Abstract
Introduction
Polycystic ovary syndrome (PCOS) is among the most prevalent endocrine disorders in women and can lead to many other disorders and chronic diseases. Thus, early diagnosis and treatment of this syndrome is important. Using probiotics, prebiotics, and synbiotics supplementations to treat PCOS seems appropriate because of their useful effects and low complications.
Aims
To assess the effects of probiotics, prebiotics, and synbiotics on hormonal indices such as testosterone, dehydroepiandrosterone sulfate (DHEA-S), sex hormone binding globulin, Free Androgen Index (FAI), and inflammatory indices, such as high sensitive C reactive protein (hsCRP), malondialdehyde (MDA), total glutathione (GSH), nitric oxide (NO), and total antioxidant capacity (TAC) as the primary outcomes and the hirsutism score as the secondary outcome.
Methods
All published articles from the beginning until 10 November 2018 in English (Cochrane Library, Web of Sciences, Google Scholar, PubMed, Scopus, and ProQuest) and Persian (SID and Magiran) databases were searched. The effect of interventions on the outcomes was reported with a standard mean difference (SMD) and confidence interval of 95%. In case of high heterogeneity, the random effect model was used instead of the fixed effect model. The statistical heterogeneity of the included clinical trials was tested using the Chi square test and I2.
Results
Thirteen studies with 855 participants with PCOS(438 women in the intervention group and 417 women in the control group) were included in the meta-analysis. Results of the meta-analysis showed that the SHBG (SMD: 0.56; 95% CI 0.26–0.86; P = 0.0002) and NO (SMD: 0.38; 95% CI 0.09–0.68; P = 0.01) concentration increased significantly in the probiotics and synbiotics groups compared to the placebo group. FAI (SMD: − 0.58; 95% CI − 0.95 to − 0.21; P = 0.002) and MDA (SMD: − 0.76; 95% CI − 1.46 to − 0.05; P = 0.03) concentration in the probiotics and synbiotics groups reduced significantly compared to the placebo group. The results of meta-analyses on other hormonal and inflammatory indices such as testosterone, DHEAS, GSH, hsCRP, TAC, and hirsutism score showed that there were no significant differences between the intervention and control groups.
Conclusion
Using synbiotics and probiotics in women with polycystic ovary syndrome improve hormonal (FAI, SHBG) and inflammatory (NO, MDA) indices in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is among the most prevalent endocrine disorders in women, which is also associated with a spectrum of symptoms [1, 2]. Hirsutism, hyperandrogenism, oligoovulation, anovulation, polycystic ovaries, and increased levels of androgen are essential for the diagnosis of PCOS [3,4,5]. The leading cause of this syndrome is unknown, but environment and genetics have been implied in its development [6]. The prevalence of this syndrome varies in different countries and depends on its clinical and biochemical properties, which vary among different races and age groups [7, 8].
PCOS results in anovulation induced infertility in about 90% of cases [9]. It is associated with insulin resistance, and hence, an increased risk of obesity and diabetes. These disorders stimulate the progression of hormonal and inflammatory disorders, and oxidative stress [10, 11], so that 50–70% of women with PCOS and insulin resistance will develop metabolic syndrome in the future, which in turn causes other chronic diseases [12,13,14]. There is also a higher risk of endometrial and breast cancer, and psychological disorders, such as depression and hypersomnia, in these patients [15, 16]. As a result, early diagnosis and treatment may prevent its short- and long-term complications [17].
There are many recommended treatment methods for PCOS, including lifestyle changes (e.g., diet, weight loss, and exercise), surgery, and pharmacotherapy. However, changing one’s lifestyle, along with the improvement of quality of life is considered to be the first therapeutic step in these patients [4, 10, 18].
Prebiotics are indigestible and unfermentable compounds that enhance the host’s health by reducing the combination and activity of harmful bacteria and increasing useful intestinal bacteria [19, 20]. Prebiotics can improve the host’s health by increasing bifidobacteria, inhibiting the growth of pathogens, moderating the immune system, inhibiting rotavirus activity, stimulating intestinal microflora activity, curing diarrhea and irritable bowel syndrome, preventing intestinal inflammation and cancer [21, 22], intervening in lipid metabolism [23], and increasing absorption of Fe, Mg, Ca, and Zn by reducing intestinal pH [24]. Inulin, resistant dextrin, oligofructose, fructooligosaccharide, galactooligosaccharide, and lactulose are among the prebiotics [25, 26].
Probiotics are non-pathological living microorganisms and adequate consumption could have healthful and beneficial effects on the host through balancing the intestinal microbes. The lactic acid-producing bacteria, in particular lactobacillus and bifidobacterium, are generally a part of the gastrointestinal ecosystem and typically reside at the distal intestine and colon after entering the GI tract [27]. Probiotics are effective in treating lactose intolerance, inflammatory bowel disease, preventing autoimmune diseases, stimulating the immune system [27, 28], reducing cholesterol probably through bile acid deconjugation [27,28,29], regulating the patient’s weight and serum lipids, reducing blood pressure, and preventing and curing infections. They are also beneficial due to their anticancer and anti-inflammatory properties, which prevent atherosclerosis and cancers [27, 28, 30, 31].
The term synbiotic refers to products including both probiotics and prebiotics [32]; for example, a product containing fructooligosaccharides and bifidobacterium. Synbiotics improve the host’s health condition via improving survival rate of the probiotic and implantation of useful intestinal microbes [33, 34].
Some studies have reported the effectiveness of prebiotics, probiotics, and synbiotics in improving hormonal and inflammatory indicators in patients with PCOS [35,36,37,38,39,40]. However, it continues to remain unknown whether these supplements are effective in improving hormonal and inflammatory indicators in patients with PCOS. Thus, this systematic review was designed to answer these questions based on clinical trials.
Objectives
The present study aimed initially to evaluate the effects of probiotics, prebiotics, and synbiotics on hormonal indicators, such as testosterone, dehydroepiandrosterone sulfate (DHEA-S), sex hormone binding globulin (SHBG), Free Androgen Index (FAI), and inflammatory indicators, such as high sensitive C-reactive protein (hsCRP), malondialdehyde (MDA), total glutathione (GSH), nitric oxide (NO), and total antioxidant capacity (TAC). The second objective of the study was to obtain the hirsutism score.
Methods
Data source and identification of studies
This review study investigated clinical trials on the effects of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indicators (primary outcomes) and the hirsutism score (secondary outcome) in women with PCOS. All Farsi and English articles published until November 2018 in the Cochrane Library, Web of Sciences, Google Scholar, ProQuest, PubMed, Scopus, SID, and Magiran database were reviewed. The references in the found articles were also used to find relevant studies. The search strategy was according to the MeSH terminology. The MeSH keywords used alone or in a combination with other terms included “PCO”, “Polycystic ovary”, “Prebiotic”, “Prebiotic supplementation”, “Probiotic”, “Probiotic supplementation”, “Synbiotic”, “Synbiotic supplementation”, “Inulin”, “Resistant Dextrin”, “Gut microflora”, “Lactobacillus” and “Probiotic bacteria”.
Inclusion and exclusion criteria
This study investigated all controlled randomized or quasi-experimental clinical trials into the effects of probiotics, prebiotics, and synbiotics on clinical and paraclinical symptoms of women with PCOS. In addition, the population, intervention, control, outcome (PICO) criteria, including participants, intervention, comparison, and outcome, were used. The inclusion criteria were women of reproductive age (15–49 years) with PCOS (diagnosed based on the Rotterdam criteria [5]), not taking probiotics, prebiotics, and synbiotics during and 3 months before the study, not taking antibiotics during the study, not having any chronic disease (e.g., Cushing’s syndrome, diabetes, hypertension, autoimmune disease, active liver disease, history of heart and kidney diseases, pancreatitis, pulmonary disease, thyroid problem, adrenal hyperplasia, hyperprolactinemia, and female infertility), no smoking, no dieting or partaking in any type of extra physical activity such as aerobics, and not using Omega 3, and multivitamin products.
The intervention included the use of different doses of probiotics, prebiotics, and synbiotics in the form of powder or capsule. The comparison group included the placebo or maltodextrin group. The outcomes included hormonal and inflammatory indicators and the hirsutism score.
Data extraction
The collected articles were carefully reviewed and two authors separately scrutinized the title and abstract for inclusion criteria. In case of inadequate information in the title and abstract of an article, it was fully reviewed by the authors. In case of contradiction, the consensus was made through discussion with a third author. The article-related data, namely time of the study, name of the author, methodology, type and consumption method of probiotics, prebiotics, and synbiotics, comparison details between treatment regimens, length of treatment, length of follow-up, participants’ characteristics, number of randomized participants, number of attritions in follow-up, primary and secondary outcomes, and reported complications, were extracted.
Assessment of risk of bias in the included studies
The two authors separately evaluated the articles based on the Cochrane handbook criteria [41] for selection, performance, detection, attrition, and reporting bias. The bias risk of each item for clinical trials were categorized as “low risk”, “high risk”, or “unclear” topics. Then judgments of the two authors were compared and any disagreement was resolved by the third author.
Statistical method
The statistical analysis was done with the software RevMan version 5.3. The effect of interventions on the outcomes was reported with a confidence interval of 95% for the difference between means. Due to the application of different methods to estimate hormonal and inflammatory levels, the standardized mean difference (SMD) was used instead of mean difference (MD). In case of high heterogeneity, the random effect model was used instead of the fixed effect model. The statistical heterogeneity of the included clinical trials was tested using the Chi square test and I2. In that, I2 > 75% and P value < 0.01 was characterized as significant heterogeneity, 0–40%: might not be important; 30–60%: may represent moderate heterogeneity, 50–90%: may represent substantial heterogeneity, and 75–100%: considerable heterogeneity [41].
Results
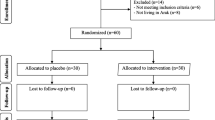
A total of 2515 articles were found in the various databases. Among them, 2457 articles were excluded because of irrelevant titles and 39 articles were duplicates. Among the 19 full-text reviewed articles, 13 were finally included (Fig. 1).
Table 1 shows the characteristics of the included studies. The sample size varied between 60 [35, 38, 40, 42, 44, 46] and 118 [47] women. The intervention groups received probiotics, prebiotics, and synbiotics in four [39, 40, 42, 43], seven [35, 36, 38, 44,45,46,47], and two studies [37, 48], respectively. In all studies, the control group received a placebo, either starch or maltodextrin. In the included studies, the Ferriman-Gallwey (FG) scoring system was used for assessing hirsutism. In four studies [35, 37, 38, 40], the hormone levels were measured with ELISA and in three studies [35, 38, 47], the hsCRP was measured with ELISA. For measuring the inflammatory indices, different methods were used such as spectrophotometry [37, 40], latex-enhanced immunonephelometry [36], immunoturbidimetry [43], a commercial kit [44], and the spectrophotometric method as described by Benzie and Strain, Griess, and Beutler [35, 38].
Risk of bias of included studies
The risk of random allocation bias was low in 11 studies [35,36,37, 39, 40, 42,43,44,45,46, 48]. The risk of allocation concealment bias was low in nine studies [35, 37, 39, 40, 42,43,44, 46, 48]. The risk of bias from the lack of blinding was low in 11 studies [35,36,37, 39, 40, 42,43,44,45,46, 49]. The risk of bias from the lack of assessor blinding was unclear in all studies. The risk of incomplete outcome bias was low in eight studies [35,36,37,38, 40, 45, 46, 48] and the risk of reporting bias was low in six studies [35, 38, 39, 42, 44, 46] (Figs. 2, 3).
Meta-analysis of included studies
The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indicators and clinical symptoms of PCOS are reported in Figs. 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13.
Hormonal indices
Testosterone
The meta-analysis results showed that the testosterone concentration in the probiotics and synbiotics groups was reduced by 0.05 ng/ml which was lower than in the placebo group; however, this reduction was not statistically significant (SMD: − 0.50; 95% CI − 1.25 to 0.25; P = 0.19) and the heterogeneity level was high (I2 = 84%; Tau2 = 0.37; Chi2 = 12.30; P = 0.002) (Fig. 4).
DHEAS
The meta-analysis results showed that the DHEAS concentration in the probiotics, prebiotics and synbiotics groups was reduced by 0.22 μg/ml, which was lower than in the placebo group; however, this reduction was not statistically significant (SMD: − 0.22; 95% CI − 0.51 to 0.07; P = 0.14) and the included studies were homogeneous (I2 = 0%; Chi2 = 1.13; P = 0.57) (Fig. 5).
SHBG
The meta-analysis results showed that the SHBG concentration in probiotics and synbiotics significantly increased by 0.56 μg/ml higher than in the placebo group (SMD: 0.56; 95% CI 0.26–0.86; P = 0.0002) and the included studies were homogeneous (I2 = 0%; Chi2 = 0.78; P = 0.68) (Fig. 6).
Fai
The meta-analysis results showed that the FAI concentration in probiotics and synbiotics were significantly reduced by 0.58 μg/ml, which was lower than in the placebo group (SMD: − 0.58; 95% CI − 0.95 to − 0.21; P = 0.002), and there was the substantial heterogeneity level (I2 = 68%; Chi2 = 3.12; P = 0.08) (Fig. 7).
Inflammatory indices
hsCRP
The meta-analysis results showed that the hsCRP concentration in probiotics, prebiotics, and synbiotics groups were reduced by 0.59 mg/dl, which was lower than in the placebo group; however, this reduction was not statistically significant (SMD: − 0.59; 95% CI − 1.60–0.42; P = 0.25), and there was the considerable heterogeneity level (I2 = 96%; Tau2 = 1.78; Chi2 = 148.31; P < 0.00001) (Fig. 8).
NO
The meta-analysis results showed that the NO concentration in probiotics and synbiotics groups significantly increased by 0.38 mg/dl higher than in the placebo group (SMD: 0.38; 95% CI 0.09–0.68; P = 0.01) and the included studies were homogeneous (I2 = 0%; Chi2 = 0.38; P = 0.83) (Fig. 9).
TAC
The meta-analysis results showed that the TAC concentration in probiotics and synbiotics groups increased by 0.30 mg/dl higher than in the placebo group; however, this increase was not statistically significant (SMD: 0.30; 95% CI − 0.58 to 1.17; P = 0.51) and the heterogeneity level was considerable (I2 = 88%; Tau2 = 0.52; Chi2 = 16.72; P = 0.0002) (Fig. 10).
GSH
The meta-analysis results showed that the GSH concentration in probiotics and synbiotics groups increased by 0.53 mg/dl, which was higher than in the placebo group; however, this increase was not statistically significant (SMD: 0.53; 95% CI − 0.00 to 1.06; P = 0.05) and there was the substantial heterogeneity level (I2 = 68%; Tau2 = 0.15; Chi2 = 6.24; P = 0.04) (Fig. 11).
MDA
The meta-analysis results showed that the MDA concentration in the probiotics and synbiotics groups was reduced by 0.72 mg/dl, which was more than in the placebo group, and this reduction was statistically significant (SMD: − 0.76; 95% CI − 1.46 to − 0.05; P = 0.03) and the heterogeneity level was considerable (I2 = 81%; Tau2 = 0.31; Chi2 = 10.42; P = 0.005) (Fig. 12).
Clinical symptoms
Hirsutism
The meta-analysis results showed that the hirsutism concentration in probiotics, prebiotics, and synbiotics groups was reduced by 0.12, which was lower than in the placebo group; however, this reduction was not statistically significant (SMD: − 0.12; 95% CI − 0.38 to 0.13; P = 0.34) and the heterogeneity was at a moderate level (I2 = 50%; Chi2 = 5.96; P = 0.11) (Fig. 13).
Discussion
According to the search results, this was the first review study on the effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indicators and clinical symptoms in women with PCOS. The meta-analysis results showed that probiotics and synbiotics significantly reduced FAI and MDA and increased NO and SHBG. The use of probiotics, prebiotics, and synbiotics in women with PCOS reduced the serum testosterone, DHEAS, and hsCRP levels and the hirsutism score as compared to the placebo group; however, this difference was not statistically significant. The consumption of probiotics and synbiotics by women with PCOS increased serums TAC and GSH levels; however, the difference with the placebo group was not significant.
An increase in metabolic indices, such as cholesterol as the prerequisite of androgenic hormone generation in these patients, resulted in an increase in serum androgen levels [37, 50]. Among the PCOS pathophysiology, glucose intolerance and insulin sensitivity had an important role in the development of this syndrome. The uptake of probiotics, prebiotics, and synbiotics balanced the colony of intestinal microbes and intestinal pH, improved intestinal decomposition and metabolism of lipids and starch, produced inflammatory cytokines, and improved intestinal digestion and absorption of nutrients [51]. They also reduced cholesterol by reducing its production in the liver, reduced blood glucose by consuming the serum insulin, and reduced insulin resistance which, in turn, reduced the production of androgens, such as testosterone, FAI, DHEAS, and SHBG levels [37, 52, 53]. According to the meta-analysis results, the consumption of probiotics, prebiotics, and synbiotics significantly reduced DHEAS; however, the consumption of probiotics and synbiotics did not significantly reduce testosterone levels. This can be attributed to the short duration of the intervention, which was between 8 and 12 weeks. Moreover, few studies measured the hormones as the outcome [35, 37, 38, 40].
This meta-analysis into the effect of probiotics, prebiotics, and synbiotics on clinical symptoms of this syndrome showed that they reduced the hirsutism score in these patients; however, this reduction was not significant. As it was mentioned, reduced levels of male sex hormone in women with PCOS may result in fewer clinical symptoms and improve hirsutism via increasing female sex hormones. As a result, body fat, weight, and male sex hormone levels decrease with reducing serum cholesterol and increasing leptin, peptide YY, glucagon-like peptide-1, and ghrelin, which may reduce clinical symptoms, such as hirsutism [54, 55]. The probable reason for insignificant reduction of hirsutism symptoms after receiving probiotics, prebiotics, and synbiotics may be its short-term use, as clinical symptoms in this syndrome are developed long after an increase in serum androgens and progress with time. As a result, the short-term consumption of these supplements may not result in rapid improvement of these symptoms. This is because; the improvement of signs may take a long time to appear after the regulation of male sex hormones in the patients’ serum [56,57,58].
Oxidative stress and inflammation increase in patients with PCOS, resulting in insulin resistance through the functional disorder of pancreatic beta cells [59]. It finally causes ovarian dysfunction, which is accelerated with unbalanced antioxidant levels [60]. Reduced hyperandrogenism is correlated with the reduction and improvement of oxidative and inflammatory stress [61, 62].
The meta-analysis results on the effect of probiotics, prebiotics, and synbiotics on inflammatory indicators showed that these compounds reduced hsCRP concentration; however, this reduction was not statistically significant. Consumption of probiotics and synbiotics resulted in a significant decrease in the MDA level. Moreover, an increase in plasma levels of TAC and GSH were observed in the conducted meta-analyses; however, these changes were not significant. Additionally, the consumption of probiotics and synbiotics resulted in a significant increase in the plasma level of NO. Of course, NO plays a dual role in the process of immunoinflammation. On the one hand, NO can kill microorganisms and has a protective effect on the body. On the other hand, NO can damage normal tissue cells to generate pathogenic effects. According to existing research, macrophages and other effector cells, including neutrophils, monocytes, and endothelial cells, are the main effector cells involved in the antimicrobial effects of NO [63].
Oxidative stress is correlated with obesity and hyperandrogenism. Synbiotics can reduce hydroperoxidase and finally increase plasma levels of nitric oxide. They also can reduce MDA by reducing blood lipids and inhibiting lipid peroxidase [64,65,66]. Probiotics may improve inflammation and oxidative stress by moderating the signaling pathway of inflammatory factors, producing antioxidant metabolites, upregulation of antioxidants activity, and downregulation of ROS-producing enzymes. As a result, the oxidative stress increases following ROS (reactive oxygen species) which may, in turn, increase hyperandrogenemia and insulin resistance. Reduced antioxidants and increased oxidative stress and aggregation of ROS have a significant role in folliculogenesis and oocyte maturity in women with PCOS and their reproductive system [67]. Probiotics may exert anti-inflammatory and anti-oxidative effects through the production of short-chain fatty acids in the intestine [68]. Prebiotics, such as oligofructose, reduce the expression of oxidative and inflammatory markers in the liver [69]. It is a mechanism through which prebiotics improve inflammation and antioxidants: the change of intestinal bacteria to butyrogenic-genera, such as peptostreptococcus, fusobacterium, bifidobacterium, which are well-known for their anti-inflammatory properties. An increase in oxidative stress results in an increase in intestinal permeability and endotoxins in blood [70]. Lipopolysaccharide is the main and most important element in the extracellular wall of Gram-negative bacteria and the main inflammatory element in obese people [69]. The lactic acid-producing bacteria have anti-oxidative properties, which eliminate free radicals and secrete antioxidants at the intestinal wall which, in turn, reduce MDA concentration in the blood [71]. However, the differences in the length of use, dose, genotype, and supplement might reduce their effectiveness. In addition, the high dose and prolong use of these supplements may result in significant changes in the inflammatory indicators.
A meta-analysis has recently investigated the effect of probiotics or synbiotics supplementation on QUICKI, triglycerides, fasting insulin, and HDL in women with PCOS and the results have shown that these supplements produce a significant effect on the symptoms of this syndrome. However, the outcomes of the present study are different from the mentioned meta-analysis [72]. This study analyzed the secondary outcomes of some studies instead of the primary outcomes. The limited number of studies into these indicators can be a plausible cause regarding their insignificance. Moreover, the sample size was calculated based on the primary outcomes, which can be a cause for insufficient sample size for evaluating the hormonal and inflammatory outcomes and insignificance of these indices in the meta-analysis.
Limitation
The scant number of studies into the effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indicators and clinical symptoms in women with PCOS was a limitation of the present meta-analysis. Therefore, results should be reported carefully. The conduction of all studies in Iran, except one [47], was another limitation of this meta-analysis. There was not a limitation on country searching in our research about the effect of probiotics, prebiotics, and synbiotics on women with PCOs, and unfortunately most of the studies have been conducted in Iran. Although the research environment in clinical trials is not very important, the effect of prebiotics, synbiotics, and probiotics on hormonal and inflammatory indicators, and clinical symptoms in women with PCOS can be affected by ethnicity, race, and climate, and factors related to the lifestyle of Iranians. Therefore, we suggest more clinical trials be done with these factors in other countries to make sure of their efficacy on PCOS patients.
On the other hand, in all of these studies, metabolic indices were also measured. The sample size was determined based on the metabolic indices in five studies, hsCRP in two studies, and testosterone in other two studies. The sample size estimation method was not mentioned in four studies. Therefore, the sample size might be insufficient for measuring the effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indicators, as well as clinical symptoms, and this could affect the results. Additionally, since the bacterial species were the same in most of the studies, we could not do the subgroup meta-analysis according to the mentioned variable.
Conclusion
This meta-analysis showed that consumption of probiotics and synbiotics had a significant effect on the control of hormonal and inflammatory indicators by significantly reducing FAI and MDA, and increasing SHBG and NO. Although probiotics and synbiotics increased the GSH and TAC levels, this increase was not statistically significant. Moreover, probiotics, prebiotics, and synbiotics reduced the testosterone, DHEAS, hsCRP, and hirsutism score; however, this reduction was not statistically significant. In conclusion, due to the limited number of studies on women with PCOS, more clinical studies are needed to determine the suitable dose, length of use, and type of the supplement.
References
Aalei Bibi SH, Naderi T (2004) Poly cystic ovarian syndrome: clinical, ultrasound and laboratory characteristics, Kerman. Iran J Endocrinol Metab 6(2):153–161
Speroff L, Mark AF (2011) Clinical gynecology endocrinology and infertility, 8th edn. Lippincott Williams & Wilkins, Philadelphia
Nadjarzadeh A, Dehghani Firouzabadi R, Vaziri N, Daneshbodi H, Lotfi MH, Mozaffari-Khosrav H (2013) The effect of omega-3 supplementation on androgen profile and menstrual status in women with polycystic ovary syndrome: a randomized clinical trial. Iran J Reprod Med 11(8):665–672
Zhang J, Li T, Zhou L, Tang L, Xu L, Wu T et al (2010) Chinese herbal medicine for subfertile women with polycystic ovarian syndrome. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007535.pub2/abstract
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 Consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Mehrabian F, Khani B, Kelishadi R, Ghanbari E (2011) The prevalence of poly cystic ovary syndrome in Iranian women based on different diagnostic criteria. EndoCrynol Polska 62(3):238–242
Kauffman RP, Baker VM, Dimarino P, Castracane VD (2002) Poly cystic ovarian syndrome and insulin resistance in white and Mexican American women: a comparison of two distinct population. An J Obstet Gynecol 187(5):1362–1369
Koivunen R, Laatikainen T, Tomas C, Huhtaniemi I, Tapanainen J, Martikainen H (1999) The prevalence of poly cystic ovaries in healthy women. Acta Obstet Gynecol Scand 78(2):137–141
Balen AH, Rutherford AJ (2007) Managing anovulatory infertility and polycystic ovary syndrome. BMJ 335:663–669
Moran LJ, Ranasinha S, Zoungas S, McNaughton SA, Brown WJ, Teede HJ (2013) The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum Reprod 28(8):2276–2283
Bizzarri M, Carlomagno G (2014) Inositol: history of effective therapy for polycystic ovary syndrome. Eur Rev Med Pharmacol Sci 18:1896–1903
Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S (2009) Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online 19(3):398–405
Meyer C, McGrath BP, Teede HJ (2005) Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab 90(10):5711–5716
Lobo RA (2001) Priorities in polycystic ovary syndrome. Med J Aust 174:554–555
Moran Lisa J, Hutchison Samantha K, Norman Robert J, Teede Helena J (2011) Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 7:4. https://doi.org/10.1002/14651858.CD007506.pub3/abstract
Salehpour S, Taherzadeh Broujeni P, Neisani Samani E (2008) Leptin, ghrelin, adiponectin, homocysteine and insulin resistance related to polycystic ovary syndrome. Int J Fertil Steril 2(3):101–104
Hoftman BH, Bradshaw KD, Cunningham FG, Halvorson LM, Schaffer JI, Schorge JO (2008) Williams gynecology. Mc Graw-Hill Medical, New York
Sheu J, Buzeny E, Reynolds R, Buzany C (2014) Polycystic ovary syndrome: a review for dermatologists: Part I. Diagn Manifest 71(5):912–919. https://doi.org/10.1016/j.amjmed.2014.04.017
Schrezenmeir J, De Vaese M (2001) Probiotics, prebiotics, and synbiotics-approaching a definition. J Nutr 73:361–364
Scavuzzi BM, Hernique FC, Miglioranza LHS, Sinmao ANC, Dichi I (2014) Impact of prebiotics, probiotics and synbioticss on components of the metabolic syndrome. Ann Nutr Disord Ther 1(2):1009 (ISSN: 2381-8891)
Roberfroid B, Bornet F, Bouley C, Cummings JH (1995) Colonic microflora:nutrition and health: summary and conclusions of an International Life Sciences Institute (ILSI) [Europe] workshop held in Barcelona, Spain. Nutr Rev 53(5):127–130
Paineau D, Payen F, Panserieu S, Coulombier G, Sobaszek A, Lartigau I et al (2008) The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br J Nutr 99(2):311–318
Ooi LG, Liong MT (2010) Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci 11(6):2499–2522. https://doi.org/10.3390/ijms11062499
Scholz-Ahrens KE, Schrezenmeir JR (2007) Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J Nutr 137(11):2513S–2523S
Scavuzzi BM, Hernique FC, Miglioranza LHS, Sinmao ANC, Dichi I (2014) Impact of prebiotics, probiotics and synbioticss on components of the metabolic syndrome. Ann Nutr Disord and Ther 1(2):1009 (ISSN: 2381-8891)
Greg Kelly N (2009) Inulin-type prebiotics: review (part2). Altern Med Rev 14(1):36–55
Homayouni Rad A (2008) Therapeutical effects of functional probiotic, prebiotic and synbiotics foods, 1st edn. Tabriz University of Medical Sciences, Tabriz
Goldin BR, Gorbach SL (2008) Clinical indications for probiotics: an overview. Clin Infect Dis 46(2):96–100
Begley M, Hill C, Gahan CGM (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72(3):1729–1738
Oelschlaeger T (2010) Mechanisms of probiotic actions—a review. Int J Med Microbiol 300(1):57–62
Flint H, Bayer E, Rincon M, Lamed R, White B (2008) Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 6(2):121–131
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125(6):1401–1412
Fooks LJ, Gibson GR (2002) Probiotics as modulators of the gut flora. Br J Nutr 88(1):S39–S49
Su P, Henriksson A, Mitchell H (2007) Prebiotics enhance survival and prolong the retention period of specific probiotic inocula in an in vivo murine model. J Appl Microbiol 103(6):2392–2400
Karamali M, Eghbalpour S, Rajabi S, Jamilian M et al (2018) Effects of probiotic supplementation on hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double blind, placebo-controlled trial. Arch Iran Med 21(1):1–7
Ghanei N, Rezaei N, Amiri GA, Zayeri F, Makki G, Nasseri E (2018) The probiotic supplementation reduced inflammation in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Funct Food 42:306–311
Gholizadeh-Shamasbi S, Dehgan P, Mohammad-Alizadeh S, Aliasgarzadeh A, Mirghafourvand M (2018) The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: a randomized, triple-blind, controlled, clinical trial. Euro J Nutr. https://doi.org/10.1007/s00394-018-1648-7
Jamilian M, Mansury Sh, Bahmani F, Heidar Z, Amirani E, Asemi Z (2018) The effects of probiotic and selenium cosupplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res 11:80. https://doi.org/10.1186/s13048-018-0457-1
Esmaeilinezhad Z, Babajafari S, Sohrabi Z, Eskandari M-H, Amooee S, Barati-Boldaji R (2018) Effect of synbiotics pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: a randomized, triple blind, controlled trial. Nut Metab Cardiovasc Dis. https://doi.org/10.1016/j.numecd.2018.07.002
Nasri Kh, Jamilian M, Asemi Z, Rahmani E, Bahmani F, Tajabadi-Ebrahimi M (2018) The effects of synbiotics supplementation on hormonal status, biomarkers of inflammation and oxidative stress in subjects with polycystic ovary syndrome: a randomized, double-blind, placebocontrolled trial. BMC Endocr Disord 18:21. https://doi.org/10.1186/s12902-018-0248-0
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. Wiley, Chichester
Samimi M, Seyed Hosseini E, Dadkhah A, Asemi Z (2018) The effects of synbiotics supplementation on metabolic status in women with polycystic ovary syndrome: a randomized double-blind clinical trial. Probiot Antimicrob Proteins. https://doi.org/10.1007/s12602-018-9405-z
Karimi E, Moini A, Yaseri M, Shirzad N, Sepidarkish M, Hossein-Boroujerdi M, Hosseinzadeh-Attar MJ (2018) Effects of synbiotics supplementation on metabolic parameters and apelin in women with polycystic ovary syndrome: a randomised double-blind placebo-controlled trial. Br J Nutr 119(4):398–406. https://doi.org/10.1017/S0007114517003920
Ahmadi Sh, Jamilian M, Karamali M, TajabadiEbrahimi M, Jafari P, Taghizadeh M, Memarzadeh MR, Asemi Z (2017) Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Hum Fertil 20(4):254–261. https://doi.org/10.1080/14647273.2017.1283446
Shoaei T, Heidari-Beni M, Ghasemi Tehrani H, Feizi A, Esmaillzadeh A, Askari Gh (2015) Effects of probiotic supplementation on pancreatic β-cell function and C-reactive protein in women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Int J Prev Med 6:27. https://doi.org/10.4103/2008-7802.153866
Shabani A, Noshadian M, Jamilian M, Chamani M, Mohammadi S, Asemi Z (2018) The effects of a novel combination of selenium and probiotic on weight loss, glycemic control and markers of cardio-metabolic risk in women with polycystic ovary syndrome. J Funct Foods 46:329–334
Rashad NM, El-Shal AS, Amin AI, Soliman MH (2017) Effects of probiotics supplementation on macrophage migration inhibitory factor and clinical laboratory feature of polycystic ovary syndrome. J Funct Foods 36:317–324
Gholizadeh-Shamasbi S, Dehgan P, Mohammad-Alizadeh S, Aliasgarzadeh A, Mirghafourvand M (2018) Effect of prebiotic on anthropometric indices in women with polycystic ovarian syndrome: a triple-blind, randomized, controlled clinical trial. Iran Red Crescent Med J 20(11):67270
Api M, Badoglu B, Akca A, Api O, Gorgen H, Cetin A (2008) Interobserver variability of modified Ferriman–Gallwey hirsutism score in a Turkish population. Arch Gynecol Obstet 279(4):473–479. https://doi.org/10.1007/s00404-008-0747-8
Khajebishak Y, Payahoo L, Homayouni Rad A, Shokrvash B (2014) The role of intestinal microbiota in health and a short review on the probiotic and prebiotic supplements in obesity prevention. Arak Med Univ J 17(90):18–26
Pan C, Zhao Y, Liao SF, Chen F, Qin S, Wu X et al (2011) Effect of selenium enriched probiotics on laying performance, egg quality, egg selenium content, and egg glutathione peroxidase activity. J Agric Food Chem 59(21):11424–11431
Aliasgharzadeh A, Khalili M, Mirtaheri E, Pourghassem Gargari B, Tavakoli F, Abbasalizadeh Farhangi M et al (2015) A combination of prebiotic inulin and oligofructose improve some of cardiovascular disease risk factors in women with type 2 diabetes: a randomized controlled clinical trial. Adv Pharm Bull 5(4):507–514
Amiri M, Golsorkhtabaramiri M, Esmaeilzadeh S, Ghofrani F, Bijani A, Ghorbani L et al (2014) Effect of metformin and flutamide on anthropometric indices and laboratory tests in obese/overweight PCOS women under hypocaloric diet. J Reprod Infertil 15(4):205–213
Carvalho BM, Abdalla Saad MJ (2013) Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm 2013:986734. https://doi.org/10.1155/2013/986734
Mehdizadeh ZT, Mirfeizi M, Mirfeizi Z, Asghari MJ, Hojat SH (2013) The effect of diet and physical activity on obese women with polycystic ovary syndrome. Med J Mashhad 56(2):77–84. https://doi.org/10.22038/MJMS.2013.846
Huang A, Brennan K, Azziz R (2010) Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil Steril 93(6):1938–1941
Wehr E, Moller R, Horejsi R, Giuliani A, Kopera D, Schweighofer N et al (2009) Subcutaneous adipose tissue topography and metabolic disturbances in polycystic ovary syndrome. Wien Klin Wochenschr 121(7–8):262–269
West S, Lashen H, Bloigu A, Franks S, Puukka K, Ruokonen A et al (2014) Irregular menstruation and hyperandrogenaemia in adolescence are associated with polycystic ovary syndrome and infertility in later life: northern Finland birth cohort 1986 study. Hum Reprod 29(10):2339–2351
Gonzalez F (2015) Nutrient-induced inflammation in polycystic ovary syndrome: role in the development of metabolic aberration and ovarian dysfunction. Semin Reprod Med 33(4):276–286
Zuo T, Zhu M, Xu W (2016) Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid Med Cell Longev 2016:8589318
Sathyapalan T, Shepherd J, Coady AM, Kilpatrick ES, Atkin SL (2012) Atorvastatin reduces malondialdehyde concentrations in patients with polycystic ovary syndrome. J Clin Endocrinol Metab 97(11):3951–3955
Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I et al (2006) Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod 21(6):1426–1431
Xue Q, Yan Y, Zhang R, Xiong H (2018) Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci 19:3805–3818. https://doi.org/10.3390/ijms19123805
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:537–546
Shakeri H, Hadaegh H, Abedi F et al (2014) Consumption of synbiotics bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type 2 diabetes. Lipids 49(7):695–701
Wang Y, Li Y, Xie J et al (2013) Protective effects of probiotic lactobacillus casei Zhang against endotoxin- and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int Immunopharmacol 15(1):30–37
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D et al (2017) Antioxidant properties of probiotic bacteria. Nutrients 9(5):521. https://doi.org/10.3390/nu9050521
Sadrzadeh-Yeganeh H, Elmadfa I, Djazayery A, Jalali M, Heshmat R, Chamary M (2010) The effects of probiotic and conventional yoghurt on lipid profile in women. Br J Nutr 103(12):1778–1783. https://doi.org/10.1017/s0007114509993801
Cani PD, Possemiers S, Van de Wiele T et al (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58(8):1091–1103
Suzuki A, Mitsuyama K, Koga H et al (2006) Bifidogenic growth stimulator for the treatment of active ulcerative colitis: a pilot study. Nutrition 22(1):76–81
Rishi P, Mavi SK, Bharrhan S et al (2009) Protective efficacy of probiotic alone or in conjunction with a prebiotic in Salmonella-induced liver damage. FEMS Microbiol Ecol 69(2):222–230
Heshmati J, Farsi F, Yosaee S, Razavi M, Rezaeinejad M, Karimie E, Sepidarkish M (2018) The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiot Antimicro Prot. https://doi.org/10.1007/s12602-018-9493-9
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
SGS, SGH, and MM participated in the study design. SGS and SGH searched the literature and selected studies, extracted data, assessed quality, and SGS drafted the manuscript. MM revised the draft and all authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do have not any conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical standards
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Shamasbi, S.G., Ghanbari-Homayi, S. & Mirghafourvand, M. The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Nutr 59, 433–450 (2020). https://doi.org/10.1007/s00394-019-02033-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02033-1