Abstract
Purpose
Fish consumption and dietary intake of n-3 polyunsaturated acids (PUFAs) may be associated with inflammatory bowel disease (IBD). We aimed to conduct a systematic review and summarize published articles on the association between fish consumption and dietary intake of n-3 PUFAs with the risk of IBD.
Methods
PubMed, Scopus, and Web of Science databases were used to conduct a comprehensive search and identify eligible literature published prior to January 2019. Fixed-effects model or random-effects models (DerSimonian–Laird method) were applied to pool the effect sizes. Cochrane Q test was used to trace the potential source of heterogeneity across studies.
Results
12 studies (5 prospective and 7 case–control) were included in the systematic review, which ten of them were eligible for inclusion in the meta-analysis. Studies were included a total sample size of 282610 participants which 2002 of them were cases of IBD [1061 Crohn’s disease (CD) and 937 ulcerative colitis (UC)]. A negative association was found between fish consumption and the incidence of CD (pooled effect size: 0.54, 95%CI: 0.31–0.96, P = 0.03). There was no relationship between total dietary n-3 PUFAs intake and IBD (pooled effect size: 1.17, 95%CI: 0.80–1.72, P = 0.41). A significant inverse association was observed between dietary long-chain n-3 PUFAs and the risk of UC (pooled effect size: 0.75, 95%CI: 0.57–0.98, P = 0.03). Moreover, no association was found between α-Linolenic acid (ALA) and IBD (pooled effect size: 1.17, 95%CI: 0.63–2.17, P = 0.62).
Conclusions
Findings showed a negative association between fish consumption and the risk of CD. Moreover, there was a significant inverse association between dietary long-chain n-3 PUFAs and the risk of UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiological studies have shown an increase in the incidence of inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC) [1]. The highest annual incidence of IBD has been reported in Europe (12.7 and 24.3 per 100,000 person-years for CD and UC) and North America (20.0 and 19.2 per 100,000 person-years for CD and UC) followed by Asia and the Middle East (5.0 and 6.3 per 100,000 person-years for CD and UC) [1]. The underlying etiology in the development of IBD appears to be a deficiency of the mucosal immune response to intestinal flora due to genetic susceptibility and environmental factors [2, 3]. However, the reported genetic contribution in the pathophysiology of IBD is approximately 25% [4], which suggests a role of environmental and lifestyle factors in the etiology of IBD [3]. Therefore, identifying the determinants of IBD is needed to develop appropriate countermeasures.
In several studies, dietary components have been associated with IBD [5, 6]. Dietary intake of fruits, vegetables, dairy products, iron, and Vitamin D has been related to the etiology of IBD [7,8,9]. Moreover, given the importance of polyunsaturated fatty acids (PUFAs) in the regulation of inflammatory processes, n-3 PUFAs [10], eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), or α-Linolenic acid (ALA) may be associated with IBD. However, studies showed inconsistent results on the association between fish consumption and n-3 PUFAs and the risk of IBD [11,12,13,14,15,16,17,18,19,20,21,22]. Some studies have shown a strong negative [13, 17, 18] or positive [16] association between fish consumption and risk of IBD, while others found associations with n-3 PUFAs but not for fish intake [22]. Moreover, some studies failed to find any association between fish [11, 19, 21] and n-3 PUFAs intake [12, 14, 15, 20] with the risk of IBD. To the best of our knowledge, there are no studies that have summarized the quantitative association between fish and n-3 PUFAs intake and the risk of IBD. A recent meta-analysis of randomized controlled trials did not support the use of omega-3 supplementation for maintenance of remission in IBD [23]. However, the role of n-3 PUFAs in the onset of IBD may differ from the treatment of IBD. Moreover, dietary intake of n-3 PUFAs in the context of foods such as fish might have a more appreciable effect. Given the conflicting results on the association between fish and n-3 PUFAs and the risk of IBD, we performed a systematic literature review and meta-analysis summarizing earlier findings.
Methods
The current study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [24]. Ethical approval for this protocol was obtained through the National Institute for Medical Research Development (Grant and Ethics Number: 977288).
Search strategy
A comprehensive literature search was performed in online PubMed, Scopus, and Web of Science for pertinent articles published prior to January 2019. The search terms included the following: (Seafood[Mesh] OR Fishes[Mesh] OR “Fish Oils”[Mesh] OR “Cod Liver Oil”[Mesh] OR “Fatty Acids, Omega-3”[Mesh] OR “Eicosapentaenoic Acid”[Mesh] OR “Docosahexaenoic Acids”[Mesh] OR Seafood[tiab] OR Fishes[tiab] OR “Fish Oils”[tiab] OR “Cod Liver Oil”[tiab] OR “Omega 3 Fatty Acid”[tiab] OR “eicosapentaenoic acid”[tiab] OR “docosahexaenoic acids”[tiab] OR “polyunsaturated FA”[tiab] OR “n-3 PUFA”[tiab] OR “n-3 Fatty Acid”[tiab] OR “n-3 Polyunsaturated Fatty Acid”[tiab] OR EPA[tiab] OR DHA[tiab] OR “docosapentaenoic acid”[tiab]) AND (“Inflammatory Bowel Diseases”[Mesh] OR “Crohn Disease”[Mesh] OR “Colitis, Ulcerative”[Mesh] OR “Inflammatory bowel diseases”[tiab] OR “Crohn disease”[tiab] OR “ulcerative colitis”[tiab]). No restriction or filter was exerted in terms of publication date while searching the aforementioned databases. The bibliographies of eligible studies and relevant reviews were also examined. To accelerate the process of screening citations, all publications were saved into an EndNote library (version X7, for Windows, Thomson Reuters, Philadelphia, PA, USA) and duplicate citations were removed.
Selection criteria
Eligible articles on the association between fish consumption, n-3 PUFAs, and IBD were defined as studies that: (a) had observational study designs, including cohort, case–control, nested case–control and cross-sectional studies, (b) were published in English, (c) included individuals ≥ 15 years of age, and (d) reported either odds ratios (ORs), hazard ratios (HRs), or relative risks (RRs) as well as 95% confidence intervals (95% CIs) for IBD in relation with fish consumption or dietary intake of n-3 PUFAs (including α-linolenic acid, EPA, and DHA). To avoid skewed findings, when redundant publications with the similar data sets and intellectual material were available, the publications with the most complete information were selected for inclusion in the analysis. As the focus of this study is on observational studies, publications that reported the supplements intake of n-3 PUFAs were not included.
Excluded studies
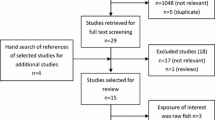
The initial search resulted in 920 publications. After removing duplicates, 727 studies remained for screening. Reviewing 727 title and abstracts, 690 were excluded, since they were short communication, letters, books, case report, review articles, animal studies, and unrelated publications. Out of 37 remaining articles for full-text evaluation, 25 studies were excluded based on the following: (a) had a non-observational study design, e.g., those with clinical design (n = 9), (b) assessed the association between fish or n-3 PUFAs with IBD in children (n = 2), (c) assessed the association between plasma levels of n-3 PUFAs and IBD (n = 5), (d) examined the association between dietary factors and fatty acids except fish and n-3 PUFAs with IBD (n = 4), (e) evaluated the association between dietary patterns and IBD (n = 2), (f) examined the association between fish or n-3 PUFAs with IBD severity not onset of IBD (n = 2), (g) reported data from the same population (n = 3), (h) reported findings based on other kinds of analysis except risk estimates along with their 95% confidence intervals (n = 4), and (i) published in a language other than English (n = 3). Two nested case–control studies had the same data set and were performed as part of the EPIC study [14, 15]. However, since one study assessed CD and the other UC, both were included in the analysis. Finally, 12 articles were eligible for inclusion in this study [11,12,13,14,15,16,17,18,19,20,21,22], five of which were prospective (three cohort [12, 13, 16] and two nested case–control [14, 15] studies), and seven had a case–control study design [11, 17,18,19,20,21,22]. A flow diagram that outlines the search strategy is provided in Fig. 1.
Data extraction
In the present study, fish consumption, dietary intake of n-3 PUFAs was regarded as the main exposure, while the risk of IBD (CD or UC) was regarded as the main outcome. In cohort studies, fish or dietary n-3 PUFAs intake at the baseline or during the recruitment study period was the key exposure variable and incidence of IBD (CD or UC) during the follow-up period was the main outcome variable. The reported risk estimates (ORs or HRs or RRs) for IBD among participants in the highest category of fish and dietary n-3 PUFAs intakes compared with those in the lowest category were extracted from all studies. Adjusted effect sizes were extracted where available. Some studies only provided risk estimates for ALA, EPA, and DHA [15, 20]. To conduct the meta-analysis on the association between long-chain n-3 PUFAs (EPA + DHA) or total n-3 PUFAs (long-chain n-3 PUFAs plus ALA) with IBD, risk estimates were pooled before data analysis. However, separate analyses were performed for ALA and long-chain n-3 PUFAs, since there were sufficient studies.
Furthermore, the following characteristics of eligible articles were extracted as follows: first author’s name, date of publication, study origin, age and gender of subjects, the number of participants who completed the study, the length of cohort studies, the type of exposure (fish, n-3 PUFAs, EPA, DHA, and ALA), outcome of interest (CD, UC, and IBD), methods used for measuring dietary intakes and assessing IBD, reported risk estimates related to CD, UC, and IBD (including ORs, RRs, HRs as well as their 95% confidence intervals) as well as any study variables that were adjusted. Each of the foregoing steps was assessed by two independent investigators. When the reviewers disagreed, the principal investigator was the tie breaker.
Quality assessment of studies
The Newcastle–Ottawa Scale (NOS) was used to determine the quality of included articles (Supplemental Table 1) [25]. Based on this star scoring system, each prospective study can be awarded a maximum of nine points based on the criteria in the following three domains: selection (a maximum of 4 points), comparability (a maximum of 2 points), and assessment of outcomes (a maximum of 3 points). Each case–control study can be awarded a maximum of ten points based on the parameters in the following three domains: selection (a maximum of 4 points), comparability (a maximum of 2 points), and assessment of exposure (a maximum of 4 points). According to NOS, one-to-three stars indicate low quality, four-to-six stars indicate moderate quality, and seven-to-nine stars indicate high quality [26]. Quality assessment was assessed independently by two authors and any disagreements were settled by discussion.
Statistical analysis
In this meta-analysis of observational studies, log RRs and standard errors (SEs) were calculated using odds ratio, relative risks and hazard ratios and their 95% confidence intervals that had been reported for IBD regarding fish consumption and dietary intake of n-3 PUFAs. At first, a fixed-effects model was used to drive the overall effect sizes. If there was significant between-studies heterogeneity, the random-effects model (DerSimonian–Laird) was applied as an alternative. Cochrane Q test and I2 were used to measure potential sources of heterogeneity across studies. In this study, I² > 50 was used as an indicator of heterogeneity among studies [27]. Subgroup analysis using a fixed-effect model was performed on the following criteria: design (prospective/case–control), geographical region (USA/Europe/Asia), gender (female/ both genders), adjustment for energy intake as a covariate (adjusted effect size/non-adjusted effect size), adjustment for body mass index (BMI) (adjusted effect size/non-adjusted effect size), adjustment for smoking (adjusted effect size/non-adjusted effect size), quality assessment score (> 6/≤ 6), and methods of IBD assessment (medical records and histology/confirmed by physicians).
Sensitivity analysis was performed to elucidate the stability of findings and to ascertain whether the final pooled effect sizes were affected by a single or several publications. In addition, plausible publication bias was specified visually by funnel plot and confirmed by the statistical evidence of Egger’s test [28]. Data analyses were performed on Stata version 11.2 (Stata Corp, College Station, TX). P values were considered significant at the level of < 0.05.
Results
Systematic review findings
Five prospective [12,13,14,15,16] (3 cohort and 2 nested case–control) and seven case–control studies [11, 17,18,19,20,21,22] were selected for inclusion in the current systematic review. Characteristics of each study are provided in Table 1. Publication date varied between 1997 and 2015. Five of the included studies were conducted in Europe [11, 14,15,16,17], five in Asia [18,19,20,21,22], and two were from USA [12, 13]. Three studies were conducted on women [12, 13, 16], while nine studies included both genders [11, 14, 15, 17,18,19,20,21,22]. None of the studies considered the gender-specific association between fish and n-3 PUFAs intake and the risk of IBD. The studies’ sample size ranged from 186 to 170,805. In total, 282,610 subjects, aged ≥ 15 years, with 2002 of them were cases of IBD (1061 CD and 937 UC) were entered in the current systematic review. The mean duration of cohort studies ranged between 10.4 and 26 years.
Ten studies used a food frequency questionnaire (FFQ) to determine dietary intakes [12,13,14,15,16,17,18,19,20, 22], out of which six were self-administered [12,13,14,15,16, 22], and four were derived from interviews [17,18,19,20]. Moreover, one study used an interview-based diet history questionnaire (DHQ) as the dietary assessment tool [21] and one study used their own questionnaire [11]. In one cohort study, dietary intake was determined only at baseline [16]. In one other study, dietary intakes during high school were evaluated using the high school-food frequency questionnaire (HS-FFQ) and statistical analyses were performed based on data from HS-FFQ [13]. However, in the study by Ananthakrishnan et al., dietary assessment was repeated throughout the recruitment period participants were categorized based on mean intake of dietary n-3 PUFAs [12]. Prospective collection of dietary intakes in the two nested case–control studies minimized recall bias [14, 15]. Moreover, to reduce the likelihood of recall bias, the case–control studies collected dietary intakes of newly diagnosed patients prior to the progression of IBD or manifestation of symptoms [11, 17,18,19,20,21,22].
Seven studies assessed the risk of IBD in relation with fish consumption [11, 13, 16,17,18,19, 21], four were related to dietary n-3 PUFAs intake [12, 14, 15, 20] and one included both fish and n-3 PUFAs intake [22]. Of the five studies evaluating dietary intake of n-3 PUFAs [12, 14, 15, 20, 22], three assessed ALA [14, 15, 20], two evaluated long-chain n-3 PUFAs (EPA + DHA) [12, 14], two assessed EPA and DHA separately [15, 20], and two assessed dietary intake of total n-3 PUFAs [12, 22]. In three studies without any reported effect size for total n-3 PUFAs, we combined the risk estimates of ALA and long-chain n-3 PUFAs (EPA + DHA) to estimate RR for total n-3 PUFAs [14, 15, 20].
Out of the 12 studies, three examined the risk of CD [11, 14, 18], three reported UC [15, 19, 20], five considered both CD and UC separately [12, 13, 17, 21, 22], and one reported IBD [16].
In the cohort studies, data regarding the onset of IBD were based on self-report that had been verified by a medical record or gastroenterologist [12, 13, 16]. In the case–control studies, data on IBD were determined based on physician diagnosis in two studies [17, 21], based on medical records that were confirmed by a physician in two other studies [19, 20], and based on laboratory investigation and imaging in one study [18]. Moreover, one study used diagnostic criteria that had been proposed by the research committee on IBD in Japan [22], and another study used the criteria that had been formulated by Lennard-Jones [11]. In addition, in two nested case–control studies, a combination of a self-report questionnaire, in-patient admission data, histopathology records, regional databases of IBD, and national databases of IBD was used and all cases were confirmed by a physician [14, 16].
All the included studies reported adjusted effect sizes along with 95% CIs regarding the association between fish and n-3 PUFAs intake and IBD. Energy intake was adjusted before analysis in seven studies [12, 13, 16, 19,20,21,22] and it also included as a covariate in six studies [12, 14,15,16, 19, 20]. BMI was adjusted for in five studies [12,13,14, 17, 20]. Vitamin D and fatty acids intakes were adjusted for in two [13, 14] and three studies [14, 15, 20], respectively. Of the eight studies which reported the association between fish consumption and IBD, two reported risk based on quartiles of fish intake [13, 22], two classified subjects based on tertiles of fish intake [16, 19], and two compared high fish intake with low intake [17, 21]. Moreover, one study reported risk for those who consumed fish more than once a week [18]. Furthermore, among the five studies which categorized subjects based on intake of n-3 PUFAs, two were based on quintiles [12, 14], two were based on quartiles [15, 22], and one was based on tertiles [20].
In the case–control studies, controls were matched to cases based on age in one study [18], both age and gender in four studies [11, 17, 19, 20], and age, gender, and site in another [22]. However, in some studies, a wide range of match characteristics was used. For example, in one study, cases and controls were matched in terms of age, gender, country of origin and residential neighborhood [21].In the two nested case–control studies, matching was based on age, gender, center, and date of recruitment into the EPIC study [14, 15]. Case–control studies recruited controls from in-patient [22], outpatient [17, 19, 20], and community-based centers [11, 18]. One study selected controls from both outpatient and community-based centers [21].
Although three studies showed an inverse association between fish consumption and the risk of CD [13, 17, 18], others did not report an association [11, 21]. Moreover, one study showed a negative association between fish consumption and the risk of UC [17], while several studies did not show any association [13, 19, 21]. Overall, included studies provided a null or negative association between fish consumption and the risk of IBD. In two studies, where fish consumption was not separated from other seafood, a positive association was detected regarding CD [22] and IBD [16]. With regard to dietary n-3 PUFAs intake, studies showed negative [12] or positive [22] associations in relation with the risk of IBD, while the other studies did not provide any significant association [14, 15, 20].
Meta-analysis findings
In total, 10 studies (4 prospective and 6 case–control) were eligible for inclusion in the current meta-analysis. Two studies were excluded from meta-analysis, since the reported risk estimate for fish consumption was not separated from seafood products [16, 22]. If the study reported the risk estimate for fish consumption separately for CD and UC, but not IBD, the reported risk estimate for CD and UC was pooled [13, 17, 21]. However, due to an adequate number of studies, CD and UC were analyzed separately (except for ALA). A total of 213,584 individuals were considered in the current meta-analysis, of which 1691 of cases of IBD (905 CD and 786 UC).
Fish consumption
The association between fish consumption and the risk of IBD based on a fixed-effects model (Fig. 2) and random-effects model (Supplementary Fig. 1) was provided. Studies included 41,601 participants with 823 cases of IBD (563 CD and 260 UC). There was no significant association between fish consumption and the risk of IBD in the fixed-effects model (pooled effect size: 0.93, 95% CI: 0.85–1.03, P = 0.18) (I2 = 76.4%, Pheterogeneity = 0.001). However, using a random-effects model, a marginally negative association was found between fish consumption and the risk of IBD (pooled effect size: 0.68, 95% CI: 0.46–1, P = 0.05) (I2 = 76.4%, Pheterogeneity = 0.001).
Moreover, the association between fish consumption and IBD was investigated in relation with CD and UC separately. No association was found between fish consumption and the risk of CD (pooled effect size: 0.94, 95%CI: 0.85–1.04, P = 0.25) or UC (pooled effect size: 0.82, 95%CI: 0.56–1.22, P = 0.33), based on the fixed-effects model (Fig. 3). Due to the existence of between-study heterogeneity with CD (I² = 80.5%, Pheterogeneity = 0.0001), the analysis was repeated with a random-effects model (Supplementary Fig. 2). There was a strong inverse association between fish consumption and the risk of CD based on the random-effects model (pooled effect size: 0.54, 95%CI: 0.31–0.96, P = 0.03).
Subgroup analysis (fish and CD)
Based on the findings from subgroup analysis, geographical region, quality assessment, and method of outcome assessment were detected as potential sources of heterogeneity. The association between fish consumption and the risk of CD was significant in Asian countries (pooled effect size: 0.54, 95%CI: 0.37–0.78, P = 0.001), in studies that were adjusted for BMI and smoking (pooled effect size: 0.35, 95%CI: 0.19–0.66, P = 0.001), high-quality studies (pooled effect size: 0.46, 95%CI: 0.25–0.87, P = 0.01), and in those studies, where IBD was confirmed by diagnosis physician (pooled effect size: 0.32, 95%CI: 0.13–0.80, P = 0.01).
Total dietary n-3 PUFAs intake
The association between total n-3 PUFAs intake and the risk of IBD based on the fixed-effects (Fig. 4) and random-effects model (Supplementary Fig. 3) was provided. Studies included 172,428 participants with 1102 cases of IBD (468 CD and 634 UC). No association was observed between total dietary n-3 PUFAs intake with the risk of IBD based on fixed-effects model (pooled effect size: 1.05, 95% CI: 0.84–1.32, P = 0.64) (I² = 57.3%, Pheterogeneity = 0.03) and random-effects model (pooled effect size: 1.17 95% CI: 0.80–1.72, P = 0.41) (I² = 57.3%, Pheterogeneity = 0.03). Moreover, there was a positive association between total dietary n-3 PUFAs intake with CD (pooled effect sizefixed model: 1.95, 95% CI: 1.06–3.61, P = 0.03) (I² = 80.1%, Pheterogeneity = 0.02), while no association was found between n-3 PUFAs intake and UC (pooled effect sizefixed model: 0.96, 95% CI: 0.75–1.22, P = 0.73) (I²= 0%, Pheterogeneity = 0.53) based on fixed-effects model. Due to existence of heterogeneity regarding CD (I² = 80.1%, Pheterogeneity = 0.02), the analysis was repeated applying random-effects model. No association was observed between dietary n-3 PUFAs intake with the risk of CD (pooled effect sizerandom model: 1.62, 95% CI: 0.38–6.87, P = 0.51) (I² = 80.1%, Pheterogeneity = 0.02) based on random-effects model.
Subgroup analysis (total n-3 PUFAs intake and CD)
Due to the limited number of studies, we were unable to conduct subgroup analysis.
Dietary long-chain n-3 PUFAs intake (EPA + DHA)
The association between long-chain n-3 PUFAs intake and the risk of IBD based on the fixed-effects model is shown in Fig. 5. Studies included 171,983 participants with 868 cases of IBD (342 CD and 526 UC). There was a significant negative association between long-chain n-3 PUFAs intake and the risk of IBD (pooled effect sizefixed model: 0.78, 95% CI: 0.63–0.97, P = 0.02) (I² = 0%, Pheterogeneity = 0.70). No association was found between Long-chain n-3 PUFAs intake and CD (pooled effect sizefixed model: 0.85, 95% CI: 0.59–1.23, P = 0.37) (I² = 0%, Pheterogeneity = 0.93), while a strong negative association was observed with UC (pooled effect sizefixed model: 0.75 95% CI: 0.57–0.98, P = 0.73) (I² = 0%, Pheterogeneity = 0.96).
ALA intake
The association between ALA intake and the risk of IBD based on the fixed-effects model is shown in Fig. 6. Studies included 171,983 participants with 868 cases of IBD (342 CD and 526 UC). There was no association between ALA intake and the risk of IBD (pooled effect sizefixed model: 1.17, 95% CI: 0.63–2.17, P = 0.62) (I² = 0%, Pheterogeneity = 0.40).
To avoid double-counting, we combined the effect sizes for CD and UC (if the study reported both of them) before data analysis to examine the association between fish consumption and IBD. However, with regard to n-3 fatty acids, the pooled effect size of the forest plot (which shows the association between n-3 fatty acids with CD and UC separately) was reported as the association between n-3 fatty acids and IBD, since the results were similar in both approaches.
Sensitivity analysis
Findings of the sensitivity analysis showed that the association between dietary n-3 PUFAs intake and the risk of IBD did not rely on a single or a few publications. However, regarding fish consumption, the study by Abubakar et al. was outside the limit (Supplementary Fig. 4). It also contributed a high weight in the forest plot (85.42% for IBD and 91.08% for CD). Therefore, we excluded that study and repeated the analysis for the association between fish consumption and risk of IBD and CD. A strong inverse association was observed between fish consumption and risk of IBD based on fixed-effects model (pooled effect size: 0.59, 95%CI: 0.46–0.77, P = 0.0001) (I² = 47.3%, Pheterogeneity = 0.01). Moreover, there was an inverse association between fish consumption and risk of CD (pooled effect size: 0.46, 95% CI: 0.33–0.65, P = 0.0001) (I² = 0%, Pheterogeneity = 0.50) and between-study heterogeneity disappeared. In the study by Abubakar et al., fish consumption was considered as secondary finding of the study and it was adjusted for only a limited number of variables.
Publication bias
Visual inspection of the funnel plot (Supplementary Fig. 5) and the results of the Egger’s test were used to examine publication bias. No evidence of publication bias was observed regarding the association between fish consumption (P = 0.08), dietary n-3 PUFAs (P = 0.30), long-chain n-3 PUFAs (P = 0.25), and ALA intake (P = 0.62) and risk of IBD.
Discussion
In the present meta-analysis of observational studies, we showed that fish consumption was inversely associated with the risk of CD. Moreover, there was a strong inverse association between dietary long-chain n-3 PUFAs intake and the risk of UC. To the best of our knowledge, this is the first meta-analysis summarizing the association between fish consumption and dietary intake of n-3 PUFAs and risk of IBD.
Previously published studies revealed that fish consumption and dietary n-3 PUFAs intake might play a role in the etiology of IBD [12, 13, 17, 18, 22]. In the present study, no association was observed between fish consumption and UC, while an inverse association was detected regarding CD. In agreement with our findings, one study revealed that following a Mediterranean diet, rich in fish and seafood, for 6 weeks resulted in reduced inflammation and normalized the gut microbiome in CD patient [29]. Moreover, in two case–control studies (with participants aged < 20), fish consumption [30] and dietary patterns high in fish [31] were associated with lower risk of CD. In contrast, a cross-sectional study showed that fish consumption was not associated with IBD progression in the patients [32]. In studies that did not find a significant association, fish consumption was considered a secondary outcome and without adjustments for multiple variables, ,[11], utilized various study designs [32], or reported the association of disease progression but not onset [32].
The significant inverse association between fish consumption and CD was observed in Asian countries, high-quality studies, and in studies that adjusted for BMI and smoking.
In the subgroup analysis, the significant inverse association between fish consumption and CD in Asian countries can be explained by several mechanisms. First, food preferences and dietary patterns differ around the world. High consumption of red and processed meat, sugar, animal fat, and sweets, which are characteristic of the Western-type dietary pattern have been shown to be associated with increased odds of CD [33, 34]. Second, the variation in intestinal microflora may differ by race. Clostridium coccoides, Bacillus species, and Bacteroides vulgatus (all subspecies have been involved in the pathogens of CD) are high in fecal microflora of Canadians, while Bifidobacteria and Eubacteria (seems to be protective against intestinal inflammation) are greater in fecal microflora of Japanese adults [35]. Moreover, higher consumption of dietary fiber in the Asian dietary pattern compared with western patterns has been associated with a lower number of Clostridium species [36]. Finally, compared with other nations, Asian patients with CD had no mutations in caspase recruitment domain-15 (CARD-15)/nucleotide-binding oligomerization domain-2 (NOD-2) [37]. The expression of this gene has an antibacterial effects in human intestinal cells [38].
In addition, the aforementioned association between fish intake and CD was detected in high-quality studies (NOS > 6 vs. NOS ≤ 6). In these high-quality studies, there was a representative sample size [13, 21], the outcome of interest was not present at the start of the study [13], the study adjusted for energy intake [21] and other covariates such as BMI and smoking [13], follow-up duration was long and only less than 20% of subjects were lost to follow-up [13], control subjects were recruited from the community [21], and ascertainment of outcome for both cases and controls was based on the same method [21].
In the present study, two out of the three studies that reported an inverse association between fish consumption and CD were adjusted for BMI and smoking [13, 17], while in those studies that did not report any association, such adjustments were not considered [11, 18, 19, 21]. There are several possible explanations which may explain the association between obesity and IBD. First, mesenteric fat increases inflammatory biomarkers, which might be integral to the inflammatory cascade involved in CD [39]. Second, excess macronutrient intake can heighten systematic oxidative stress [40, 41]. The study by Aljada et al. [40], showed that feeding people a large meal portion resulted in the induction of nuclear factor kappa beta (NFkB) activity (lasted for about 4–5 h after a meal), indicating that prolonged overeating might lead to chronic oxidative stress. Third, the prevalence of insulin resistance in obese individuals can impede the anti-inflammatory effects of insulin [42]. Moreover, a meta-analysis showed that obese individuals have altered gut microbes, with a relatively reduced proportion of Bifidobacterium and Bacteroidetes [43]. Smoking may contribute to the etiology of IBD by affecting immunity [44], reducing antioxidant capacity and increasing the generation of free oxygen radicals, decreasing mucosal blood flow in the rectum [45], enhancing thrombotic tendency in intestinal cells [46], and alteration of gut permeability and motility [47].
In the current study, no association was found between total dietary n-3 PUFAs and ALA intake with IBD. Moreover, no relationship was observed between long-chain n-3 PUFAs with the incidence of CD, while there was an inverse association with UC. Therefore, it appears that the beneficial effect of n-3 PUFAs on IBD, which was reported in the previously published studies, may be driven primarily by long-chain n-3 PUFAs (EPA + DHA). The protective effect of long-chain n-3 PUFAs in relation with the development of UC have been shown in both human [48] and animal studies [49,50,51,52]. In contrast, a meta-analysis of clinical studies did not support the role of fish oil consumption on remission maintenance in IBD patients [23]. It is noteworthy that trials did not consider other fatty acids or used oleic acid as a placebo which might imbue a beneficial effect on IBD. For example, an intervention study on IBD patients revealed that the reduction in the ratio of n-6/n-3 PUFAs was effective in remission of the disease [53]. Furthermore, it is likely that long-chain n-3 PUFAs can influence the onset of IBD. An experimental study showed that pretreatment of endothelial cells with long-chain n-3 PUFAs reduced adhesion molecules in the presence of Interleukin-1β (IL-1β), as an inflammatory stimulus, while such results were not observed when the experiment was performed on an activated cell [54]. Moreover, in a mouse model, administration of n-3 PUFAs before induction of intestinal inflammation impeded the progression of colitis [55]. On the other hand, since the concentration of arachidonic acid (AA) in plasma [56], neutrophils [57], and colonic mucosa [58] is higher in IBD patients, the efficacy of fish oil consumption on IBD patients may appear in different and higher doses than has been examined in trial studies to date.
Based on a review of the literature, it appears that long-chain n-3 PUFAs have a competitive action toward AA and inhibit eicosanoids production from AA. Long-chain n-3 PUFAs reduce the level of leukotriene B4 in the intestinal membrane and inhibit gene expression of adhesion molecules [59]. With inhibiting activation of toll-like receptor-4, n-3 PUFAs play an important role in reducing intestinal inflammation [60]. In addition, long-chain n-3 PUFAs inhibit nuclear factor kappa beta and peroxisome proliferator-activated receptor gamma, which are fundamental for intracellular inflammatory cascade [61].
In the current study, the negative association between fish consumption and the incidence of CD was not supported through dietary n-3 PUFAs intake. However, there are some hypotheses why the aforementioned inverse association between long-chain n-3 PUFAs intake and UC was not observed after fish consumption. First, the relative contribution and biological effect of nutritional elements in the broader context of food and diet might change. Second, fish contain different amounts of long-chain n-3 PUFAs, and therefore, participants may have consumed fish with low long-chain n-3 PUFAs. However, the type of fish was not reported in the included studies. Third, fish as a meat is a source of animal protein. A cohort study showed that high intake of protein, particularly from animal sources (meat and fish but not dairy products and eggs), was associated with increased risk of IBD, and with dose–response association regarding UC [16]. The underlying mechanism could be due to protein fermentation by colonic microflora, which results in an increase of toxic end products including hydrogen sulfide, ammonia, and phenolic compounds [62]. These toxic metabolites can alter intestinal cell membranes and cause loss of barrier integrity as had been seen in the etiology of UC [63].
To date, there are four studies that have explored the association between EPA and DHA with IBD, separately [14, 15, 20, 48]. Three studies showed an inverse association between DHA with the risk of IBD, while no association was found between EPA and IBD [14, 15, 48]. Based on the evidence, both DHA and EPA metabolized into lipid mediators called resolvin which has an anti-inflammatory effect in the etiology of IBD [64]. Although the underlying reason is unknown, greater dietary choices for DHA may be a reason for detecting the relationship regarding DHA. Moreover, some studies have reported better efficacy for DHA compared to EPA in suppressing inflammation [65]. Other studies reported EPA had pro-inflammatory properties, since it could metabolize into leukotriene B5 (with pro-inflammatory activity) [66] and prostaglandin E3 [67] (disturb epithelial barrier of the intestine).
In this study, our objective was to investigate whether fish consumption was associated with the risk of IBD. The relationship between n-3 PUFAs and IBD was examined to determine whether the association between fish consumption, as a rich source of n-3 PUFAs, and IBD was mediated through n-3 PUFAs. Future studies should not neglect n-6 PUFAs. Dietary intake of n-6 fatty acids can potentiate inflammatory processes by increasing inflammatory biomarkers, such as prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and leukotriene B4 (LTB4) [68], which may exacerbate the onset of many diseases including IBD [68]. However, despite experimental evidence, clinical data regarding the effectiveness of n-6 fatty acids are controversial [23, 69]. Moreover, in some of the epidemiological studies, the association between PUFAs and IBD was not investigated based on different subtype of PUFAs (including n-6 and n-3 fatty acids) [13, 21].
In addition, another important factor which is needed to be taken into account is n-6-to-n-3 PUFAs ratio, since n-3 and n-6 fatty acids are considered competitive substrates [70]. There has also been an increase in the n-6-to-n-3 ratio is recent decades [70, 71] and is highly variable [72].
To the best of our knowledge, this is the first meta-analysis on the association between fish consumption and dietary intake of n-3 fatty acids with IBD. In all the studies, data on IBD were collected based on medical records or confirmation by a physician, which is more reliable than by self-report. There are several limitations in the current that requires discussion. Adjustment of energy intake, BMI, and smoking is necessary for examining the association between dietary intakes and the risk of IBD. However, out of 12 included studies, only 6 [12, 14,15,16, 19, 20] and 5 [12,13,14, 17, 20] included energy and BMI as covariates, respectively, and only seven controlled for smoking [12,13,14,15, 17, 20, 22]. Moreover, other determinants such as vitamin D and fatty acids intake were not adjusted for in several studies. Vitamin D which is present in similar foods that contain EPA and DHA was only controlled for only in two studies [13, 14]. Fatty acids which may influence the metabolism of each other and also inflammatory cascades were adjusted for only in three studies [14, 15, 20]. Therefore, adjustment of the foregoing factors might attenuate the pooled risk estimates to some extent. In addition, due to the limited number of studies, we were unable to explore the independent effects of EPA and DHA with IBD. Finally, different statistical methods were applied in the included studies to evaluate the association between fish consumption and dietary n-3 PUFAs intake with IBD.
Conclusion
There was an inverse association between fish consumption and the risk of CD. Moreover, an inverse association was observed between dietary long-chain n-3 PUFAs intake and the incidence of UC. Although the current meta-analysis provided a comprehensive review of the association between fish consumption and dietary intake of n-3 PUFAs with IBD, additional well-designed studies, considering subtypes of fish and n-3 PUFAs, are needed.
References
Molodecky NA, Soon S, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142(1):46–54.e42
Cho JH (2008) The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8(6):458
Jantchou P, Monnet E, Carbonnel F (2006) Environmental risk factors in Crohn’s disease and ulcerative colitis (excluding tobacco and appendicectomy). Gastroenterol Clin Biol 30(6–7):859–867
Cho JH, Abraham C (2007) Inflammatory bowel disease genetics: Nod2. Annu Rev Med 58:401–416
Lee D, Albenberg L, Compher C, Baldassano R, Piccoli D, Lewis JD, Wu GD (2015) Diet in the pathogenesis and treatment of inflammatory Bowelá diseases. Gastroenterology 148(6):1087–1106
Cashman KD, Shanahan F (2003) Is nutrition an aetiological factor for inflammatory bowel disease? Eur J Gastroenterol Hepatol 15(6):607–613
Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H, Schulze M (2012) Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr 51(6):637–663
Mouli VP, Ananthakrishnan AN (2014) vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther 39(2):125–136
Oldenburg B, Koningsberger J, Van Berge Henegouwen G, Van Asbeck B, Marx J (2001) Iron and inflammatory bowel disease. Aliment Pharmacol Ther 15(4):429–438
Mori TA, Beilin LJ (2004) Omega-3 fatty acids and inflammation. Curr Atheroscler Rep 6(6):461–467
Abubakar I, Myhill DJ, Hart AR, Lake IR, Harvey I, Rhodes JM, Robinson R, Lobo AJ, Probert CS, Hunter PR (2007) A case-control study of drinking water and dairy products in Crohn’s disease—further investigation of the possible role of Mycobacterium avium paratuberculosis. Am J Epidemiol 165(7):776–783
Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, Willett WC, Richter JM, Chan AT (2014) Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 63(5):776–784
Ananthakrishnan AN, Khalili H, Song M, Higuchi LM, Richter JM, Nimptsch K, Wu K, Chan AT (2015) High school diet and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 21(10):2311–2319
Chan S, Luben R, Olsen A, Tjonneland A, Kaaks R, Lindgren S, Grip O, Bergmann M, Boeing H, Hallmans G (2014) Association between high dietary intake of the n – 3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn’s disease. Aliment Pharmacol Ther 39(8):834–842
Investigators IiES (2009) Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case–control study within a European prospective cohort study. Gut 58(12):1606–1611
Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault M-C, Carbonnel F (2010) Animal protein intake and risk of inflammatory bowel disease: the E3N prospective study. Am J Gastroenterol 105(10):2195
Maconi G, Ardizzone S, Cucino C, Bezzio C, Russo AG, Porro GB (2010) Pre-illness changes in dietary habits and diet as a risk factor for inflammatory bowel disease: a case-control study. World J Gastroenterol WJG 16(34):4297
Pugazhendhi S, Sahu MK, Subramanian V, Pulimood A, Ramakrishna BS (2011) Environmental factors associated with Crohn’s disease in India. Indian J Gastroenterol 30(6):264–269
Rashvand S, Somi MH, Rashidkhani B, Hekmatdoost A (2015) Dietary protein intakes and risk of ulcerative colitis. Med J Islam Repub Iran 29:253
Rashvand S, Somi MH, Rashidkhani B, Hekmatdoost A (2015) Dietary fatty acid intakes are related to the risk of ulcerative colitis: a case–control study. Int J Colorectal Dis 30(9):1255–1260
Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T (1997) Pre-illness dietary factors in inflammatory bowel disease. Gut 40(6):754–760
Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama T, Inaba Y, Miyake Y, Sasaki S, Okamoto K (2005) Dietary risk factors for inflammatory bowel disease A multicenter case–control Study in Japan. Inflamm Bowel Dis 11(2):154–163
Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM (2011) Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis 17(1):336–345
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341
Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P (2016) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute, 2009. (Available in March)
Mozaffari H, Djafarian K, Mofrad M, Shab-Bidar S (2018) Dietary fat, saturated fatty acid, and monounsaturated fatty acid intakes and risk of bone fracture: a systematic review and meta-analysis of observational studies. Osteoporos Int 2018:1–13
Higgins JP, Green S (2011) Cochrane handbook for systematic reviews of interventions, vol 4. Wiley, Hoboken
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Marlow G, Ellett S, Ferguson IR, Zhu S, Karunasinghe N, Jesuthasan AC, Han DY, Fraser AG, Ferguson LR (2013) Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum Genomics 7(1):24
Amre DK, D’souza S, Morgan K, Seidman G, Lambrette P, Grimard G, Israel D, Mack D, Ghadirian P, Deslandres C (2007) Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am J Gastroenterol 102(9):2016
D’souza S, Levy E, Mack D, Israel D, Lambrette P, Ghadirian P, Deslandres C, Morgan K, Seidman EG, Amre DK (2007) Dietary patterns and risk for Crohn’s disease in children. Inflamm Bowel Dis 14(3):367–373
Tasson L, Canova C, Vettorato MG, Savarino E, Zanotti R (2017) Influence of diet on the course of inflammatory bowel disease. Dig Dis Sci 62(8):2087–2094
Andersen V, Olsen A, Carbonnel F, Tjønneland A, Vogel U (2012) Diet and risk of inflammatory bowel disease. Dig Dis Sci 44(3):185–194
Gentschew L, Ferguson LR (2012) Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Mol Nutr Food Res 56(4):524–535
Benno Y, Suzuki K, Suzuki K, Narisawa K, Bruce WR, Mitsuoka T (1986) Comparison of the fecal microflora in rural Japanese and urban Canadians. Microbiol Immunol 30(6):521–532
Benno Y, Endo K, Miyoshi H, Okuda T, Koishi H, Mitsuoka T (1989) Effect of rice fiber on human fecal microflora. Microbiol Immunol 33(5):435–440
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411(6837):603
Hisamatsu T, Suzuki M, Reinecker H-C, Nadeau WJ, McCormick BA, Podolsky DK (2003) CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124(4):993–1000
Flores A, Burstein E, Cipher DJ, Feagins LA (2015) Obesity in inflammatory bowel disease: a marker of less severe disease. Dig Dis Sci 60(8):2436–2445
Aljada A, Mohanty P, Ghanim H, Abdo T, Tripathy D, Chaudhuri A, Dandona P (2004) Increase in intranuclear nuclear factor κB and decrease in inhibitor κB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr 79(4):682–690
Chapman-Kiddell CA, Davies PS, Gillen L, Radford-Smith GL (2010) Role of diet in the development of inflammatory bowel disease. Inflamm Bowel Dis 16(1):137–151
Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25(1):4–7
Walters WA, Xu Z, Knight R (2014) Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett 588(22):4223–4233
Miller LG, Goldstein G, Murphy M, Ginns LC (1982) Reversible alterations in immunoregulatory T cells in smoking: analysis by monoclonal antibodies and flow cytometry. Chest 82(5):526–529
Srivastava E, Russell M, Feyerabend C, Rhodes J (1990) Effect of ulcerative colitis and smoking on rectal blood flow. Gut 31(9):1021–1024
Thomas GA, Rhodes J, Green JT, Richardson C (2000) Role of smoking in inflammatory bowel disease: implications for therapy. Postgrad Med J 76(895):273–279
Prytz H, Benoni C, Tagesson C (1989) Does smoking tighten the gut? Scand J Gastroenterol 24(9):1084–1088
John S, Luben R, Shrestha SS, Welch A, Khaw K-T, Hart AR (2010) Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol 22(5):602–606
Hekmatdoost A, Wu X, Morampudi V, Innis SM, Jacobson K (2013) Dietary oils modify the host immune response and colonic tissue damage following Citrobacter rodentium infection in mice. Am J Physiol Gastrointest Liver Physiol 304(10):G917–G928
Hekmatdoost A, Mirshafiey A, Feizabadi MM, Djazayeri A (2009) Polyunsaturated fatty acids, microflora and colitis. Ann Nutr Metabol 55(4):325–325
Hekmatdoost A, Feizabadi MM, Djazayery A, Mirshafiey A, Eshraghian MR, Yeganeh SM, Sedaghat R, Jacobson K (2008) The effect of dietary oils on cecal microflora in experimental colitis in mice. Indian J Gastroenterol 27(5):186–189
Nieto N, Torres MI, Ríos A, Gil A (2002) Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J Nutr 132(1):11–19
Uchiyama K, Nakamura M, Odahara S, Koido S, Katahira K, Shiraishi H, Ohkusa T, Fujise K, Tajiri H (2010) N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm Bowel Dis 16(10):1696–1707
Collie-Duguid E, Wahle K (1996) Inhibitory effect of fish oil N-3 polyunsaturated fatty acids on the expression of endothelial cell adhesion molecules. Biochem Biophys Res Commun 220(3):969–974
Shores DR, Binion DG, Freeman BA, Baker PR (2010) New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm Bowel Dis 17(10):2192–2204
Esteve-Comas M, Nunez M, Fernández-Bañares F, Abad-Lacruz A, Gil A, Cabre E, Gonzalez-Huix F, Bertran X, Gassull M (1993) Abnormal plasma polyunsaturated fatty acid pattern in non-active inflammatory bowel disease. Gut 34(10):1370–1373
Kawakami Y, Okada H, Murakami Y, Kawakami T, Ueda Y, Kunii D, Sakamoto Y, Shiratori Y, Okita M (2007) Dietary intake, neutrophil fatty acid profile, serum antioxidant vitamins and oxygen radical absorbance capacity in patients with ulcerative colitis. J Nutr Sci Vitaminol 53(2):153–159
Nishida T, Miwa H, Shigematsu A, Yamamoto M, Iida M, Fujishima M (1987) Increased arachidonic acid composition of phospholipids in colonic mucosa from patients with active ulcerative colitis. Gut 28(8):1002–1007
Marion-Letellier R, Savoye G, Beck PL, Panaccione R, Ghosh S (2013) Polyunsaturated fatty acids in inflammatory bowel diseases: a reappraisal of effects and therapeutic approaches. Inflamm Bowel Dis 19(3):650–661
Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH (2005) Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol 174(9):5390–5397
Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ (2003) NF-κB inhibition by ω-3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am J Physiol Lung Cell Mol Physiol 284(1):L84–L89
Hughes R, Magee E, Bingham S (2000) Protein degradation in the large intestine: relevance to colorectal cancer. Curr Issues Intestinal Microbiol 1(2):51–58
Roediger W (2008) Nitric oxide from dysbiotic bacterial respiration of nitrate in the pathogenesis and as a target for therapy of ulcerative colitis. Aliment Pharmacol Ther 27(7):531–541
Weylandt KH, Chiu C-Y, Gomolka B, Waechter SF, Wiedenmann B (2012) Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat 97(3–4):73–82
Depner CM, Philbrick KA, Jump DB (2013) Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr–/– mouse model of western diet-induced nonalcoholic steatohepatitis1–3. J Nutr 143(3):315–323
Goldman D, Pickett W, Goetzl E (1983) Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun 117(1):282–288
Rodríguez-Lagunas MJ, Ferrer R, Moreno JJ (2013) Effect of eicosapentaenoic acid-derived prostaglandin E3 on intestinal epithelial barrier function. Prostaglandins Leukot Essent Fatty Acids 88(5):339–345
Patterson E, Wall R, Fitzgerald G, Ross R, Stanton C (2012) Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metabol 2012:539426
Turner D, Steinhart AH, Griffiths AM (2007) Omega 3 fatty acids (fish oil) for maintenance of remission in ulcerative colitis. Cochrane Database of Syst Rev 18(3):CD006443
Scaioli E, Liverani E, Belluzzi A (2017) The imbalance between n-6/n-3 polyunsaturated fatty acids and inflammatory bowel disease: a comprehensive review and future therapeutic perspectives. Int J Mol Sci 18(12):2619
Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR (2011) Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr 93(5):950–962
Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR (2003) Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 38(4):391–398
Acknowledgements
This study was supported by National Institute for Medical Research Development (Grant and Ethics Number: 977288).
Author information
Authors and Affiliations
Contributions
LA and HM designed the study. Searching processes, data extraction, statistical analysis, and manuscript drafting performed by HM and reviewed by ED, BL, NB, and LA. LA supervised all the study processes and checked the search strategy processing and statistical analysis. The final version of the manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mozaffari, H., Daneshzad, E., Larijani, B. et al. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: a systematic review and meta-analysis of observational studies. Eur J Nutr 59, 1–17 (2020). https://doi.org/10.1007/s00394-019-01901-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-01901-0