is an important incretin hormone that increases β-cell mass by stimulating replication and neogenesis, as well as by reducing apoptosis by activation of several signalling pathways see [10]. Several transcription factors and signalling molecules are involved in the anti-apoptotic and proliferative actions of GLP-1. In MIN6 cells (a line cell of β-cells) exposed to hydrogen peroxide, activation of GLP-1 receptor (GLP-1R) by exendin-4 inhibits apoptosis, and this effect depends on 3′,5′-cyclic adenosine monophosphate (cAMP) and phosphoinositide 3-kinase (PI3K) in association with the upregulation of β-cell lymphoma-2 (Bcl-2) [11]. Moreover, GLP-1 inhibits forkhead box transcription factor (Fox) subclass 1 (FoxO1), a transcriptional effector of insulin signalling and insulin-like growth factor 1, promoting its phosphorylation and consequent nuclear exclusion. Inhibition of FoxO1 increases the expression of pancreatic and duodenal homeobox 1 (PDX-1), with antiapoptotic, neogenic and proliferative effects [12]. The Wnt signalling pathway, initially discovered for its role in tumourigenesis, has as its key effector the bipartite transcription factor β-cat/TCF, formed by free β-catenin (β-cat) and a TCF protein, such as transcription factor 7-like 2 (TCF7L2) [13]. Exendin-4 stimulates the Wnt/β-cat/TCF7L2 pathway through cAMP/PKA signalling and increases cyclin D1 transcription, contributing to β-cell proliferation. Finally, GLP-1 induces the expression of transcription factors through the β-catenin signalling pathway, contributing to an increase in β-cell mass [14,15,16].
In intestinal L cells, GLP-1 is produced from the cleavage of proglucagon by the enzyme prohormone convertase 1/3 (PC1/PC3), whereas in pancreatic α-cells, proglucagon is cleaved into glucagon by the action of prohormone convertase 2 (PC2) [17]. In normal pancreatic α-cells, little or no PC1/PC3 is detected [18,19,20,21]. However, in stress conditions or during β-cell regeneration, PC1/PC3 expression is upregulated in α-cells [19,20,21,22]. There is a significant increase in PC1/PC3 and GLP-1 expression in α-cells from diabetic rats [20], in murine models of insulin resistance [ob/ob, db/db, NOD (nonobese diabetic)] and in situations typically associated with islet cell expansion (pregnancy or neonatal stage) [19]. The increased GLP-1 expression in islets under stress and/or from β-cell damage suggests that this hormone produced in the islet contributes to cell survival [16, 22,23,24]. Furthermore, the positive regulation of GLP-1 in α-cells that are undergoing expansion (pregnancy and neonatal phases) or regeneration (ob/ob, db/db, NOD) models of β-cells suggests that GLP-1 produced in pancreatic islet cells promotes cell proliferation [10, 25].
We have shown that female and male rats that are protein-restricted during intrauterine and lactation phases have a monophasic pattern of insulin secretion [26, 27]. However, in female rats, the first phase of insulin secretion is preserved and potentiated, while in males, it is abolished. Interestingly, GLP-1 potentiates the first phase of insulin secretion. Thus, we evaluated whether the intra-islet production of GLP-1 and its modulator effect on apoptosis, proliferation and neogenesis were altered by early protein restriction. Because the effects of GLP-1 are limited due its short half-life (1–2 min) as result of cleavage by the enzyme dipeptidyl peptidase 4 (DPP-4), we used a GLP-1R agonist, exendin-4, which is resistant to the action of this enzyme and is already used in the treatment of diabetic patients.
Material and methods
The experimental procedures were performed in accordance with the guidelines of the Brazilian Society for Science in Laboratory Animals (SBCAL) and were approved by the Ethics Committee at the Federal University of Mato Grosso (protocol number: 23108.015308/14-1).
Animals and diets
Male (n = 6) and virgin female (n = 25) Wistar rats (85–90 days old) were obtained from the Cuiabá University breeding colony. Pairing was performed by housing males with females overnight (1 male to 4 females), and pregnancy was confirmed based on the examination of vaginal smears for the presence of sperm. Pregnant females were separated at random and maintained from the first day of pregnancy until the end of lactation on isocaloric diets containing either 17% (control diet) or 6% protein (low-protein diet), constituting control (C, n = 12 rats) and protein-restricted (PR, n = 13 rats), respectively. Spontaneous delivery took place on day 22 of pregnancy, after which, at 3 days of age, large litters were reduced to eight pups to ensure a standard litter size per mother. At weaning (4 weeks after birth), female pups were divided into two groups: C, consisting of offspring born to and suckled by mothers fed a control diet and who were subsequently fed the same diet after weaning until 90 days of age; and PR, consisting of the offspring of mothers fed a protein-restricted diet and who were subsequently fed the control diet after weaning until 90 days of age. In this phase, rats were maintained in collective cages (three or four rats/cage).
The diets were isocaloric, as described in Table 1. Food intake was recorded three times/week during the experimental period. The pre-weighed diet was provided, and after 48 h, rats were briefly removed from their cages. The amount of food remaining, including any food on the bottom of the cages, was recorded. Food intake was calculated as the weight (in grams) of diet provided less that recovered. It is expressed in absolute or relative values.
Body weight was recorded at 3 and 28 days of age, and after weaning, the measurement of body weight was performed once a week.
At 75 days of age, the rats were divided into four groups according to treatment with the GLP-1 receptor agonist and the mother’s protein intake: non-treated control (NTC, n = 11 rats), consisting of rats receiving daily saline injections (0.9% sodium chloride solution); treated control (TC, n = 13 rats), consisting of rats treated with the GLP-1 receptor agonist; non-treated protein restricted (NTPR, n = 13 rats), consisting of rats from the protein-restricted group receiving daily saline injections; and treated protein-restricted (TPR, n = 15 rats), consisting of rats from the protein-restricted group treated with the GLP-1 receptor agonist. Throughout the treatment, rats were housed alone in a cage.
During the experimental period, rats had free access to food and water and were housed at 22 °C with a 12 h light/dark cycle.
Treatment
In the TC and TPR groups, the GLP-1 receptor agonist exendin-4 (Sigma-Aldrich, USA, E7144) was administered intraperitoneally at a dose of 0.3 μg/kg (1.1 mL/kg body weight) dissolved in 0.9% saline solution. The NTC and NTPR groups received 0.3 μg/kg (1.1 mL/kg body weight) of 0.9% saline solution. This procedure was performed daily at 5:00 pm for a period of 15 days.
Oral glucose-tolerance test (OGTT)
After a 12 h fast, glucose (200 g/L) was administered orally at a dose of 2 g/kg of body weight. Blood samples were obtained from the cut tip of the tail 0, 15, 30, 60 and 120 min later for the determination of blood glucose using an Accu‐Chek glucometer and serum insulin concentration by ELISA. The stimulation index was calculated as the insulin concentration 15 min after glucose load divided by the insulin concentration before the glucose load.
Euthanasia, collection of biological material, biochemical and hormonal analyses
In the present study, we used two cohorts of rats. One was for the oral glucose tolerance test, measurement of pancreatic insulin content and histological procedures. Another cohort of animals was used for real-time PCR. A representative sample of each cohort was used for serum biochemical analyses.
One day after the oral glucose tolerance test, rats were euthanized after a 12 h fast for collection of biological material. Initially, rats were narcotized in a CO2 chamber and euthanized by decapitation. The pancreas was removed, dissected, and weighed for morphometric and immunohistochemical analyses or for measurement of insulin content. Pancreatic tissue was homogenized using buffer containing 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA and 1% Triton X 100 and stored at − 80 °C [25]. Samples of pancreas homogenate were diluted (1:10,000) for insulin determination. Pancreatic insulin was analysed by specific enzyme-linked immunoassays (Insulin Total ELISA, EZRMI-13K, Millipore, USA) following the manufacturer’s instructions. All commercial assay kits have been shown to exhibit a high degree of specificity. For real-time PCR, the pancreases were removed, dissected and digested with collagenase.
Blood samples were collected in tubes without additives and without anticoagulants (BD Vacutainer, Becton and Dickinson, Brazil) and were centrifuged for 20 min at 2465 ×g. The serum was separated and stored at − 80 °C for the subsequent measurement of urea, alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) using commercial kits (Bioclin/Quibasa, Brazil).
Histological processing
Five rats from each group were used for the histological studies. Pancreatic tissues were fixed in 4% paraformaldehyde solution for 24 h at 4 °C, dehydrated in increasing concentrations of ethanol, clarified in xylol, and embedded in paraffin using a histological processor (MTP 100 Slee, Mainz, Germany). Tissue was serially sectioned at 3 µm thickness throughout its length using a microtome (Leica RM2125, Leica Biosystems Nussloch, Germany) and mounted on slides with an adhesive surface. Ten pancreatic sequential sections were deparaffinized in an oven at 60 °C for 2 h, followed by immersion in xylol and descending concentrations of ethanol. After, the slides were rehydrated and stained with haematoxylin and eosin for determination of pancreatic areas using a light microscope at 5× magnification (Axio Scope. A1, Carl Zeiss, Oberkochen, Germany). Pancreatic areas were used for determination of β- and α-cell masses as described previously [28]. For immunofluorescence, after antigen retrieval at 70 °C for 1 h using citrate buffer (pH 6.0), the sections were blocked using 5% albumin serum and incubated overnight at 4 °C with primary antibodies. In the same section were simultaneously used (1) mouse monoclonal anti-Ki67 antibody (Dako, Carpinteria, USA; M7249; 1:50), guinea pig polyclonal anti-insulin antibody (Dako, Carpinteria, USA; A0564; 1:200) and rabbit polyclonal anti-glucagon antibody (Dako, Carpinteria, USA; A0565; 1:200); (2) mouse monoclonal anti-PC2 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, USA; SC-374140; 1:100) and rabbit polyclonal anti-glucagon antibody; (3) mouse monoclonal anti-PC1, 3 antibody (Abcam plc, England; ab55543, 1: 33) and rabbit polyclonal anti-glucagon antibody; (4) mouse monoclonal anti-GLP-1 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, USA;SC-80602; 1:200) and rabbit polyclonal anti-glucagon antibody; (5) rabbit polyclonal anti-GLP-1R antibody (Thermo Fisher Scientific, USA; PA5-72433, 1:100) and guinea pig polyclonal anti-insulin antibody; or (6) mouse monoclonal anti-β-catenin antibody (Abcam plc, England; ab6301, 1:100) and rabbit polyclonal anti-glucagon antibody. Rabbit monoclonal anti-FoxO1 antibody (Cell Signaling Technology, USA; #2880S, 1:100), mouse monoclonal anti-PDX-1 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, USA; sc-390792, 1:120) and rabbit polyclonal anti-cleaved caspase-3 antibody (Cell Signaling Technology, USA; #9661S, 1:200) were used in sections separately. The sections were subsequently incubated for 1 h at room temperature with an appropriate secondary antibody (Alexa Fluor AF-555 goat anti-mouse, Alexa Fluor 647 goat anti-guinea pig IgG, or Alexa Fluor 488 goat anti-rabbit. Thermo Fisher Scientific, USA). 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich, USA, D9564, 1:3000) was used as a cell nucleus marker. The antibody dilution and methods were previously tested and validated in our laboratory.
Propidium iodide (PI) was used to identify morphologically altered nuclei, which are indicative of cell death, as previously described [29]. To this end, deparaffinized and rehydrated pancreas sections were incubated for 40 min in a dark, humidified chamber in a solution of PI (Sigma-Aldrich, USA; P4170, 20 μg/mL) and ribonuclease A (Sigma-Aldrich, USA; R6513, 100 μg/mL). The labelled apoptotic nuclei of endocrine cells in the pancreas sections were counted in digitized islet images.
At least 10 fields from each section were photographed to make morphometric measurements using an Axio Scope A1 microscope (Carl Zeiss, Germany). Image capture and analysis were performed by a blinded observer.
Morphometry
For determination of the islet and β-cell areas, entire pancreatic sections stained for insulin were imaged at 20× magnification using an Axio Scope A1 microscope (Carl Zeiss, Germany) equipped with a motorized stage. A tile image of the tissue section was generated using AxioVision version 4.8.2 software (Carl Zeiss). The areas of the pancreas stained positive for insulin were digitally quantified with a colour-based threshold using AxioVision software. The α-cell area was obtained by subtracting the β-cell area from the total islet area. The α- and β-cell relative areas were calculated by the ratio between the α- and β-cell areas and the pancreas area. The islet relative area was obtained by the ratio between the islet area and the total pancreatic tissue area and multiplying the result by 100. The α- and β-cell masses were calculated by multiplying the total β-cell volume (the product of the sum of the entire α- or β-cell and the total pancreas area) by the pancreas weight. The sizes of the β-cells were obtained by dividing the β-cell area by the number of β-cells. It is important to emphasize that this method overestimates size because the actual number of cells is probably greater than the number counted since not all cells are sectioned in their nuclei [30].
The endocrine cell proliferation frequency was assessed by dividing the total number of endocrine cells by the number of Ki67-positive endocrine cells in one islet and multiplying the result by 100. The ratio between proliferation and α- or β-cell mass was obtained by dividing the frequency of Ki67-positive endocrine cells by the α- or β-cell mass.
The index of apoptotic cells was obtained by dividing the number of apoptotic nuclei labelled with PI or apoptotic cells labelled with cleaved caspase-3 in one islet by the total number of endocrine cells in one islet and multiplying the result by 100. The ratio between apoptosis and α- or β-cell mass was obtained by dividing the frequency of cells labelled with cleaved caspase-3 by the α- or β-cell mass. The ratio between proliferation and apoptosis in endocrine cells was obtained by dividing the frequency of Ki67-positive endocrine cells by the number of endocrine cells labelled with cleaved caspase-3.
GLP-1 fluorescence was measured using AxioVision 4.9.1 software (Carl Zeiss). Colocalization analysis was carried out using the “JACoP” plugin in ImageJ and expressed as Pearson’s correlation coefficient. Pearson’s correlation coefficient measures the pixel-by-pixel covariance in the signal levels of two images, subtracting the mean intensity from each pixel’s intensity value. Hence, Pearson’s correlation coefficient is independent of signal levels and signal offset (background) [31]. A value near or equal to − 1.0 represents no colocalization, while a value near or equal to 1.0 represents full colocalization of two antigens. Data from five to seven sections were averaged for each pancreas.
Islet isolation
The pancreas was removed from each rat and digested with collagenase (Boehringer Mannheim). Briefly, the pancreas was inflated with Hanks solution containing 0.7–0.9 g/L collagenase and then excised and maintained at 37 °C for 20 min. The tissue was harvested, and the islets were handpicked under a stereomicroscope for quantitative real-time PCR. Pancreatic islet pools were used; each contained approximately 300 islets and was obtained from two rats.
RNA extraction and quantitative real-time PCR
Total RNA from 300 islets was extracted using TRIzol® (Invitrogen, Brazil) and homogenized for 1 min using a vortex. Total RNA was isolated and quantified using a spectrophotometer. Complementary DNA was prepared using 3 μg of total RNA and reverse transcriptase and transcribed into cDNA using a TaqMan mRNA reverse transcription kit (Applied Biosystems, LTU, Ref: 4368814). The primers used in the experiments were standard TaqMan primers (Applied Biosystems, USA). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA was used as an endogenous control (TaqMan Rodent GAPDH Control Reagents, Ref: 4352338E). The genes analysed were GLP-1R (GenBank Rn00562406_m1), Bcl-2 (GenBank Rn99999125-m1), caspase 3 (GenBank Rn00563902-m1) and Pdx-1 (GenBank Rn00755591-m1). Real-time polymerase chain reaction was carried out in a StepOne polymerase chain reaction cycler (Applied Biosystems, USA). The polymerase chain reaction conditions were 95 °C for 10 min, followed by 45 cycles at 95 °C for 10 s and 60 °C for 45 s. Real-time data were analysed using a Sequence Detector System 1.7 (Applied Biosystems, USA). Relative quantitation of gene expression was performed by comparing the efficiency of amplification of each gene of interest using the ΔCt method, as described by the manufacturer (Applied Biosystems, USA). The results are expressed as fold inductions compared with endogenous controls.
Statistical analysis
The number of rats used was determined by the resource equation method [32]. The results are expressed as the means ± SD for the number of indicated rats (n). Levene’s test for the homogeneity of variances was initially used to check the fit of the data to parametric assumptions. The Shapiro–Wilk test was used to test data normality. When necessary, data were transformed to correct for variance heterogeneity. Body weight at birth, cumulative food intake during the recovery phase, PC1/PC3/glucagon colocalization and PC2/glucagon colocalization from the C and R groups were compared with the unpaired t test. Changes in body weight during lactation and during the growth phase after weaning, as well as blood glucose and serum insulin concentration curves, were evaluated by two-way repeated-measures ANOVA. A two-way ANOVA (effects of nutritional status and exendin-4 treatment) was used to evaluate the in vivo data, mRNA expression, biochemical and hormonal data, and histological and morphometric data. The relative frequency distribution of islet size was evaluated and classified as follows: small islets-area ≤ 10,000 μm2; medium islets-area > 10,000 and ≤ 25,000 μm2; and large islets-area > 25,000 μm2. A three-way ANOVA was used to evaluate the effects of the factors tested (nutritional status (NS), exendin-4 treatment and islet size classification (Clas)) using a customized model (Clas, NS × Clas, exendin-4 × Clas, NS × exendin-4 × Clas). When necessary, these analyses were followed by Tukey’s test to determine the significance of individual differences. The level of significance was set at P < 0.05. The data were analysed using the Statistica software package (Statsoft).
Results
At 3 days of age, the body weights of the PR and C groups were similar, and at 28 days of age, both groups had higher body weights than at 3 days (P < 0.0001). However, the body weight of the PR group was lower than that of the C group at 28 days of age (P < 0.0001) (Fig. 1a). During the phase of growth after weaning, the PR group showed a lower body weight compared to the C group at all times evaluated (Fig. 1b). The cumulative food intake from 28 days until 75 days was significantly lower in the PR than in the C rats (P < 0.05) (Fig. 1c). During the 15 days treatment with exendine-4 or saline, body weight gain did not differ among groups (Fig. 1d). At 90 days of age, protein-restricted rats showed lower body weight compared to control rats (F1,42 = 15.91, P < 0.0003) (Fig. 1e). In this phase, the cumulative food intake was significantly higher in the protein-restricted groups than in the control groups (F1,16 = 20.81, P < 0.0003) (Fig. 1f). Food intake relative to 100 g body weight was higher in the protein-restricted groups compared to the control groups (F1,16 = 92.6, P < 0.00001) but lower in the treated groups compared to the non-treated groups (F1,16 = 6.33, P < 0.02) (Fig. 1g).
a Changes in body weight during lactation of offspring born to and suckled by mothers fed a control (n = 20 rats) or a low-protein diet (n = 26 rats). b Changes in body weight and c cumulative food intake during the growth phase after the weaning of offspring born to and suckled by mothers fed a control diet and who were subsequently fed the same diet after weaning (C group, n = 20 rats) or of offspring born to and suckled by mothers fed a low-protein diet and who were subsequently fed a control diet after weaning (PR group, n = 26 rats). d Body weight gain, e body weight at 90 days age, f cumulative food intake, and g relative food intake of non-treated and treated rats from the control (NTC group, n = 5; TC group, n = 5) and protein-restricted groups (NTPR group, n = 5; TPR group, n = 5). Values are the mean with standard deviation. *Indicates effect of nutritional status (two-way ANOVA, P < 0.05). #Indicates effect of treatment (two-way ANOVA, P<0.05) §Indicates a significant difference (independent samples t test, P < 0.05). Different superscript letters denote significant differences (Tukey’s test P < 0.05)
The basal blood glucose and serum insulin concentrations did not differ among the groups (Fig. 2a–d). The blood glucose concentration was similar in all groups after a glucose load (Fig. 2a, b). 15 min after the glucose load, the serum insulin concentration was similar in the NTPR and TPR groups but was higher in the TC group than in the NTC group (Fig. 2c, d). The stimulation index was significantly higher in the TC group than in the NTC group (P < 0.03) and in both groups this variable did not differ to the NTPR and TPR groups (Fig. 2e).
a Blood glucose concentration curves of non-treated and treated rats from the control groups and b of non-treated and treated rats from the protein- restricted groups. c Serum insulin concentration curves of non-treated and treated rats from the control groups and d of non-treated and treated rats from the protein-restricted groups. e Stimulation index obtained from the glucose oral tolerance test of non-treated and treated rats from the control or protein-restricted groups. a,bMean values are significantly different (Tukey’s test, P < 0.05)
The ALP, AST and ALT concentrations were lower in the treated groups compared to the non-treated groups (F1,16 = 5.90, P < 0.0273; F1,15 = 5.98, P < 0.02; F1,16 = 9.70, P < 0.0067, respectively). The urea concentration did not differ among groups (Table 2).
The absolute pancreas weight was lower in the protein-restricted groups than in the control groups (F1,16 = 10.79, P < 0.0047). The pancreas weight relative to 100 g of body weight did not differ among the groups. The pancreatic insulin content per gram of pancreas and the content per milligram of protein was higher in the treated groups compared to the non-treated groups (F1,12 = 4.81, P < 0.0488; F1,12 = 12.30, P < 0.0043, respectively). The protein-restricted groups exhibited higher pancreatic insulin content per kilogram of body weight than the control groups (F1,12 = 17.16, P < 0.0014) (Table 3).
Regardless of the factors tested, 65% of the islets were small, 20% were medium, and 15% were large. ANOVA revealed significant effects of nutritional status (NS × classification) and the interaction between nutritional status and treatment with exendin-4 (NS × exendin-4 × classification) on the relative frequency distribution of islet size. Thus, nutritional status on its own and in conjunction with exendin-4 decreased the relative frequency of smaller islets and increased the frequency of larger islets. However, the NS × exendin-4 × classification interaction showed that this pattern was observed only in the control group (Fig. 3a). The β-cell size was similar among groups (Fig. 3b). The β-cell area relative to the total pancreas area was higher in the treated groups than in the non-treated groups (F1,9 = 5.14, P < 0.049) (Fig. 3c). The β-cell mass was lower in the protein-restricted groups compared to the control groups (F1,9 = 14.32, P < 0.0043) but higher in the treated groups compared to the non-treated groups (F1,9 = 10.43, P < 0.01) (Fig. 3d). GLP-1 fluorescence was higher in the protein-restricted groups than in the control groups (F1,16 = 27.23, P < 0.0001) (Fig. 3e). The GLP-1 fluorescence/α-cell mass ratio was higher in the protein-restricted groups than in the control groups, independent of treatment (F1,15 = 10.74, P < 0.005) (Fig. 3f). The islet and α-cell areas relative to the total pancreas area were similar among groups (data not shown).
a Relative frequency distribution of islet size, b β-cell size, c area of β-cells, d β-cell mass, e GLP-1 fluorescence, and f GLP-1/α-cell mass ratio of non-treated and treated rats from the control and protein-restricted groups. Values are means with standard deviations represented by vertical bars (n = 3–5 rats). *Indicates the effect of nutritional status (two-way ANOVA, P < 0.05). #Indicates the effect of treatment (two-way ANOVA, P < 0.05)
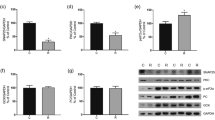
PC1/PC3/glucagon colocalization was lower in the PR group than in the C group (Fig. 4a and Fig. 8 in ESM). The PR and C groups exhibited similar PC2/glucagon colocalization (Fig. 4b, Fig. 8 in ESM). GLP-1/glucagon colocalization was higher in islets from the NTPR group than in islets from the NTC group (P < 0.001) and was higher in the TPR group than in the TC group (P < 0.02). Moreover, GLP-1/glucagon colocalization was higher in islets from the TC group compared to the NTC group (P < 0.0003) and did not differ between the TPR and NTPR groups (Fig. 4c, Fig. 9 in ESM). The colocalization of GLP-1R/insulin did not differ among groups (Fig. 4d, Fig. 10 in ESM).
a PC1/PC3/glucagon colocalization and b PC2/glucagon colocalization in islets from rats maintained on a control diet or low-protein diet during the intrauterine life and lactation. Values are means with standard deviation, represented by vertical bars (n = 5 rats per group). §Indicates a significant difference (independent samples t test, P < 0.05). c GLP-1/glucagon colocalization and d GLP-1R/insulin colocalization in the islets of non-treated and treated rats from the control (NTC = 5 and TC = 5) and recovered groups (NTR = 5 and TR = 5). a–cMean values are significantly different (Tukey’s test, P < 0.05)
PDX-1/DAPI colocalization was similar in all groups (Fig. 5a, Fig. 11 in ESM). FoxO1/DAPI colocalization was higher in the treated groups than in the non-treated groups (F1,15 = 7.60, P < 0.014) (Fig. 5b, Fig. 12 in ESM). β-Catenin/DAPI colocalization was higher in the protein-restricted groups than in the control groups (F1,16 = 12.47, P < 0.0028), as well as in the treated groups compared to the non-treated groups (F1,16 = 39.56, P < 0.0000) (Fig. 5c, Fig. 13 in ESM).
a PDX-1/DAPI colocalization, b FoxO1/DAPI colocalization, and c β-catenin/DAPI colocalization in the islets of non-treated and treated rats from the control and protein-restricted groups. Values are means with standard deviation, represented by vertical bars (n = 3–5 rats). *Indicates the effect of nutritional status (two-way ANOVA, P < 0.05). #Indicates the effect of treatment (two-way ANOVA, P < 0.05)
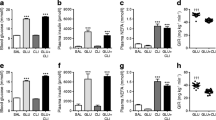
The frequency of PI-positive endocrine cells was higher in the protein-restricted groups compared to the control groups (F1,16 = 14.92, P < 0.0014) but lower in the treated groups compared to the non-treated groups (F1,16 = 9.02, P < 0.0084) (Fig. 6a, Fig. 14 in ESM). The NTPR group showed a higher frequency of cleaved caspase-3-positive endocrine cells than the NTC group (P < 0.0002). The frequency of cleaved caspase-3-positive endocrine cells was lower in the TPR group compared to the NTPR group (P < 0.0001), but the frequency in the NTPR group was similar to that in the TC group. There was no difference in the frequency of cleaved caspase-3-positive endocrine cells between the TC and NTC groups (Fig. 6b, Fig. 14 in ESM). The cleaved caspase-3/α-cell mass ratio was higher in the NTPR group compared to the NTC, TC and TPR groups (P < 0.029, P < 0.014 and P < 0.025, respectively) (Fig. 6c). The cleaved caspase-3/β-cell mass was higher in the protein-restricted groups compared to the control groups (F1,15 = 4.86, P < 0.043) but lower in the treated groups compared to the non-treated groups (F1,15 = 24.01, P < 0.0002) (Fig. 6d). The frequency of Ki67-positive endocrine cells was higher in the protein-restricted groups compared to the control groups (F1,16 = 22.2, P < 0.0002) (Fig. 6e, Fig. 15 in ESM). The Ki67/α-cell mass ratio and Ki67/β-cell mass ratio were higher in the protein-restricted groups than in the control groups (F1,15 = 8.22, P < 0.011; F1,15 = 9.93, P < 0.0066) (Fig. 6f, g). The Ki67/cleaved caspase-3 ratio was similar among the NTPR, NTC and TC groups, which all exhibited a lower Ki67/cleaved caspase-3 ratio compared to the TPR group (P < 0.002; P < 0.002; P < 0.003) (Fig. 6h). The cluster areas relative to the total pancreas area were higher in the treated groups compared to the non-treated groups (F1,11 = 6.73, P < 0.025) (Fig. 6i).
a Frequency of propidium iodide (PI)-positive endocrine cells, b frequency of cleaved caspase-3-positive endocrine cells, c cleaved caspase-3/α-cell mass ratio, d cleaved caspase-3/β-cell mass ratio, e frequency of Ki67-positive endocrine cells, f Ki67-positive endocrine cell/α-cell mass ratio, g Ki67-positive endocrine cell/β-cell mass ratio, h Ki67-positive endocrine cell/cleaved caspase-3 ratio, and i endocrine cell cluster area in relation to pancreas area in the islets of non-treated and treated rats from the control (NTC and TC) and protein-restricted (NTR and TR) groups. Values are means with standard deviation, represented by vertical bars (n = 4–5 rats). *Indicates the effect of nutritional status (two-way ANOVA, P < 0.05). #Indicates the effect of treatment (two-way ANOVA, P < 0.05). a–cMean values are significantly different (Tukey’s test, P < 0.05)
The Glp-1R and Bcl2 mRNA contents in the TPR, NTPR and TC groups were similar. However, these groups showed higher Glp-1R (P < 0.03; P < 0.0005; P < 0.007, respectively) and Bcl2 mRNA contents (P < 0.021; P < 0.02; P < 0.03, respectively) than the NTC group (Fig. 7a, b). The caspase-3 mRNA content was higher in islets from the protein-restricted groups compared to the control groups (F1,11 = 8.07, P < 0.016) (Fig. 7c). There was no difference among groups in Pdx-1 transcript (Fig. 7d).
a Glp-1R, b Bcl2, c caspase-3 and d Pdx-1 mRNA contents in the islets of non-treated and treated rats from control (NTC and TC) and protein-restricted (NTR and TR) groups. Values are means with standard deviation, represented by vertical bars (n = 4–5 rats). *Indicates the effect of nutritional status (two-way ANOVA, P < 0.05). a,bMean values are significantly different (Tukey’s test, P < 0.05)
Discussion
We evaluated the intra-islet GLP-1 production and its modulator effect on the regulator mechanisms of β-cell mass in adult female rats that were protein-restricted during the intrauterine and lactation stages or not. Although the protein-restricted group exhibited low PC1/PC3/glucagon colocalization, intra-islet GLP-1 production was increased in both protein-restricted groups compared to control groups, as shown by GLP-1 fluorescence, the GLP-1 fluorescence/α-cell mass ratio and GLP-1/glucagon colocalization. There are at least two plausible explanations for this paradox: (1) low PC1/PC3 expression was counter-regulated by its enhanced activity, since PC1/PC3, through its various domains, is capable of controlling its own enzymatic activity [33]; and (2) there was decreased DPP4 activity, as shown by the presence of this enzyme within α-cells [34].
As expected, exendin-4 produced an anorexigenic effect in both the control and protein-restricted groups, but not enough to interfere with body weight. It is interesting to note the difference in absolute food intake before vs. during treatment with exendin-4, especially in the protein-restricted groups. We attributed the increased dietary intake in protein-restricted groups during treatment to the synergistic effect of protein restriction on intrauterine life and lactation and the stress caused by the isolation of rats in individual cages. Both situations increase the expression of the neurotransmitter NPY in the hypothalamus [34, 36], a potent orexigenic peptide.
Based on the results from the oral glucose tolerance test, the control group treated with exendin-4 exhibited an elevated insulin stimulation index compared to the non-treated control group, despite the unaltered GLP-1 fluorescence in pancreatic islets. Curiously, the increase in the intra-islet GLP-1 did not produce the expected incretin effect in our protein-restricted groups. However, in a previous study [27], we verified that isolated islets from protein-restricted rats displayed a kinetics of insulin secretion characterized by a rapid increase after exposure to 11.1 mmol/L glucose (first-phase secretion), which resulted in a significant enhancement in the area under the insulin curve compared to the control rats. In the same study, an increase in glucose concentration from 2.8 to 11.1 mmol/L induced biphasic insulin secretion with a rapid first‐phase release after exposure to high glucose, followed by a decrease and then an increase during the second phase in islets from control rats. The discrepancies between the results of stimulatory indexes evaluated in the present study and the kinetics of insulin secretion verified previously [27] are not surprising. In vivo, β-cells are under the influence of glucose and other nutrients, hormones, and neurotransmitters, whereas in in vitro, islets are stimulated by glucose alone. Moreover, changes in peripheral insulin levels do not exclusively reflect alterations in insulin secretion because they are influenced by liver insulin extraction rates [37]. Thus, the intra-islet GLP-1 production verified in our protein-restricted animals and the kinetics of insulin secretion previously observed corroborate the idea that GLP-1 participates in making the first phase particularly prominent [38]. Although the increase in intra-islet GLP-1 content seems to have an impact on the increase of the first secretory phase, which is considered beneficial for metabolic health, the loss of the typical biphasic pattern in our model indicates that these islets are dysfunctional.
Interestingly, the protein-restrict rats exhibited reduced β-cell mass due to increased apoptosis, which was confirmed by an increase in caspase-3 transcripts, cleaved caspase-3 expression, cleaved caspase-3/β-cell mass ratio and frequency of PI-positive endocrine cells. It is noteworthy that these effects were observed despite increased mRNA Glp-1R and Bcl2 expression, which could contribute to cellular survival. At least in relation to GLP-1R, alterations in GLP-1R/insulin colocalization were not observed. Although these results appear controversial, it is known that transcription and translation are not always correlated because there are many processes governed by these mechanisms that can be regulated by hormones, nutrients such as amino acids, and energy state [39]. Moreover, it is important to emphasize that mRNA Glp-1R expression was determined in pancreatic islets and that its content was assessed exclusively in β-cells. The elevated α-cell apoptosis observed in the non-treated protein-restricted group, as shown by the cleaved caspase-3/α-cell mass ratio, was not reflected in the α-cell mass, possibly due to increased α-cell proliferation.
The proliferative effect of GLP-1 was observed in our protein-restricted animals, as shown by the elevated frequency of Ki67-positive endocrine cells, increased the ratios of Ki67/α-cell mass and Ki67/β-cell mass. This effect was possibly modulated by the β-catenin pathway, whose increase in nuclear localization contributes to the expression of genes that encode cyclins, contributing to the proliferation of β-cells [14]. Although studies have indicated that active β-catenin promotes the increase in FoxO1 in the nucleus and consequently decreases Pdx-1 transcription [40, 41], in the present study, these effects were not observed. Previous findings regarding elevated adenylate cyclase and PKA contents in islets from protein-restricted rats [42] support the hypothesis that β-catenin participates in the increased β-cell proliferation.
Exendin-4 treatment did not impair hepatic function; instead, it reduced the levels of hepatic enzymes related to insulin resistance, corroborating the report of its suppressor effect on hepatic glucose production [43]. Additionally, exendin-4 treatment increased the relative frequency of large islets, especially in the control group, and the relative area and absolute mass of β-cells in both treated groups. These effects resulted from the reduced apoptosis in the protein-restricted rats and increased neogenesis in both groups. Curiously, the activation of neogenesis was not mediated by PDX-1, since its transcription and nuclear localization were unaltered by exendin-4. In addition, in the present study, the increase in FoxO1 nuclear localization by exendin-4 did not correlate with apoptosis, contradicting the report that increased FoxO1 nuclear localization decreases cellular survival [44]. Although exendin-4 did not influence β-cell proliferation, its anti-apoptotic effect was able to increase the proliferation/apoptosis ratio only in the protein-restricted group, but not enough to restore the β-cell mass.
Taking into consideration the results obtained in the present study, we conclude that protein restriction in early life increased intra-islet GLP-1 production and β-cell proliferation, possibly mediated by the β-catenin pathway. Exendin-4 prevented apoptosis and increased neogenesis. Therefore, GLP-1 receptor analogues could be a therapeutic option to mitigate structural damage to pancreatic cells caused by adverse intrauterine and neonatal environments.
References
Berney DM, Desai M, Palmer DJ, Greenwald S, Brown A, Hales CN et al (1997) The effects of maternal protein deprivation on the fetal rat pancreas: major structural changes and their recuperation. J Pathol 183:109–115. https://doi.org/10.1002/(SICI)1096-9896(199709)183:1%3c109:AID-PATH1091%3e3.0.CO;2-B
Bonner-Weir S (2001) beta-cell turnover: its assessment and implications. Diabetes 50:S20–S24. https://doi.org/10.2337/diabetes.50.2007.s20
Fujimoto K, Hanson PT, Tran H, Ford EL, Han Z, Johnson JD et al (2009) Autophagy regulates pancreatic beta cell death in response to Pdx1 deficiency and nutrient deprivation. J Biol Chem 284:27664–27673. https://doi.org/10.1074/jbc.M109.041616
Bonner-Weir S, Aguayo-Mazzucato C, Weir GC (2016) Dynamic development of the pancreas from birth to adulthood. Ups J Med Sci 121:155–158. https://doi.org/10.3109/03009734.2016.1154906
Bonner-Weir S, Guo L, Li WC, Ouziel-Yahalom L, Lysy PA, Weir GC et al (2012) Islet neogenesis: a possible pathway for beta-cell replenishment. Rev Diabet Stud 9:407–416. https://doi.org/10.1900/RDS.2012.9.407
Renehan AG, Booth C, Potten CS (2001) What is apoptosis, and why is it important? BMJ 322:1536–1538. https://doi.org/10.1136/bmj.322.7301.1536
Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T et al (1998) Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med 187:587–600. https://doi.org/10.1084/jem.187.4.587
Rojas J, Bermudez V, Palmar J, Martínez MS, Olivar LC, Nava M et al (2018) Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J Diabetes Res 2018:9601801. https://doi.org/10.1155/2018/9601801
Petrik J, Reusens E, Arany C, Remacle C, Coelho C, Hoet JJ et al (1999) A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat. Endocrinology 140:4861–4873. https://doi.org/10.1210/endo.140.10.7042
Lee Y, Jun H (2014) Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 63:9–19. https://doi.org/10.1016/j.metabol.2013.09.010
Hui H, Nourparvar A, Zhao X, Perfetti R (2003) Glucagon-like peptide-1 inhibits apoptosis of phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 144:1444–1455. https://doi.org/10.1210/en.2002-220897
Buteau J, Spatz ML, Accili D (2006) Peptide-1 effects on pancreatic b-cell mass. Diabetes 55:1190–1196. https://doi.org/10.2337/db05-0825
Ip W, Chiang YA, Jin T (2012) The involvement of the Wnt signaling pathway and TCF7L2 in diabetes mellitus: the current understanding, dispute, and perspective. Cell Biosci 2:28. https://doi.org/10.1186/2045-3701-2-28
Liu Z, Habener JF (2008) Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J Biol Chem 283:8723–8735. https://doi.org/10.1074/jbc.M706105200
Elghazi L, Gould AP, Weiss AJ, Barker DJ, Callaghan J, Opland D et al (2012) Importance of b-Catenin in glucose and energy homeostasis. Sci Rep 2:1–12. https://doi.org/10.1038/srep00693
Figeac F, Uzan B, Faro M, Chelali N, Portha B, Movassat J (2010) Neonatal growth and regeneration of b-cells are regulated by the Wnt/b-catenin signaling in normal and diabetic rats. Am J Physiol Endocrinol Metab 298:245–256. https://doi.org/10.1152/ajpendo.00538.2009
Rouille Y, Westermarktt G, Martin SK, Steiner DF (1994) Proglucagon is processed to glucagon by prohormone convertase PC2 in aTC1-6 cells. Biochemistry 91:3242–3246
Hansen AMK, Nordestgaard DNE, Heller RS, Gotfredsen CF, Maedler K, Fels JJ et al (2011) Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus—an adaptive response to hyperglycaemia? Diabetologia 54:1379–1387. https://doi.org/10.1007/s00125-011-2080-1
Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M (2010) Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of β-cell regeneration. Islets 2:149–155
Nie Y, Nakashima M, Brubaker PL, Li Q, Perfetti R, Jansen E et al (2000) Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J Clin Invest 105:955–965. https://doi.org/10.1172/JCI7456
O’Malley TJ, Fava GE, Zhang Y, Fonseca VA, Wu H (2014) Progressive change of intra-islet GLP-1 production during diabetes development. Diabetes Metab 30:661–668. https://doi.org/10.1002/dmrr.2534.PROGRESSIVE
Whalley NM, Pritchard LE, Smith DM, White A (2011) Processing of proglucagon to GLP-1 in pancreatic α-cells: Is this a paracrine mechanism enabling GLP-1 to act on β-cells? J Endocrinol 211:99–106. https://doi.org/10.1530/JOE-11-0094
Huang C, Yuan L, Cao S (2015) Endogenous GLP-1 as a key self-defense molecule against lipotoxicity in pancreatic islets. Int J Mol Med 36:173–185. https://doi.org/10.3892/ijmm.2015.2207
Liu Z, Stanojevic V, Avadhani S, Yano T, Habener JF (2011) Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances beta cell survival. Diabetologia 54:2067–2076. https://doi.org/10.1007/s00125-011-2181-x
Moffett RC, Vasu S, Thorens B, Drucker DJ, Flatt PR (2014) Incretin receptor null mice reveal key role of GLP-1 but not GIP in pancreatic beta cell adaptation to pregnancy. PLoS One 9:1–10. https://doi.org/10.1371/journal.pone.0096863
Marin L, Silva HBF, Damin G, Ignacio-Souza LM, Reis SRL, de Oliveira CAM et al (2018) Nutritional recovery from a low-protein diet during pregnancy does not restore the kinetics of insulin secretion and Ca(2+) or alterations in the cAMP/PKA and PLC/PKC pathways in islets from adult rats. Appl Physiol Nutr Metab 43:1257–1267. https://doi.org/10.1139/apnm-2017-0629
Marin BK, Reis SRL, Ramalho AFS, Lemes SF, Marin L, Vanzela EC et al (2019) Protein restriction in early life increases intracellular calcium and insulin secretion, but does not alter SNARE proteins expression during pregnancy. Exp Physiol 104:1029–1037. https://doi.org/10.1113/EP087045
Matveyenko AV, Singh I, Shin BC, Georgia S, Devaskar SU (2010) Differential effects of prenatal and postnatal nutritional environment on ß-cell mass development and turnover in male and female rats. Endocrinology 151:5647–5656. https://doi.org/10.1210/en.2010-0978
Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S (1997) Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138:1736–1741. https://doi.org/10.1210/endo.138.4.5069
Movassat J, Saulnier C, Portha B (1995) Beta-cell mass depletion precedes the onset of hyperglycemia in the GK rat, a genetic model of non-insulindependent diabetes mellitus. Diabetes Metab 21:365–370
Dunn KW, Kamocka MM, McDonald JH (2011) A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Physiol 300:C723–C742. https://doi.org/10.1152/ajpcell.00462.2010
Charan J, Kantharia ND (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4:303–306. https://doi.org/10.4103/0976-500X.119726
Rabah N, Gauthier D, Dikeakos JD, Reudelhuber TL, Lazure C (2007) The C-terminal region of the proprotein convertase 1⁄3(PC1⁄3) exerts a bimodal regulation of the enzyme activity in vitro. FEBS J 3:3482–3491. https://doi.org/10.1111/j.1742-4658.2007.05883.x
Poulsen MD, Hansen GH, Dabelsteen E, Hoyer PE, No OVE, Sjt H et al (1993) Dipeptidyl peptidase IV is sorted to the secretory granules in pancreatic islet A-cells. J Histochem Cytochem 41:81–88
Plagemann A, Waas T, Harder T, Rittel F, Ziska T, Rohde W (2000) Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats. Neuropeptides 34:1–6. https://doi.org/10.1054/npep.1999.0778
Krolow R, Noschang C, Arcego DM, Huffell AP, Marcolin ML, Benitz AN et al (2013) Sex-specific effects of isolation stress and consumption of palatable diet during the prepubertal period on metabolic parameters. Metabolism 62:1268–1278. https://doi.org/10.1016/j.metabol.2013.04.009
Henquin JC, Nenquin M, Stiernet P, Ahren B (2006) In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: pattern and role of cytoplasmic Ca2+ and amplification signals in beta-cells. Diabetes 55:441–451. https://doi.org/10.2337/diabetes.55.02.06.db05-1051
Thomaseth K, Pavan A, Pacini G, Ahre B, Glucagon-like B (2007) Glucagon-like peptide-1 accelerates the onset of insulin action on glucose disappearance in mice. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/ajpendo.00303.2006
Kimball SR, Jefferson LS (2006) New functions for amino acids : effects on gene transcription and translation. Am J Clin Nutr 83:500–507. https://doi.org/10.1093/ajcn/83.2.500S
Kitamura T, Nakae J, Kitamura Y, Kido Y, Iii WHB, Wright CVE et al (1839) The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. Rapid Publ 110:1839–1847. https://doi.org/10.1172/JCI200216857.tion
Lv L, Chen H, Sun J, Lu D, Chen C, Liu D (2015) PRMT1 promotes glucose toxicity-induced b cell dysfunction by regulating the nucleo-cytoplasmic trafficking of PDX-1 in a FOXO1-dependent manner in INS-1 cells. Endocrine. https://doi.org/10.1007/s12020-015-0543-8
Ignácio-Souza LM, Reis SR, Arantes VC, Botosso BL, Veloso RV, Ferreira F et al (2013) Protein restriction in early life is associated with changes in insulin sensitivity and pancreatic β-cell function during pregnancy. Br J Nutr 109:236–247. https://doi.org/10.1017/S000711451200089X
Prigeon RL, Quddusi S, Paty B, D’Alessio DA (2003) Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285:701–707. https://doi.org/10.1152/ajpendo.00024.2003
Hou G, Li C, Huan Y, Liu S, Liu Q, Liu M et al (2017) The PI3K/Akt1-FoxO1 translocation pathway mediates EXf effects on NIT-1 cell survival. Exp Clin Endocrinol Diabetes 125:669–676. https://doi.org/10.1055/s-0043-117048
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951. https://doi.org/10.1093/jn/123.11.1939
Acknowledgements
The authors are grateful to Celso Roberto Afonso for his excellent technical assistance and Carlos Henrique Fregadolli for their contribution to the statistical analyses.
Funding
This work was supported by the Brazilian foundation CAPES-Brasil (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil, Finance Code 001; PROCAD 160638) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant no. 308588/2016-9). This work is part of a dissertation presented by Chaiane Aline da Rosa as a partial requirement for a Master’s degree in Nutrition, Food and Metabolism at the College of Nutrition, UFMT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da Rosa-Santos, C.A., da Costa Rodrigues, P., Silva, L.R. et al. Early protein restriction increases intra-islet GLP-1 production and pancreatic β-cell proliferation mediated by the β-catenin pathway. Eur J Nutr 59, 3565–3579 (2020). https://doi.org/10.1007/s00394-020-02192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02192-6