Abstract
Purpose
Hyperinsulinemia is linked to the development of various chronic diseases, especially obesity given to the role of insulin responses in body fat accumulation; hence, the current study aimed to examine the association of insulinemic potential of the diet with general and abdominal obesity among a large population of Iranian adults.
Methods
This cross-sectional study was carried out among 8691 adult participants aged 18–55 years. Dietary data were collected using a validated dish-based 106-item semi-quantitative food frequency questionnaire. Dietary insulin index (DII) was computed through considering food insulin index values published earlier. Dietary insulin load (DIL) was also calculated using a standard formula. Assessment of anthropometric measures was conducted through a self-administered questionnaire. General obesity was defined as body mass index ≥ 25 kg/m2, and abdominal obesity as waist circumference ≥ 94 cm for men and ≥ 80 cm for women.
Results
Mean age of study participants was 36.8 ± 8.1 years; 60.3% were women. Compared with the lowest quintile, women in the highest quintile of DIL were less likely to be abdominally obese (OR 0.73; 95% CI 0.57–0.92). Such significant association was not seen after controlling for potential confounders (OR 0.86; 95% CI 0.44–1.67). Neither in crude nor in adjusted models, we observed a significant association between DIL and general obesity among men and women. In terms of dietary insulin index, men in the top quintile of DII were more likely to be generally overweight or obese compared with those in the bottom quintile (OR 1.27; 95% CI 1.00–1.62). This association became non-significant after controlling for demographic characteristics (OR 1.14; 95% CI 0.84–1.56). Furthermore, in thefully adjusted model, women in the top quintile of DII were more likely to have general obesity compared with those in the bottom quintile (OR 1.40; 95% CI 1.07–1.84).
Conclusions
We found that adherence to a diet with a high DII was associated with greater odds of general obesity among women, but not in men. Although such information might help to draw conclusions on the practical relevance of the shown findings, further studies, specifically of prospective design, are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postprandial hyperinsulinemia may be relevant in the context of the prevention and management of various chronic diseases such as obesity, type 2 diabetes, cardiovascular disease and some cancers [1,2,3,4,5]. Dietary patterns that provoke excessive insulin secretion are believed to increase oxidative stress and accelerate the course of beta-cell dysfunction, which would, in turn, result in an increased risk of obesity and type 2 diabetes [6]. Recent studies have demonstrated that high-carbohydrate diets, which elicit high postprandial glucose and insulin responses, contribute to undesirable lipid profiles, greater body fat, and the development of insulin resistance [7,8,9].
It is now well accepted that carbohydrate is the major macronutrient that directly increases postprandial blood glucose levels and thus the main dietary stimulus for postprandial insulin secretion [10]. However, other dietary factors such as protein and fat also play a role. Almost foods high in protein or fat can enhance insulin secretion despite producing relatively small blood glucose responses [11]. Postprandial insulin responses, accordingly, are not always proportional to blood glucose concentrations because the insulinogenic effect of foods can be mediated by other factors including fructose, certain amino acids and fatty acids. Therefore, the classification of the relative insulinemic effects of all dietary components and their metabolic interactions is of both theoretical and practical significance, allowing a more comprehensive approach to determine insulin responses. [12, 13]. To systematically quantify postprandial insulin responses for all of these insulinotropic factors, a food insulin index (FII) has been suggested; a novel algorithm that ranks foods directly based on the insulin response in healthy subjects relative to an isoenergetic reference food (analogous to the GI, either glucose or white bread) [14]. Dietary Insulin Index (DII) is computed based on FII value of each food by considering the energy content and the consumption frequency of that food item. Findings from epidemiological studies indicated that FII is a more accurate predictor of observed insulin responses to composite meals than carbohydrate content [15]. In addition, Bell et al. found that DII was associated with improved postprandial glycemia without increased risk of hypoglycemia [16]. However, a cross-sectional study reported no association between the DII and glycemic control among healthy adults [17].
The prevalence of obesity has raised globally [18]; so that according to World Health Organization estimates, more than 1.9 billion adults around the world, are overweight. Of these, over 650 million are obese [19]. Although the prevalence of obesity in Asian population is lower than that in western nations, it has been increased at an alarming rate over the past decades [20]. Despite the association of DII with several chronic conditions in earlier studies, we are aware of no study examining the association between dietary insulin index and obesity. Examining the association of DII with chronic conditions is particularly relevant for obesity since stimulating greater insulin secretion results in augmented body fat accumulation through reducing fat oxidation and increasing carbohydrate oxidation. We hypothesized that a greater DII is associated with greater risk of obesity. Therefore, the purpose of the current study was to examine the association of insulinemic potential of the diet with general and abdominal obesity among a large population of Iranian adults.
Study population and methods
Participants
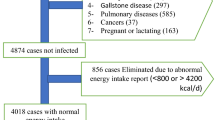
This cross-sectional study was done in the framework of the Study on the Epidemiology of Psychological Alimentary Health and Nutrition (SEPAHAN), a project that was performed in a large population of Iranian adults working in 50 different health centres in Isfahan province. Detailed information about the study design, participants and data collection method has been published previously [21]. At the beginning of the study, trained staff, who were taught completely by the principal investigators of the project regarding the rationale and methodological aspects of SEPAHAN, provided enough information to participants about the contents of questionnaires and study aims, while they were handing out the questionnaires [21]. A self-administrated questionnaire containing detailed information on anthropometric measurements, socio-demographic characteristics, physical activity and dietary intake was sent to 10,087 people aged 18–55 years, and 8691 subjects returned the completed questionnaire (response rate: 86.6%) [21]. In the current analysis, we excluded participants who had reported total daily energy intake of outside the range of 800–4200 kcal (n = 787) [22]. We also excluded individuals with missing data from the analysis (outcome and covariate variables) (n = 1322) [21]. These exclusions left 6582 and 5203 persons with complete data for the current analysis on general and abdominal obesity, respectively. All subjects provided written informed consent forms prior to study enrolment. The Bioethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran, approved the study [21].
Dietary intake assessment
Usual dietary intakes of participants were evaluated using a validated Willett-format [23]. Dish-based 106-item Semi-quantitative Food Frequency Questionnaire (DS-FFQ) which was designed particularly for Iranian adults [24]. Detail information on design, food items, and validity of this FFQ were reported elsewhere [24]. The food items included in the DS-FFQ was classified into five different categories: (1) mixed dishes (cooked or canned, 29 items); (2) grain-based foods (different types of bread, cakes, biscuits and potato, ten items); (3) dairy products (dairies, butter and cream, nine items); (4) fruits and vegetables (22 items); and (5) miscellaneous food items and beverages (including sweets, fast foods, nuts, desserts and beverages, 36 items). Participants were asked to determine their intakes of foods and mixed dishes based on multiple choice frequency response categories varying from “never or less than once a month” to “12 or more times per day”. The frequency response categories for the food list varied from 6 to 9 choices. We omitted the high-frequency categories for foods consumed infrequently, while for common foods with high consumption, the number of multiple choice categories increased. The most popular serving sizes that were usual among Iranian adults were used in the questionnaire. Finally, we estimated the daily intakes of all foods and dishes and converted to grams per day via the booklet of household measures [25]. Nutritionist IV software was used to obtain nutrient contents of foods and dishes based on the US Department of Agriculture’s (USDA) national nutrient databank. Then, we computed daily nutrient intakes of each participant by summing up the nutrient contents of all foods and dishes [26].
The validity of the DS-FFQ was investigated in a subgroup of 200 randomly selected participants of SEPAHAN project [24, 27]. All participants in the validation study completed the DS-FFQ at study baseline and 6 months later. During this validation study, participants provided three detailed dietary records that were used as a gold standard. Based on results from this study, the DS-FFQ could provide reasonably valid and reliable measures of long-term dietary intakes in Iranian population; for instance, dietary carbohydrate intake obtained from DS-FFQ was significantly correlated with the one obtained from the average of three dietary records (r = 0.81).
Calculation of dietary insulin index and load
After considering the components of mixed dishes, we converted all items in the DS-FFQ into a separate food item. Food insulin index (FII) refers to the incremental insulin area under the curve over 2 h in response to the consumption of a 1000-kJ portion of the test food divided by the area under the curve after ingestion of a 1000-kJ portion of the reference food. Food insulin index for each food item was obtained from previous studies published by Brand-Miller et al. [28]. For food items in the current study that was not available in the food list published by Brand-Miller et al., we used the FII of similar food items. To determine dietary insulin load (DIL), we first calculated the insulin load of each food by the following formula:
By summing up the insulin load of each food, DIL was obtained for each person. Then, we calculated dietary insulin index (DII) for each participant by dividing DIL by total energy intake.
Anthropometric assessment
Data on height, weight and waist circumference (WC) were gathered using a self-administered questionnaire. Body mass index (BMI) was calculated as weight in kilograms divided by the height in meters squared. Participants were classified into two categories based on their BMI: normal weight (≤ 24.9 kg/m2) and overweight or obese (≥ 25 kg/m2). Abdominal obesity was defined based on waist circumference. Abdominal overweight and obesity were identified based on the criteria proposed by Lean et al. and the National Cholesterol Education Program (NCEP), respectively [29, 30]. Participants were categorized into 2 groups: normal (< 80 cm for women, < 94 cm for men) and abdominaly obese (≥ 80 cm for women and ≥ 94 cm for men).
The validity of self-reported weight, height and WC was examined in a pilot study on 200 participants from the same population. In the validation study, self-reported values of anthropometric indices were compared with actual measured values. The correlation coefficients for self-reported weight, height and WC versus corresponding measured values were 0.95 (P < 0.001), 0.83 (P < 0.001) and 0.60 (P < 0.001), respectively. The correlation coefficient for computed BMI from self-reported values and the one from measured values was 0.70 (P < 0.001). These data indicated that the self-reported values of anthropometric measures provide a reasonable measure for these indices.
Assessment of covariates
We used a self-administered questionnaire to collect information about age, gender, marital status (single/married), education (high school diploma or below/university graduated), smoking status (non-smoker/former smoker/current smoker), family size (≤ 4/> 4 members), breakfast consumption and home ownership (owner/non-owner). Those who were consuming breakfast < 4 times/week were defined as breakfast skippers. To assess physical activity levels of participants, we used the General Practice Physical Activity Questionnaire (GPPAQ), a simple questionnaire reflecting an individual’s current physical activity [31]. In the current analysis, we categorized participants as having < 1 h/week or ≥ 1 h/week of moderate physical activity.
Statistical analysis
We first obtained energy-adjusted DIL and DII by the use of residual method. Then, we categorized men and women by quintiles of energy-adjusted DIL and DII. One-way analysis of variance (ANOVA) was used to examine significant differences in continuous variables across quintiles of DIL and DII. Chi square test was applied to assess the distribution of men and women in terms of categorical variables across quintiles of DIL and DII. To compare food and nutrient intakes across quintiles of DIL and DII, we used analysis of covariance (ANCOVA). To find the association of DIL and DII with general or abdominal obesity, binary logistic regression was used in different models. In the first model, age (continuous) and energy intake (continuous) were adjusted for. Then we additionally adjusted for marital status (single/married), education (high school diploma or below/above high school diploma), smoking status (non-smoker/former smoker/current smoker), family size (≤ 4/> 4 members), physical activity (< 1 h/week/≥ 1 h/week), breakfast skipping (< 4/≥ 4 times/week) and home ownership (owner/non-owner). In all analyses, the first quintile of DIL and DII was considered as the reference category. To compute the overall trend of odds ratios across increasing quintiles of DIL and DII, we used these quintiles as an ordinal variable in the logistic regression models. All statistical analyses were separately done for both genders using SPSS software (version 19.0; SPSS Inc, Chicago IL). P values were considered significant at < 0.05.
Results
Mean age of study participants was 36.8 ± 8.1 years; 60.3% were women. The prevalence of general obesity among men and women was 9.0 and 9.6%, respectively. Abdominal obesity was prevalent among 13.4% of men and 34.1% of women.
General characteristics of men and women across quintiles of DIL and DII are provided in Table 1. Men in the highest quintile of DIL and DII were more likely to be university graduated and breakfast skipper compared with those in the lowest quintile. Women in the top quintile, compared with those in the bottom quintile of DIL, were more likely to be university graduated, of small family size, breakfast skipper and have a lower BMI, and less likely to be old, married and generally obese. Conversely, women in the fifth quintile of DII were more likely to be abdominally obese and breakfast skipper compared with those in the first quintile.
Selected food and nutrient intakes of men and women across quintiles of DIL and DII are presented in Table 2. Men and women in the top quintile of DIL and DII had significantly different intakes of some food groups and nutrients compared with those in the bottom quintile. For instance, men in the highest quintile of DII had greater intakes of grains, carbohydrate, fiber, fructose, and folate as well as lower intakes of white meat and fat compared with those in the lowest quintile.
Multivariable-adjusted odds ratios for general and abdominal obesity across quintiles of DIL and DII in men and women are shown in Table 3. With regards to DIL, no significant association with general and abdominal obesity was seen among men. In addition, after controlling for confounders, women in the highest quintile of DIL had no greater odds of general and abdominal obesity compared to those in the first quintile. Despite for decreasing odds ratios across increasing quintiles of DIL in women in crude model (P-trend = 0.02), we did not observe such a trend when covariates were taken into account (P-trend = 0.15).
As shown in Table 3, men in the top quintile of DII were not more likely to be generally overweight or obese compared with those in the bottom quintile after controlling for demographic characteristics. However, women in the highest quintile of DII were more likely to be generally overweight or obese compared with those in the first quintile even after taking potential confounders into account. Despite a significant increasing trend of odds ratios across increasing quintiles of DII among women in models 1 and 2, similar trend was not seen in the crude model. In addition to general obesity, no overall significant association was observed between DII and abdominal obesity in women.
Discussion
This cross-sectional study provided the first evidence on the association of DIL and DII with the risk of general and abdominal obesity. Our findings demonstrated that a greater DII might be positively associated with greater odds of general obesity in women, but not in men. No significant association was found between DII and abdominal obesity. In addition, there was no significant association between DIL and general or abdominal obesity either before or after taking potential confounders into account.
DII, that has received great attention in recent years, has been shown to be an appropriate indicator than glycemic index (GI) in predicting the risk of chronic conditions. It is directly based on insulin response, not other mediators [15, 32]. In the current study, we observed a significant positive correlation between DII and odds of general obesity among women, but not in men, in the highest quintile compared with those in the lowest quintile. However, the trend of odds ratios was not significant. This indicates that DII might not be directly associated with obesity until it reaches specific high values. This could be explained by the highly dynamic process of insulin secretion which is regulated by various factors including nutrients, hormones, and neuronal inputs [33]. Similar to our observations, the DONALD study, an ongoing cohort study carried out in Germany, demonstrated that higher DIL and DII during puberty was associated with a higher percent of body fat, but not BMI, in young adulthood [34]. Besides, it has been reported that DII was also positively associated with lower plasma HDL and higher triglyceride concentrations, especially in obese individuals; however, no significant association was found between DII and inflammation and several cancers [17, 35, 36]. Hyperinsulinemia and insulin resistance play an important role in the development of obesity, such that several studies have indicated that a diet inducing elevated insulin response may contribute to the development of several metabolic risk factors, including obesity [6, 37]. In addition, recent observational studies have suggested that diets which provoke lower insulin secretion have a beneficial effect on various chronic conditions and may prevent the development of unfavorable body composition [7, 38]. Overall, it seems that further studies should be done in this area to shed light on this issue.
Mean DII and DIL values in the current study were 43.1 and 107,930 in men, and 42.4 and 98,275 in women, respectively. Men and women in the highest quintile of DII had DII values of greater than 49 and 47, respectively. Mean DII value was 54.6 in men and 51.9 in women, respectively. These values about DII are comparable to other studies. For instance, in the study of Nurses’ Health Study and the Health Professionals Follow-Up Study, median DII was 41.7 in men, and 42.8 in women, respectively [17, 35, 39]. However, mean DIL values in our study were almost 1000 times greater than the ones reported in other studies. This difference can be explained by the different methods used to calculate DIL values in different studies. In the current study, we used each gram of a given food in the formula to compute DIL. Others used each serving of that food. This could result in a big difference between studies. High consumption of carbohydrate-containing foods, among Iranian people might provide another reason for this difference. Compared with other nations, Iranians take almost 62% of their energy from carbohydrate-rich foods [40]. Differences in the type of dietary assessment methods may be another reason. In the current study, we applied a dish-based FFQ to examine dietary intakes due to the culture of dietary habits among Iranians. DIL values were developed to assess the total quantity of insulinogenic food intake but they were not designed to measure meal frequency and food combinations.
In the current study, we found a gender difference in the relationship between DII and obesity. The underlying mechanism for this gender discrepancy is unknown; however, it might be explained, at least in part, by the influence of gonadal steroids on body composition and appetite [41]. Some studies have shown that the effects of changes in estrogen levels on body composition and appetite may be mediated by increases in the hypothalamic expression of the orexigenic peptides neuropeptide Y (NPY) and agouti-related peptide (AgRP) [42, 43]. Another reason for this gender disparity might be the difference in plasma adiponectin concentrations among females and males [44]. At any particular body size or body fat distribution, adiponectin concentrations are greater in women than in men. This finding might also be attributed to the accuracy of reported dietary intakes among women than in men. In the current study, women had greater intakes of various food groups and nutrients compared with men across the quintiles of DIL.
The potential mechanisms through which DII might influence obesity in unknown. A high DII may facilitate obesity by stimulating more insulin secretion, which may reduce fat oxidation and increase carbohydrate oxidation, leading to increased fat storage [45]. Several studies have suggested that reduced blood glucose fluctuations may lead to prolonged satiety and decreased energy intake [46, 47]. Indeed, high-DII foods are rapidly digested, absorbed and transformed into glucose [47], which results in transient surges in blood glucose and insulin. This can cause the rapid fall in glucose excursion which is often associated with reduced satiety, quick return of hunger sensations and excessive caloric intake [47]. However, that is not always the case for all foods [48]. For instance, in terms of yoghurt, which is a high-DII food, it is surprisingly digested and absorbed slowly [49]. Moreover, not all high-DII foods are converted into glucose. In addition, based on the involvement of gastrointestinal hormones, in particular incretin, low-DII diet has been shown to play an important role in improving glycemic control [50]. Experimental studies have indicated that increased levels of cholecystokinin may favour a quick absorption of carbohydrates and consequently induce a progressive accumulation of fat [51].
The association between dietary insulin indices and obesity may be attributed to estrogen deficiency-related mechanisms. Recent investigations suggested that in case of estrogen deficient, estrogen receptors are involved in different genomic, nongenomic, and mitochondrial mechanisms through which they disturb insulin signaling and substrate oxidation [52]. In addition, consumption of high glycemic index diets results in insulin resistance, which is associated with major depression [53]. Such psychological conditions might lead to obesity through different mechanisms.
The main strength of this study was its large sample size of adults. In addition, we controlled the analyses for several potential confounders to reach an independent association between DII and DIL and obesity. Gender-stratified analysis along with the use of a validated FFQ for dietary assessment are other strengths of this study. However, this study had several limitations. The main limitation is the cross-sectional design which prohibits inferring a causal link between DIL and DII and obesity. As in cross-sectional studies data on exposure and outcome are gathered simultaneously in a specific time point, prospective studies are necessary to confirm our findings. In addition, participants with obesity may have reduced their dietary intakes to lose weight. However, such residual confounding effects may attenuate the risk estimates, therefore, actual results may be even stronger than those obtained. Although we controlled for several covariates, taking other factors including menopausal status, hormone therapy and psychological factors into account might also be needed to reach an independent association between DII and DIL and general and abdominal obesity. In addition, despite the use of a validated FFQ for dietary assessment, some degree of measurement errors and misclassification may occur. This is also the case about the outcome of interest in the current study. Even though our validation study revealed a significant correlation between self-reported and measured data on anthropometric indicators, some sort of errors might also occur in this case [54]. Finally, due to the limited number of foods with a tested FII value, for foods that were not available in the database, we used the values for similar foods; thus, further FII testing is needed in order to confirm our findings in this study.
In conclusion, adherence to a diet with a high DII might be positively associated with greater odds of general obesity in women, but not in men. No significant association was found between DII and abdominal obesity. The take-home message of this study would be the recommendation to restrict insulin-stimulating nutrients in the dietary patterns as an approach for preventing obesity. Given that our findings are preliminary, we hope they stimulate further studies, especially of prospective design, to shed light on this issue.
Abbreviations
- FII:
-
Food insulin index
- GI:
-
Glycemic index
- DII:
-
Dietary insulin index
- SEPAHAN:
-
Studying the epidemiology of psycho-alimentary health and nutrition
- DS-FFQ:
-
Dish-based 106-item semi-quantitative food frequency questionnaire
- USDA:
-
US Department of Agriculture waist circumference
- DIL:
-
Dietary insulin load
- WC:
-
Waist circumference
- BMI:
-
Body mass index
- NCEP:
-
National cholesterol education program
- GPPAQ:
-
General practice physical activity questionnaire
- ANOVA:
-
One-way analysis of variance
- ANCOVA:
-
Analysis of covariance
- OR:
-
Odds ratio
References
Bell SJ, Sears B (2003) Low-glycemic-load diets: impact on obesity and chronic diseases. Crit Rev Food Sci Nutr 43:357–377. https://doi.org/10.1080/10408690390826554
Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC (1997) Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 20:545–550. https://doi.org/10.2337/diacare.20.4.545
Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE (2000) A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr 71:1455–1461. https://doi.org/10.1093/ajcn/71.6.1455
Larsson SC, Orsini N, Wolk A (2005) Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 97:1679–1687. https://doi.org/10.1093/jnci/dji375
Giovannucci E, Michaud D (2007) The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 132:2208–2225. https://doi.org/10.1053/j.gastro.2007.03.050
Ludwig DS (2000) The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287:2414–2423. https://doi.org/10.1001/jama.287.18.2414
Tay J, Thompson CH, Luscombe-Marsh ND, Wycherley TP, Noakes M, Buckley JD, Wittert GA, Yancy WS, Brinkworth GD (2018) Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: a 2-year randomized clinical trial. Diabetes Obes Metab 20(4):858–871. https://doi.org/10.1111/dom.13164
Papakonstantinou E, Orfanakos N, Farajian P, Kapetanakou AE, Makariti IP, Grivokostopoulos N, Ha MA, Skandamis PN (2017) Short-term effects of a low glycemic index carob-containing snack on energy intake, satiety, and glycemic response in normal-weight, healthy adults: results from two randomized trials. Nutrition 42:12–19. https://doi.org/10.1016/j.nut.2017.05.011
Ellery THP, Sampaio HAC, Carioca AAF, Silva BYDC, Alves JAG, Da Silva Costa F, Araujo Júnior E, Melo MLP (2019) Association between dietary glycemic index and excess weight in pregnant women in the first trimester of pregnancy. Rev Bras Ginecol Obstet 41(1):4–10. https://doi.org/10.1055/s-0038-1676096
Wu T, Giovannucci E, Pischon T, Hankinson SE, Ma J, Rifai N, Rimm EB (2004) Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in US women. Am J Clin Nutr 80(4):1043–1049. https://doi.org/10.1093/ajcn/80.4.1043
Moghaddam E, Vogt JA, Wolever TM (2006) The effects of fat and protein on glycemic responses in nondiabetic humans vary with waist circumference, fasting plasma insulin, and dietary fiber intake. J Nutr 136:2506–2511. https://doi.org/10.1093/jn/136.10.2506
Nuttall FQ, Gannon MC (l991) Plasma glucose and insulin response to macronutrients in nondiabetic and NIDDM subjects. Diabetes Care l4:824-838.https://doi.org/10.2337/diacare.14.9.824
Ranganath L, Norris F, Morgan L, Wright J, Marks V (1999) The effect of circulating non-esterified fatty acids on the entero-insular axis. Eur J Clin Invest 29(1):27-32.https://doi.org/10.1046/j.1365-2362.1999.00426.x
Bao J, de Jong V, Atkinson F, Petocz P, Brand-Miller JC (2009) Food insulin index: physiologic basis for predicting insulin demand evoked by composite meals. Am J Clin Nutr 90:986-992.https://doi.org/10.3945/ajcn.2009.27720
Bell KJ, Petocz P, Colagiuri S, Brand-Miller JC (2016) Algorithms to improve the prediction of postprandial insulinaemia in response to common foods. Nutrients 8(4):210. https://doi.org/10.3390/nu8040210
Bell KJ, Bao J, Petocz P, Colagiuri S, Brand-Miller JC (2015) Validation of the food insulin index in lean, young, healthy individuals, and type 2 diabetes in the context of mixed meals: an acute randomized crossover trial. Am J Clin Nutr 102:801–806. https://doi.org/10.3945/ajcn.115.112904
Nimptsch K, Brand-Miller JC, Franz M, Sampson L, Willett WC, Giovannucci E (2011) Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am J Clin Nutr 94(1):182–190. https://doi.org/10.3945/ajcn.110.009555
Rummo P, Kanchi R, Perlman S, Elbel B, Trinh-Shevrin C, Thorpe L (2018) Change in obesity prevalence among New York City adults: the NYC health and nutrition examination survey, 2004 and 2013–2014. J Urban Health. https://doi.org/10.1007/s11524-018-0310-2
World Health Organization (2016) WHO Media center. Obesity and overweight. World Health Organization, Geneva. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Kilpi F, Webber L, Musaigner A, Aitsi-Selmi A, Marsh T, Rtveladze K, McPherson K, Brown M (2013) Alarming predictions for obesity and non-communicable diseases in the Middle East. Public Health Nutr 17(5):1078–1086. https://doi.org/10.1017/S1368980013000840
Adibi P, Keshteli AH, Esmaillzadeh A, Afshar H, Roohafza H, Bagherian-Sararoudi R, Daghaghzadeh H, Soltanian N, Feinle-Bisset C, Boyce PH et al (2012) The study on the epidemiology of psychological, alimentary health and nutrition (SEPAHAN): overview of methodology. J Res Med Sci 17:292–298
Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, Willett WC (2002) Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr 76(3):535–540. https://doi.org/10.1093/ajcn/76.3.535
Willett W (2013) Nutritional epidemiology. Oxford University Press, Oxford
Keshteli AH, Esmaillzadeh A, Rajaie S, Askari G, Feinle-Bisset CH, Adibi P (2014) A dish based semi-quantitative food frequency questionnaire for assessment of dietary intakes in epidemiologic studies in Iran: design and development. Int J Prev Med 5(1):29–36
Ghaffarpour M, Houshiar-Rad A, Kianfar H (1999) The manual for household measures, cooking yields factors and edible portion of foods. Nashre Olume Keshavarzy, Tehran
Kimura Y, Wada T, Okumiya K, Ishimoto Y, Fukutomi E, Kasahara Y, Chen W, Sakamoto R, Fujisawa M, Otsuka K, Matsubayashi K (2012) Eating alone among community-dwelling Japanese elderly: association with depression and food diversity. J Nutr Health Aging 16:728–731. https://doi.org/10.1007/s12603-012-0067-3
Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L, Keshteli AH, Feizi A, Feinle-Bisset CH, Adibi P (2016) Nutrient patterns and their relation to general and abdominal obesity in Iranian adults: findings from the SEPAHAN study. Eur J Nutr 55(2):505–518. https://doi.org/10.1007/s00394-015-0867-4
Holt S, Miller J, Petocz P (1997) An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. Am J Clin Nutr 66(5):1264–1276. https://doi.org/10.1093/ajcn/66.5.1264
Lean ME, Han TS, Morrison CE (1995) Waist circumference as a measure for indicating need for weight management. BMJ 311:158–161. https://doi.org/10.1136/bmj.311.6998.158
National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A (2002) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 106:3143–3421
Department of Health (2006) The general practice physical activity questionnaire. Department of Health, London. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_063812
Sung KC, Lee MY, Kim YH, Huh JH, Kim JY, Wild SH, Byrne CD (2018) Obesity and incidence of diabetes: effect of absence of metabolic syndrome, insulin resistance, inflammation and fatty liver. Atherosclerosis 275:50–57. https://doi.org/10.1016/j.atherosclerosis.2018.05.042
Seino Susumu, Shibasaki Tadao, Minami Kohtaro (2011) Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest 121:2118–2125. https://doi.org/10.1172/JCI45680
Joslowski G, Goletzke J, Cheng G, Günther AL, Bao J, Brand-Miller JC, Buyken AE (2012) Prospective associations of dietary insulin demand, glycemic index, and glycemic load during puberty with body composition in young adulthood. Int J Obes (Lond) 36(11):1463–1471. https://doi.org/10.1038/ijo.2011.241
Prescott J, Bao Y, Viswanathan AN, Giovannucci EL, Hankinson SE, De Vivo I (2014) Dietary insulin index and insulin load in relation to endometrial cancer risk in the Nurses’ Health Study. Cancer Epidemiol Biomark Prev 23(8):1512–1520. https://doi.org/10.1158/1055-9965.EPI-14-0157
Bao Y, Nimptsch K, Wolpin BM, Michaud DS, Brand-Miller JC, Willett WC, Giovannucci E, Fuchs CS (2011) Dietary insulin load, dietary insulin index, and risk of pancreatic cancer. J Clin Nutr 94(3):862–868. https://doi.org/10.3945/ajcn.110.011205
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA et al (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112(12):1821–1830. https://doi.org/10.1172/JCI19451
Mirmiran P, Esfandiari S, Bahadoran Z, Tohidi M, Azizi F (2016) Dietary insulin load and insulin index are associated with the risk of insulin resistance: a prospective approach in tehran lipid and glucose study. J Diabetes Metab Disord 20:15–23. https://doi.org/10.1186/s40200-016-0247-5
Bao Y, Nimptsch K, Meyerhardt JA, Chan AT, Ng K, Michaud DS et al (2010) Dietary insulin load, dietary insulin index, and colorectal cancer. Cancer Epidemiol Biomark Prev 19:3020–3026. https://doi.org/10.1158/1055-9965
Bahreynian M, Esmaillzadeh A (2012) Quantity and quality of carbohydrate intake in Iran: a target for nutritional intervention. Arch Iran Med 15:648–649
Lovejoy JC, Sainsbury A, Stock Conference Working G (2009) Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 10:154–167. https://doi.org/10.1111/j.1467-789X.2008.00529.x
Pelletier G, Li S, Luu-The V, Labrie F (2007) Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol 19:426–431. https://doi.org/10.1111/j.1365-2826.2007.01548.x
Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N (2007) Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56:1051–1058. https://doi.org/10.2337/db06-0015
Adamczak M, Rzepka E, Chudek J, Wiecek A (2005) Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin Endocrinol (Oxf) 62(1):114–118. https://doi.org/10.1111/j.1365-2265.2004.02182.x
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846. https://doi.org/10.1038/nature05482
Hellström PM (2013) Satiety signals and obesity. Curr Opin Gastroenterol 29(2):222–227. https://doi.org/10.1097/MOG.0b013e32835d9ff8
Llewellyn CH, Trzaskowski M, van Jaarsveld CHM, Plomin R, Wardle J (2014) Satiety mechanisms in genetic risk of obesity. JAMA Pediatr 168(4):338–344. https://doi.org/10.1001/jamapediatrics.2013.4944
Ostman EM, Liljeberg Elmstahl HG, Bjorck IM (2001) Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr 74:96–100. https://doi.org/10.1093/ajcn/74.1.96
Flint A, Møller BK, Raben A, Pedersen D, Tetens L, Holst JJ et al (2004) The use of glycaemic index tables to predict glycaemic index of composite breakfast meals. Br J Nutr 91:979–989. https://doi.org/10.1079/BJN20041124
Ezcurra M, Reimann F, Gribble FM, Emery E (2013) Molecular mechanisms of incretin hormone secretion. Curr Opin Pharmacol 13:922–927. https://doi.org/10.1016/j.coph.2013.08.013
Chaumontet Catherine, Azzout-Marniche Dalila, Blais Anne, Chalvon-Dermersay Tristan et al (2015) Rats prone to obesity under a high-carbohydrate diet have increased post-meal CCK mRNA expression and characteristics of rats fed a high-glycemic index diet. Front Nutr 9:22. https://doi.org/10.3389/fnut.2015.00022
Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K et al (2006) Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49:588–597. https://doi.org/10.1007/s00125-005-0105-3
Moulton CD, Pickup JC, Ismail K (2015) The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol 3:461–471. https://doi.org/10.1016/S2213-8587(15)00134-5
Aminianfar A, Saneei P, Nouri M, Shafiei R, Keshteli AH, Esmaillzadeh A, Adibi P. The validity of self-reported height, weight, body mass index and waist circumference in Iranian adults. Int J Prev Med (In press)
Funding
The study was financially supported by National Institute for Medical Research Development (977045).
Author information
Authors and Affiliations
Contributions
JAS, OS and AE contributed to the conception and design of the study, data collection, and statistical analysis and drafting of the manuscript; AHK, HA and PA contributed in data collection and manuscript drafting. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declared any personal or financial conflicts of interest.
Rights and permissions
About this article
Cite this article
Anjom-Shoae, J., Keshteli, A.H., Sadeghi, O. et al. Association between dietary insulin index and load with obesity in adults. Eur J Nutr 59, 1563–1575 (2020). https://doi.org/10.1007/s00394-019-02012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02012-6