Abstract
Purpose
Excessive exposure of glucocorticoids activates adipose lipolysis, increases circulating free fatty acids, and contributes to ectopic lipid deposition in liver and skeletal muscle. Our previous study demonstrated that maternal betaine supplementation attenuates glucocorticoid-induced hepatic lipid accumulation in rat offspring. However, it is unclear whether maternal betaine supplementation is effective in preventing glucocorticoid-induced lipolysis in the adipose tissue of offspring.
Methods
In this study, 20 pregnant rats were fed with basal or betaine-supplemented (10 g/kg) diets throughout gestation and lactation, and the offspring rats were raised on the basal diet from weaning till 3 months of age followed by daily intraperitoneal injection of saline or 0.1 mg/kg dexamethasone (DEX) for 3 weeks.
Results
Chronic DEX treatment significantly (P < 0.05) decreased serum corticosterone level and increased proinflammatory cytokines, such as TNFα, IL-1β, and IL-6. Meanwhile, GR protein content in adipose tissue was increased in response to DEX treatment, which was associated with a significant (P < 0.05) up-regulation of ATGL and HSL expression at both mRNA and protein levels. All these DEX-induced changes were significantly (P < 0.05) attenuated in progeny rats derived from betaine-supplemented dams. Furthermore, DEX-induced hypomethylation of ATGL and HSL gene promoters was reversed by maternal betaine supplementation.
Conclusions
Taken together, these results suggest that maternal betaine supplementation is effective in alleviating glucocorticoid-induced lipolysis in adipose tissue with modification of DNA methylation on the promoter of lipolytic genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoids (GCs) are steroid hormones released from the adrenal glands upon activation of hypothalamic–pituitary–adrenal (HPA) axis in response to stress signals. In humans, glucocorticoids are the most effective anti-inflammatory drugs for immunosuppressive therapy [1]. However, excessive GCs exposure is reported to induce insulin resistance, obesity, and Cushing syndrome characterized by high levels of circulating cortisol [2, 3]. Adipose tissue plays a pivotal role in regulating whole-body glucose and lipid homeostasis. GCs have been reported to increase the transcription of genes encoding lipolytic enzymes, including adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) that catalyze the hydrolysis of triglyceride to free fatty acids (FFAs) [2, 4]. Activation of adipose lipolysis elevates circulating FFAs, leading to ectopic lipid deposition in liver and skeletal muscle [5]. Therefore, adipose lipolysis is considered as a potential target for alleviating GC-induced ectopic lipid deposition.
As an important methyl donor, betaine plays an important role in preventing non-alcoholic fatty liver disease (NAFLD) [6]. Recently, increasing evidences show that betaine supplementation prevents liver lipid deposition induced by high-fat diet, fructose, or vitamin B6 deficiency [7,8,9,10]. It has been well-documented that betaine rectifies the impaired methylation status in adipose tissue and thereby contributes to hepatoprotection in alcoholic or non-alcoholic fatty liver disease [11, 12]. Accumulating evidences indicate that nutritional changes in maternal diet, such as fat [13] or protein [14, 15] content, during pregnancy and lactation contribute to the metabolic programming of the offspring, through epigenetic regulation, especially DNA methylation [16]. Our previous reports demonstrated that maternal betaine supplementation during gestation and lactation improves hepatic cholesterol and lipid metabolism in neonatal piglets via epigenetic modifications [17, 18]. Besides, maternal betaine exposure attenuates glucocorticoid-induced hepatic lipid accumulation in offspring rats [19]. However, the previous studies focus predominantly on liver rather than adipose tissue. The role of maternal betaine exposure in chronic glucocorticoid-induced adipose lipolysis in offspring remains unclear. Previously, we have described a study in which adult rats born to control and betaine-supplemented dams were subjected to 3 weeks of DEX treatment to generate a chronic stress model. Offspring derived from betaine-supplemented mothers showed attenuation of DEX-induced hepatic lipid accumulation. Here, a subset of animals from this same study was used to address the hypothesis that maternal betaine supplementation reverses DEX-induced lipolysis in adipose tissue by altering the expression of key lipolytic genes. We show that maternal betaine supplementation protected rat offspring from DEX-induced alterations in serum proinflammatory cytokines, NEFA, corticosterone, and insulin concentrations, as well as ATGL and HSL expressions in adipose tissue at both mRNA and protein levels. Moreover, maternal intake of betaine prevented DEX-induced hypomethylation on the promoter of affected genes.
Materials and methods
Ethics statement
All procedures with animals were approved by the Animal Ethics Committee of Nanjing Agricultural University, with the project number 2016YFD0500502. The sampling procedures followed the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 set by the Ministry of Science and Technology, China.
Animals and experimental design
Three-month-old female Sprague–Dawley rats were obtained and raised in the laboratory animal center of Jiangsu University. They were kept under a controlled temperature of 22 ± 0.5 °C with a humidity of 50 ± 5% and a 12L:12D lighting cycle. Food and water were provided ad libitum throughout the experiment.
After 1 week of acclimatization, rats were mated and conception was confirmed by the presence of a vaginal smear plug. Twenty pregnant rats were randomly assigned to two dietary treatment groups (n = 10/group) throughout gestation and lactation: control group, received a standard diet, and betaine group, received a standard diet supplemented with 10 g/kg betaine (98% purity, B2629, Sigma-Aldrich Co., LLC, USA). After parturition, litter size was adjusted to ten pups (5 males and 5 females). At 3 months of age, 20 female offspring rats were selected, respectively, from both control group (Con) and betaine group (Bet), and each group was divided into two subgroups, being subject to daily intraperitoneal injection of physiological saline or dexamethasone (Dex) (D4902, Sigma-Aldrich Co., LLC., USA) at a dose of 0.1 mg/kg body mass for 3 weeks. After 3 weeks, all rats from four groups (Con–Con, Con–Dex, Bet–Con, and Bet–Dex) were killed with pentobarbital sodium. Serum samples were collected and stored at − 20 °C. Epididymal adipose tissues were immediately dissected, snap frozen in liquid nitrogen, and stored at − 80 °C for further analyses. The flowchart of the experiment is shown in Fig. 1.
Serum biochemical parameters
The serum concentrations of glucose (ECH0105102), total triglyceride (ECH0105151), total cholesterol (ECH0103152), high density lipoprotein-cholesterol (HDL-C) (ECH0105161), and low-density lipoprotein-cholesterol (LDL-C) (ECH0105162) were measured with a biochemical automatic analyzer (Hitachi 7020, HITACHI, Tokyo, Japan) using commercial assay kits purchased from Maccura Biotechnology Co., Ltd (Chengdu, China). Serum concentration of non-esterified fatty acid (NEFA) was determined using the Wako NEFA acyl-coenzyme A synthetase and acyl-coenzyme A oxidase assay method.
Determination of serum proinflammatory cytokines and corticosterone
Serum concentrations of proinflammatory cytokines including interleukin-1β, interleukin-6, and tumor necrosis factor α were determined by ELISA kits (Nanjing Angel Gene Bioengineering Co., Ltd, Nanjing, China). In addition, serum corticosterone concentration was measured using a commercial ELISA kit (No. ADI-900-097, Enzo, USA) according to the manufacturers’ instruction.
Total RNA isolation and real-time PCR
Total RNA was isolated from 100 mg frozen adipose tissue samples with TRIzol reagent (Invitrogen, USA) and reverse-transcribed according to the manufacturer’s protocol (Vazyme Biotech, Nanjing, China). Diluted cDNA (2 μL, 1:25) was used for real-time PCR that was performed in an Mx3000P System (Stratagene, USA). All primers (Table 1) were synthesized by Generay Biotech (Shanghai, China). 18S was chosen as a reference gene. The \(2^{{ - \Delta \Delta C_{\text{T}} }}\) method was used to analyze real-time PCR data.
Western blot analysis
Total protein was extracted from 200 mg frozen adipose tissue samples as previously described [20]. Protein concentration was measured with the Pierce BCA Protein Assay kit (No. 23225, Thermo Scientific) according to the manufacturer’s instruction. Western blot analysis of ATGL (BS7989, Bioworld, USA, diluted 1:1000), p-HSL (Ser855) (BS4234, Bioworld, USA, diluted 1:1000), AKT (AP0059, Bioworld, USA, diluted 1:1000), AMPK (BS6271, Bioworld, USA, diluted 1:1000), ACC (4190, Cell Signaling Technology, USA, diluted 1:1000), FAS (3189, Cell Signaling Technology, USA, diluted 1:1000), and GR (24050-1-AP, Proteintech, USA, diluted 1:1000) were carried out. The β-actin (AP0060, Bioworld, USA, diluted 1:5000) was used as internal control.
Methylated DNA immunoprecipitation (MeDIP) analysis
Methylated DNA immunoprecipitation analysis was performed as previously described [21]. In brief, 1 µg purified genomic DNA was fragmented to a mean size of 300 bp by sonication, heat denatured, and immunoprecipitated with 5-mc antibody (ab10805, Abcam, UK) overnight at 4 °C. The immunoprecipitated DNA captured by pretreated protein A/G agarose (sc-2003, Santa Cruz Biotechnology) was recovered with proteinase K digestion followed by phenol–chloroform–isoamyl alcohol (25:24:1) purification. The recovered DNA fractions were diluted 1:50 and used to amplify the proximal promoter sequences of rat ATGL and HSL genes by real-time PCR with specific primers (Table 1).
Statistical analysis
For all parameters involving four groups, two-way ANOVA was performed to assess the main effects of betaine and glucocorticoid, as well as their interactions using general linear model, followed by LSD post hoc analysis to evaluate differences between specific groups. Data were presented as mean ± SEM. All statistical analyses were performed with the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). A P value < 0.05 was considered statistically significant.
Results
Body weight and adipose tissue weight
Chronic DEX administration significantly decreased the body weight and daily food intake (P < 0.05), which was not reversed by maternal betaine exposure. No significant changes were observed in epididymal adipose tissue weight or adipose index after betaine or DEX treatment (Table 2).
Serum biochemical parameters
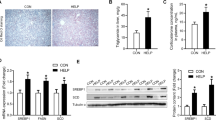
Chronic DEX treatment significantly (P < 0.05) increased serum total triglyceride level and decreased serum total cholesterol, HDL-C and LDL-C levels. Maternal betaine supplementation did not protect the progeny rats from these DEX-induced changes. Serum glucose and NEFA concentrations were not affected by either prenatal betaine exposure or postnatal dexamethasone injection. However, maternal betaine exposure significantly alleviated DEX-induced increase in insulin and decrease in corticosterone levels (Table 3).
Serum concentrations of IL-1β, IL-6, and TNFα
DEX-induced increase of serum IL-6 and TNFα concentrations was partially or completely ameliorated by maternal betaine exposure in progeny rats (Table 4). However, serum IL-1β level did not show significantly difference among four groups.
Expression of lipolytic genes in adipose tissue
ATGL and HSL are key lipolytic enzymes in adipose tissue. Maternal betaine intake significantly (P < 0.05) alleviated DEX-induced up-regulation of ATGL expression at both mRNA and protein levels (Fig. 2a, b). Similar results were observed in HSL mRNA and p-HSL protein expression (Fig. 2c, d). In addition, maternal betaine exposure markedly (P < 0.05) decreased the DEX-induced up-regulation of lipogenic genes including ACC and FAS at mRNA level, while no significant differences were observed at protein level among four groups (Fig. 3).
Expression of lipolytic genes in adipose tissue. a ATGL mRNA expression in adipose tissue; b ATGL protein expression in adipose tissue; c HSL mRNA expression in adipose tissue; d p-HSL protein expression in adipose tissue. Values are mean ± SEM (n = 6). Different letters indicate statistical significance (P < 0.05)
GR, AMPK, and AKT protein contents in adipose tissue
In addition, DEX challenge significantly increased glucocorticoid receptor (GR) protein content in adipose tissue, which was diminished by maternal betaine supplementation (Fig. 4b). However, no significant differences were observed in AMPK and AKT protein contents in adipose tissue (Fig. 4c, d).
GR, AMPK, and AKT protein expression in adipose tissue. a Western blot bands; b GR protein expression in adipose tissue; c AMPK protein expression in adipose tissue; d AKT protein expression in adipose tissue. Values are mean ± SEM (n = 6). Different letters indicate statistical significance (P < 0.05)
DNA methylation status on the promoter of lipolytic genes in adipose tissue
Methylated DNA immunoprecipitation analysis revealed that DEX administration significantly decreased the level of DNA methylation on the promoter of ATGL and HSL genes, which was significantly (P < 0.05) attenuated in progeny rats derived from betaine-supplemented dams (Fig. 5a, b).
Discussion
Numerous studies show that dietary betaine supplementation could ameliorate non-alcoholic fatty liver disease [7, 22] and alcoholic fatty liver disease [11, 23]. At present, studies on the alleviation effect of betaine have been mainly focused on liver, while adipose tissue was rarely studied. In this study, we confirmed that maternal betaine supplementation is effective in the treatment of chronic DEX-induced lipolysis in offspring rats. Interestingly, serum NEFA concentration was not affected by either betaine or DEX. However, a previous study showed that DEX administration markedly increased plasma free fatty acid levels [24]. Given that DEX-induced increase in hepatic lipid accumulation was alleviated by maternal betaine supplementation [19], we speculate that NEFA released from adipose tissue may be transported to liver or muscle for ectopic lipid deposition. Therefore, a cross-talk between adipose and other tissues (such as liver and muscle) may play a vital role in regulating serum NEFA homeostasis. In addition, maternal betaine exposure protected the progeny rats from DEX-induced chronic low-grade inflammation, which is agreement with the previous findings [8, 25] and also supports the previous report that dietary betaine mitigated high-fat-diet-induced IL-6 expression in adipose tissue [26]. Moreover, maternal betaine exposure partially alleviated DEX-induced decrease of serum endogenous corticosterone concentration in rat offspring.
ATGL and HSL are two main lipases responsible for the hydrolysis of TG [4]. Numerous studies have shown that glucocorticoid exposure induces adipose lipolysis both in vivo and in vitro [24, 27]. In addition, chronic glucocorticoid challenge also enhanced lipogenic activity in white adipose tissue [28]. In the present study, DEX-induced up-regulation of ATGL and HSL expression at mRNA and protein levels was remarkedly diminished by maternal betaine exposure, which is consistent with a recent study [11]. However, maternal betaine intake completely abolished DEX-induced increase of lipogenic genes including ACC and FAS at mRNA level. Surprisingly, neither betaine nor DEX affected ACC or FAS at protein level, which implicates a more complex mechanism involving post-transcriptional regulation. Thus, maternal betaine exposure alleviated DEX-induced lipolysis rather than lipogenesis in adipose tissue of rat offspring. Nevertheless, the mechanism underlying attenuation action of betaine on DEX-triggered lipolysis remains elusive. Increasing evidences suggest that epigenetic modifications play a central role in regulating lipid metabolism [29, 30]. We previously reported that maternal betaine treatment could alter hepatic DNA methylation, histone methylation, and micro-RNA expression in neonatal piglets [17, 31]. In addition, it has been reported that the action of betaine in ameliorating NAFLD is associated with modification of DNA methylation [32, 33]. Similar to the above results, we found that maternal betaine rectified the DEX-induced hypomethylation of ATGL and HSL gene promoters in adipose tissue of rat offspring.
The role of GCs mediated mainly through intracellular glucocorticoid receptor (GR). GR is a transcriptional factor that can bind to the glucocorticoid response elements (GREs) of target genes to induce transactivation and transrepression (named genomic pathway) [4]. In addition to above-mentioned model, GCs may also mediate a rapid non-genomic AMPK or JNK signaling pathways [34,35,36]. However, we did not detect any significant changes in AKT or AMPK in response to either betaine or DEX. In contrast, DEX-induced increase in GR protein was completely abolished in progeny rats derived from betaine-exposed dams. Due to limited quantity of adipose samples, we did not carry out chromatin immunoprecipitation assay to detect the GR binding on lipolytic genes. However, the previous studies found that RU486, a GR antagonist, could attenuate corticosterone-induced lipolytic rates and reduce ATGL and HSL protein content in 3T3-L1 cell line [5, 37]. In addition, we have reported that maternal betaine ameliorated hepatic lipid accumulation by reducing the GR binding to GREs located on the promoter of lipogenic genes [19].
In summary, we provide the evidence that maternal betaine protects rat offspring from glucocorticoid-induced adipose lipolysis by preventing promoter DNA hypomethylation of lipolytic genes including ATGL and HSL. The results of our study indicate the protective effects of maternal betaine exposure on glucocorticoid-induced metabolic dysfunctional in adipose tissue of rat offspring.
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AKT:
-
Serine/threonine-specific protein kinase
- AMPK:
-
AMP-activated protein kinase
- ATGL:
-
Adipose triglyceride lipase
- FAS:
-
Fatty acid synthase
- GR:
-
Glucocorticoid receptor
- HSL:
-
Hormone sensitive lipase
- IL-1β:
-
Interleukin 1β
- IL-6:
-
Interleukin 6
- NAFLD:
-
Non-alcoholic fatty liver disease
- TNFα:
-
Tumor necrosis factor α
References
Baschant U, Tuckermann J (2010) The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol 120(2–3):69–75. https://doi.org/10.1016/j.jsbmb.2010.03.058
Geer EB, Islam J, Buettner C (2014) Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinol Metab Clin N Am 43(1):75–102. https://doi.org/10.1016/j.ecl.2013.10.005
Do TTH, Marie G, Heloise D, Guillaume D, Marthe M, Bruno F, Marion B (2018) Glucocorticoid-induced insulin resistance is related to macrophage visceral adipose tissue infiltration. J Steroid Biochem Mol Biol. https://doi.org/10.1016/j.jsbmb.2018.08.010
Wang JC, Gray NE, Kuo T, Harris CA (2012) Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci 2(1):19. https://doi.org/10.1186/2045-3701-2-19
Campbell JE, Peckett AJ, D’Souza AM, Hawke TJ, Riddell MC (2011) Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am J Physiol Cell Physiol 300(1):C198–C209. https://doi.org/10.1152/ajpcell.00045.2010
Day CR, Kempson SA (2016) Betaine chemistry, roles, and potential use in liver disease. Biochem Biophys Acta 6:1098–1106. https://doi.org/10.1016/j.bbagen.2016.02.001
Bingul I, Aydin AF, Basaran-Kucukgergin C, Dogan-Ekici I, Coban J, Dogru-Abbasoglu S, Uysal M (2016) High-fat diet plus carbon tetrachloride-induced liver fibrosis is alleviated by betaine treatment in rats. Int Immunopharmacol 39:199–207. https://doi.org/10.1016/j.intimp.2016.07.028
Ge CX, Yu R, Xu MX, Li PQ, Fan CY, Li JM, Kong LD (2016) Betaine prevented fructose-induced NAFLD by regulating LXRalpha/PPARalpha pathway and alleviating ER stress in rats. Eur J Pharmacol 770:154–164. https://doi.org/10.1016/j.ejphar.2015.11.043
Kitagawa E, Yamamoto T, Fujishita M, Ota Y, Yamamoto K, Nakagawa T, Hayakawa T (2017) Choline and betaine ameliorate liver lipid accumulation induced by vitamin B6 deficiency in rats. Biosci Biotechnol Biochem 81(2):316–322. https://doi.org/10.1080/09168451.2016.1240604
Kovacevic S, Nestorov J, Matic G, Elakovic I (2017) Fructose and stress induce opposite effects on lipid metabolism in the visceral adipose tissue of adult female rats through glucocorticoid action. Eur J Nutr 56(6):2115–2128. https://doi.org/10.1007/s00394-016-1251-8
Dou X, Xia Y, Chen J, Qian Y, Li S, Zhang X, Song Z (2014) Rectification of impaired adipose tissue methylation status and lipolytic response contributes to hepatoprotective effect of betaine in a mouse model of alcoholic liver disease. Br J Pharmacol 171(17):4073–4086. https://doi.org/10.1111/bph.12765
Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z (2010) Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 298(5):G634–G642. https://doi.org/10.1152/ajpgi.00249.2009
Xu M, Che L, Yang Z, Zhang P, Shi J, Li J, Lin Y, Fang Z, Che L, Feng B, Wu D, Xu S (2016) Effect of high fat dietary intake during maternal gestation on offspring ovarian health in a pig model. Nutrients 8(8):E498. https://doi.org/10.3390/nu8080498
Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA (2007) Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr 97(3):435–439. https://doi.org/10.1017/S0007114507352392
Vanselow J, Kucia M, Langhammer M, Koczan D, Metges CC (2016) Maternal high-protein diet during pregnancy, but not during suckling, induced altered expression of an increasing number of hepatic genes in adult mouse offspring. Eur J Nutr 55(3):917–930. https://doi.org/10.1007/s00394-015-0906-1
Cheng Z, Zheng L, Almeida FA (2017) Epigenetic reprogramming in metabolic disorders: nutritional factors and beyond. J Nutr Biochem 54:1–10. https://doi.org/10.1016/j.jnutbio.2017.10.004
Cai D, Jia Y, Lu J, Yuan M, Sui S, Song H, Zhao R (2014) Maternal dietary betaine supplementation modifies hepatic expression of cholesterol metabolic genes via epigenetic mechanisms in newborn piglets. Br J Nutr 112(9):1459–1468. https://doi.org/10.1017/S0007114514002402
Cai D, Wang J, Jia Y, Liu H, Yuan M, Dong H, Zhao R (2016) Gestational dietary betaine supplementation suppresses hepatic expression of lipogenic genes in neonatal piglets through epigenetic and glucocorticoid receptor-dependent mechanisms. Biochem Biophys Acta 1:41–50. https://doi.org/10.1016/j.bbalip.2015.10.002
Zhao N, Yang S, Jia Y, Sun B, He B, Zhao R (2018) Maternal betaine supplementation attenuates glucocorticoid-induced hepatic lipid accumulation through epigenetic modification in adult offspring rats. J Nutr Biochem 54:105–112. https://doi.org/10.1016/j.jnutbio.2017.12.003
Liu X, Wang J, Li R, Yang X, Sun Q, Albrecht E, Zhao R (2011) Maternal dietary protein affects transcriptional regulation of myostatin gene distinctively at weaning and finishing stages in skeletal muscle of Meishan pigs. Epigenetics 6(7):899–907
Kiefer H (2015) Genome-wide analysis of methylation in bovine clones by methylated DNA immunoprecipitation (MeDIP). Methods Mol Biol 1222:267–280. https://doi.org/10.1007/978-1-4939-1594-1_20
Sookoian S, Puri P, Castano GO, Scian R, Mirshahi F, Sanyal AJ, Pirola CJ (2017) Nonalcoholic steatohepatitis is associated with a state of betaine-insufficiency. Liver Int 37(4):611–619. https://doi.org/10.1111/liv.13249
Kharbanda KK, Todero SL, King AL, Osna NA, McVicker BL, Tuma DJ, Wisecarver JL, Bailey SM (2012) Betaine treatment attenuates chronic ethanol-induced hepatic steatosis and alterations to the mitochondrial respiratory chain proteome. Int J Hepatol 2012:962183. https://doi.org/10.1155/2012/962183
Kuo T, Chen TC, Lee RA, Nguyen NHT, Broughton AE, Zhang D, Wang JC (2017) Pik3r1 is required for glucocorticoid-induced perilipin 1 phosphorylation in lipid droplet for adipocyte lipolysis. Diabetes 66(6):1601–1610. https://doi.org/10.2337/db16-0831
Olli K, Lahtinen S, Rautonen N, Tiihonen K (2013) Betaine reduces the expression of inflammatory adipokines caused by hypoxia in human adipocytes. Br J Nutr 109(1):43–49. https://doi.org/10.1017/S0007114512000888
Airaksinen K, Jokkala J, Ahonen I, Auriola S, Kolehmainen M, Hanhineva K, Tiihonen K (2018) High-fat diet, betaine, and polydextrose induce changes in adipose tissue inflammation and metabolism in C57BL/6J mice. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201800455
Li T, Guo K, Qu W, Han Y, Wang S, Lin M, An S, Li X, Ma S, Wang T, Ji S, Hanson C, Fu J (2016) Important role of 5-hydroxytryptamine in glucocorticoid-induced insulin resistance in liver and intra-abdominal adipose tissue of rats. J Diabetes Investig 7(1):32–41. https://doi.org/10.1111/jdi.12406
Chimin P, Farias Tda S, Torres-Leal FL, Bolsoni-Lopes A, Campana AB, Andreotti S, Lima FB (2014) Chronic glucocorticoid treatment enhances lipogenic activity in visceral adipocytes of male Wistar rats. Acta Physiol 211(2):409–420. https://doi.org/10.1111/apha.12226
Lee J, Kim Y, Friso S, Choi SW (2017) Epigenetics in non-alcoholic fatty liver disease. Mol Aspects Med 54:78–88. https://doi.org/10.1016/j.mam.2016.11.008
Keating ST, El-Osta A (2015) Epigenetics and metabolism. Circ Res 116(4):715–736. https://doi.org/10.1161/CIRCRESAHA.116.303936
Cai D, Jia Y, Song H, Sui S, Lu J, Jiang Z, Zhao R (2014) Betaine supplementation in maternal diet modulates the epigenetic regulation of hepatic gluconeogenic genes in neonatal piglets. PLoS One 9(8):e105504. https://doi.org/10.1371/journal.pone.0105504
Wang LJ, Zhang HW, Zhou JY, Liu Y, Yang Y, Chen XL, Zhu CH, Zheng RD, Ling WH, Zhu HL (2014) Betaine attenuates hepatic steatosis by reducing methylation of the MTTP promoter and elevating genomic methylation in mice fed a high-fat diet. J Nutr Biochem 25(3):329–336. https://doi.org/10.1016/j.jnutbio.2013.11.007
Wang L, Chen L, Tan Y, Wei J, Chang Y, Jin T, Zhu H (2013) Betaine supplement alleviates hepatic triglyceride accumulation of apolipoprotein E deficient mice via reducing methylation of peroxisomal proliferator-activated receptor alpha promoter. Lipids Health Dis 12:34. https://doi.org/10.1186/1476-511X-12-34
Motta K, Barbosa AM, Bobinski F, Boschero AC, Rafacho A (2015) JNK and IKKbeta phosphorylation is reduced by glucocorticoids in adipose tissue from insulin-resistant rats. J Steroid Biochem Mol Biol 145:1–12. https://doi.org/10.1016/j.jsbmb.2014.09.024
He J, Xu C, Kuang J, Liu Q, Jiang H, Mo L, Geng B, Xu G (2015) Thiazolidinediones attenuate lipolysis and ameliorate dexamethasone-induced insulin resistance. Metab Clin Exp 64(7):826–836. https://doi.org/10.1016/j.metabol.2015.02.005
Troncoso R, Paredes F, Parra V, Gatica D, Vasquez-Trincado C, Quiroga C, Bravo-Sagua R, Lopez-Crisosto C, Rodriguez AE, Oyarzun AP, Kroemer G, Lavandero S (2014) Dexamethasone-induced autophagy mediates muscle atrophy through mitochondrial clearance. Cell Cycle 13(14):2281–2295. https://doi.org/10.4161/cc.29272
Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, Xu G (2009) Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol 23(8):1161–1170. https://doi.org/10.1210/me.2008-0464
Acknowledgements
The present study was supported by the National Key Research and Development Program of China (2016YFD0500502), the National Basic Research Program of China (2012CB124703), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control.
Author information
Authors and Affiliations
Contributions
NZ contributed to hormone and gene assays, data analysis, and drafting of the manuscript. SY was responsible for animal care, breeding and sampling. BS and YF provided technical support. RZ contributed to conception, experimental design, data interpretation, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Rights and permissions
About this article
Cite this article
Zhao, N., Yang, S., Sun, B. et al. Maternal betaine protects rat offspring from glucocorticoid-induced activation of lipolytic genes in adipose tissue through modification of DNA methylation. Eur J Nutr 59, 1707–1716 (2020). https://doi.org/10.1007/s00394-019-02025-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02025-1