Abstract
Purpose
Hibiscus sabdariffa L. is commonly used as an ingredient for herbal teas and food supplements. Several studies demonstrated the beneficial effects of Hibiscus sabdariffa L. extracts (HSE); however, the bioactive components and their mode of action still remain unclear. Caenorhabditis elegans (C. elegans) was used to study health-related effects and the underlying molecular mechanisms of HSE in this model organism as well as effects of hydroxycitric acid (HCA), a main compound of HSE, and its structural analogue isocitric acid (ICA).
Methods
Survival and locomotion were detected by touch-provoked movement. Thermotolerance was analysed using the nucleic acid stain SYTOX green, and intracellular ROS accumulation was measured via oxidation of H2DCF. Localisation of the transcription factors DAF-16 and SKN-1 was analysed in transgenic strains (DAF-16::GFP, SKN-1::GFP). The involvement of DAF-16 and SKN-1 was further investigated using loss-of-function strains as well as gene silencing by feeding RNAi-inducing bacteria. Protection against amyloid-β toxicity was analysed using a transgenic strain with an inducible expression of human amyloid-β peptides in body wall muscle cells (paralysis assay).
Results
HSE treatment resulted in a prominent extension of lifespan (up to 24%) and a reduction of the age-dependent decline in locomotion. HCA, a main compound of HSE increased lifespan too, but to a lesser extent (6%) while ICA was not effective. HSE and HCA did not modulate resistance against thermal stress conditions and did not exert antioxidative effects: HSE rather increased intracellular ROS levels, suggesting a pro-oxidative effect of the extract in vivo. HSE and HCA increased the nuclear localisation of the pivotal transcription factors DAF-16 and SKN-1 indicating an activation of these factors. Consistent with this result, lifespan prolongation by HSE was dependent on both transcription factors. In addition to the positive effect on lifespan, HSE treatment also elicited a (strong) protection against amyloid-ß induced toxicity in C. elegans in a DAF-16- and SKN-1-dependent manner.
Conclusion
Our results demonstrate that HSE increases lifespan and protects against amyloid-β toxicity in the model organism C. elegans. These effects were mediated, at least in parts via modulation of pathways leading to activation/nuclear localisation of DAF-16 and SKN-1. Since HCA, a main component of HSE causes only minor effects, additional bioactive compounds like flavonoids or anthocyanins as well as synergistic effects of these compounds should be investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hibiscus sabdariffa L. (H. sabdariffa) belongs to the family of Malvaceae and originates from tropical and subtropical regions of the world. Dried calyces of the plant are used as food supplement, beverage and as natural colouring agent in the food and cosmetic industries [1]. H. sabdariffa flowers are also traditionally used as a medicinal herb. Antihypertensive and antihyperlipidemic effects were shown in clinical trials which qualifies the plant as a functional food [2, 3]. Moreover, animal models and cell culture studies indicate antioxidative, anti-inflammatory, antidiabetic as well as anticancerogenic effects of H. sabdariffa extracts [4]. Recently it was shown that oral H. sabdariffa supplements (combination with collagen peptides, vitamin C, and Aristotelia chilensis extract) reduce markers of dermal skin ageing in female patients indicating a protective effect against age-related degenerative processes [5]. Nade et al. showed that H. rosa sinensis improved short-term and long-term memory deficits in aged swiss albino mice (32 weeks old) in a way that these animals were comparable to young untreated animals (8 weeks old) [6]. Neuroprotective effects of H. sabdariffa (ethyl acetate fraction) were also reported in streptozotocin-(STZ) induced diabetic mice [7]. The authors suggested the reduction in oxidative stress markers as well as acetylcholinesterase activity as possible modes of action. Neuromodulatory effects were also detectable in swiss albino mice treated with H. rosa sinensis as anxiolytic effects mediated by ionotropic GABA receptors were observed [8]. H. sabdariffa extracts were shown to inhibit dopaminergic cell death in primary midbrain cultures treated with rotenone and furthermore, a H. asper leaves extract increased antioxidant enzyme activity in a 6-hydroxydopamine-lesioned rat model of Parkinson’s disease [9, 10].
Hibiscus sabdariffa contains various bioactive compounds, e.g. organic acids such as hydroxycitric acid (HCA), ascorbic acid and hibiscus acid, anthocyanins like delphinidin-3-sambubioside (hibiscin) and cyanidin-3-sambubioside (gossypicyanin), polysaccharides, as well as flavonoids and phenolic acids [1]. The protective effects of the plant are mostly attributed to the anthocyanins and flavonoids because of their multiple biological actions, e.g. antioxidative effects or interaction with enzymes and signalling pathways [4, 11, 12]. However, there is only limited knowledge about the mode of action of other components such as HCA.
We used the model organism Caenorhabditis elegans in order to study protective effects of a Hibiscus sabdariffa L. water extract (HSE) in vivo. The nematode serves as model to study ageing as well as age-related diseases on a molecular basis. For various human genes, orthologues are known in C. elegans enabling researchers to study evolutionary conserved signalling pathways and molecular processes involved in human diseases [13, 14]: More than 50% (10,678/20,310) of the human protein-coding genome has recognizable orthologues in the nematode supported by current versions of orthology-prediction methods. Several transgenic strains have been engineered to study human neurodegenerative diseases in the nematode [15]. Additionally, C. elegans is used to investigate the biological functions of food compounds [16, 17]. Using this model system, Fitzenberger et al. already showed that HSE was able to protect against stress induced by high glucose concentrations [18]. They concluded that delphinidin-3-sambubioside and cyanidin-3-sambubioside were not responsible for this effect, which indicates that other constituents may be active. We investigated the effect of HSE as well as the main compound HCA on ageing and age-related parameters in C. elegans (antioxidative effects in vivo, stress resistance, protection against Aβ-toxicity). To identify specific effects of the HSE compound HCA, the structural analogue isocitric acid (ICA) was also investigated.
Materials and methods
Materials
SYTOX Green was obtained from Molecular Probes (Eugene, OR, USA) and Levamisole hydrochloride, Tetracycline hydrochloride, Streptomycin sulphate from AppliChem (Darmstadt, Germany). Hibiscus sabdariffa L. dry extract (HSE) (100% native) was provided by Plantextrakt GmbH & Co. KG (Vestenbergsgreuth, Germany). All other chemicals were of analytical grade and purchased from SIGMA (Deisenhofen, Germany).
C. elegans strains maintenance and treatment
Caenorhabditis elegans strains used in this study (wild-type N2 var. Bristol, CF1038 [daf-16(mu86) I.], EU1 [skn-1(zu67) IV/nT1 [unc-?(n754) let-?] (IV;V).], TJ356 [zIs356 IV (pdaf-16-daf-16::gfp; rol-6)], LD001 [ldIs007 pskn-1::skn-1b/c::gfp; rol-6]) and CL4176 [(pAF29)myo-3p::A-beta (1–42)::let-? 3′UTR) + pRF4 rol-6 (su1006)] and OP50/OP50-1 bacterial strains were provided by the Caenorhabditis Genetics Centre (CGC) and RNAi strains were from the C. elegans RNAi collection from Ahringer. Except for CL4176, which was kept at 16 °C, all other strains were maintained at 20 °C on 94 mm nematode growth medium (NGM) agar plates containing a lawn of E. coli var. OP50. For the CL4176 and EU1 strains synchronisation was performed by egg laying and for all other strains by bleaching according to standard protocols [19]. Compound treatment of C. elegans was conducted in liquid NGM as described earlier [20, 21] with minor modifications: HSE, ICA and HCA stock solutions were prepared in liquid nematode growth media (NGM) and added to the incubation media (NGM, 50 µg/ml streptomycin and 109/ml OP50-1 E. coli) in the desired final concentration. The control group received the respective amount NGM. The worms were incubated with the extract, ICA or HCA together with OP50-1 E. coli. It was verified that HSE does not change the bacterial growth rate and does not change the pharyngeal pumping rate, e.g. that the effects were mediated indirectly by caloric restriction (Supplementary Fig. 1). Furthermore, we verified that HSE has no impact on the nematode size (length and area) as well as number of offspring (Fig. 1e). However, it cannot be excluded that the HSE-mediated effects were mediated by compounds generated by autoxidation or bacterial metabolism.
Stress resistance
Synchronized L4 larvae and young adult animals (N2) were treated with concentrations of HSE ranging from 0.25 to 1 mg/ml, 2 mM HCE or ICA in the incubation medium for 48 h at 20 °C. Animals were washed in PBST and single nematodes were transferred into wells of a 384-well microtiter plate containing 1 µM SYTOX Green nucleic acid stain, then thermal stress (37 °C) was applied. Fluorescence was measured in a plate reader (Synergy MX, BioTek Instruments, Inc.) every 15 min for 12 h. When the fluorescence values (excitation: 485 nm; emission: 535 nm) of individual nematodes exceeded a defined cut-off value, the animals were scored as dead.
Measurement of ROS accumulation
Synchronized L4 larvae and young adults (N2) were treated with different HSE concentrations or 2 mM HCA or ICA for 48 h in the incubation medium [2.2] at 20 °C, washed in PBST and transferred into a 384-well microtiter plate. Subsequently, 50 µM H2DCF-DA-solution was added to each well and fluorescence intensities (excitation: 485 nm; emission: 535 nm) were recorded every 15 min for 12 h under thermal stress (37 °C) in a plate reader (Synergy MX, BioTek Instruments, Inc.).
Intracellular localisation of DAF-16::GFP and SKN-1::GFP
Intracellular localisation of DAF-16 and SKN-1 was detected using the transgenic strains TJ356 and LD001. Synchronized L4 larvae and young adult animals of the corresponding strains were treated with different HSE concentrations or 2 mM HCA or ICA for 1 h in the incubation medium at 20 °C, respectively. Nematodes were transferred to a microscope slide, sedated with levamisole and cellular localisation of DAF-16::GFP/SKN-1::GFP was detected by means of a fluorescence microscope (Eclipse Ni with Intensilight C-HGFI, Nikon) equipped with a GFP filter. As a positive control a short heat stress (37 °C 5 min) was conducted to induce nuclear DAF-16::GFP localisation in the TJ356 nematodes. For the nuclear translocation of SKN-1::GFP the LD001 nematodes were treated with the compounds or 1 mM H2O2 (positive control) for 1 h. Only in the SKN-1::GPF-translocation experiment, heat-inactivated (30 min 65 °C) OP50-1 E. coli were used to inactivate bacterial catalase and allow H2O2 to induce SKN-1 translocation in the nematode.
Lifespan and locomotion
For the analysis of the lifespan at 25 °C the wild-type strain N2 and the loss-of-function mutants CF1038 and EU1 were used. 40 synchronized L4 larvae and young adult animals were transferred into liquid media (incubation medium: 2.2) with different HSE concentrations or 2 mM HCA or ICA (day 0 of the lifespan). During the first 9 days, medium contained 120 µM 5-fluoro-2-deoxyuridine (FUDR) to prevent the hatching of viable progeny. The media were exchanged 5 days a week and the survival of the animals was measured by touch-provoked movement. Lost or ruptured animals were censored. Locomotion analyses were conducted simultaneously at days 8, 14 and 18 for the wild-type strain N2 and only at day 8 for the mutant strains due to a shorter lifespan. Briefly, nematodes were classified according to their mobility after shaking the petri dish: (A) nematodes moved freely, (B) nematodes moved after prodding with a platinum wire, (C) nematodes moved scarcely their head or tail after prodding and (D) nematodes showed no movement (dead).
Aβ-paralysis
The transgenic C. elegans strain CL4176 with temperature-inducible expression of the human Aβ3–42 was used to analyse Aβ toxicity in C. elegans. Synchronized eggs were incubated in liquid media with different HSE concentrations or 1 mM caffeine as a positive control at 16 °C. Following 64 h of treatment, 40 larvae were transferred to 60 mm NGM agar plates with a lawn of OP50 E. coli and set at 25 °C to induce transgene expression. Body movement of the nematodes was analysed every other hour 26 h after the temperature up-shift. Nematodes that showed no movement or only moved the head after prodding with a platinum wire were considered as paralysed. Lost or ruptured animals were censored. For RNAi experiments E. coli var. HT115 expressing daf-16, skn-1 dsRNA or empty vector were applied and 50 µg/ml ampicillin, 12 µg/ml tetracycline and 1 mM Isopropyl β-d-1-thiogalactopyranoside (IPTG) were added to the incubation media. Eggs of the CL4176 strain were treated for 40 h at 16 °C with different HSE concentrations and the hatched larvae were transferred to 60 mm RNAi plates (NGM plates with 50 µg/ml ampicillin, 12 µg/ml tetracycline, 1 mM IPTG) seeded with HT115 E. coli expressing the respective dsRNA. Paralysis was tested according to the standard experiment. In order to validate the efficiency of the daf-16 knockdown, eggs of TJ356 nematodes were collected and incubated with daf-16 RNAi or empty vector HT115 E. coli in the RNAi incubation media at 20° for 40 h. Subsequently, nematodes were analysed by means of a fluorescence microscope (Nikon) equipped with a GFP filter. Images of 30 larvae were taken with a connected camera and the optical density (OD) of the animals was measured with ImageJ. Skn-1 knock-down efficiency was verified by measuring the reproduction rate of CL4176 nematodes. Briefly, eggs of CL4176 nematodes were exposed to empty vector or skn-1 RNAi expressing bacteria in liquid RNAi incubation media at 16 °C for 40 h. Afterwards 10 larvae were transferred to 60 mm RNAi plates. After the end of the reproductive period the nematodes were removed, and the living progeny and non-hatched eggs were counted.
Statistics
Statistical significance was determined by one-way ANOVA with Dunnett’s post-hoc test or two-way ANOVA with Tukey’s post-hoc test while lifespan analyses, thermal stress and Aβ paralysis assays were calculated using Kaplan–Meier survival analyses with log-rank test (PASW Statistics for Windows, SPSS Inc.; Chicago, USA; GraphPad Prism 6, La Jolla, USA).
Results
Effects of HSE on lifespan and stress resistance
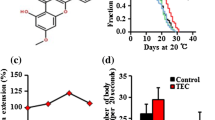
HSE increased the lifespan of the nematode dose-dependently: the median lifespan was enhanced by 24% and 6% after incubation with 1.0 and 0.5 mg/ml HSE, respectively. The lowest concentration (0.25 mg/ml) was not able to increase the lifespan significantly (Fig. 1a; Table 1). The concentrations used for our studies (0.25, 0.5 and 1 mg/ml) are only slightly higher than concentrations used for studies in mice: Seung et al. used the ethyl acetate fraction from Hibiscus sabdariffa L. in a concentration of 100 and 200 mg/kg of body weight [7]. HSE was used up to 1 mg/ml due to acidification of the incubation medium (< pH 6.0) at higher concentrations. In addition to prolongation of the lifespan, incubation with HSE prevented the age-related decline of locomotion of the nematodes. As shown in Fig. 1b, the amount of agile and freely moving nematodes diminishes dramatically with time: While at day 8 approximately 45% of the control nematodes move freely (category A), at day 14 no nematodes in this category are detectable. However, after an incubation with HSE (0.5 mg/ml and 1 mg/ml), the amount of agile nematodes is significantly increased (60% and 65% category A) at day 8 and the amount of dead nematodes is significantly decreased at days 14 and 18. The highest HSE concentration (1 mg/ml) also significantly prevents locomotion decline at day 18 (category C). We next investigated if the nematodes are also more resistant against thermal stress (SYTOX assay), but this was not the case (Fig. 1c). Congruent with this result, no antioxidative effects of HSE were detected in C. elegans under thermal stress conditions (Fig. 1d). On the contrary, HSE (1 mg/ml) even increases the accumulation of ROS in the animals, suggesting a pro-oxidative effect of the extract. Since the antioxidant compound N-acetylcysteine was able to diminish the HSE-mediated effects (increased nuclear localisation of SKN-1 and DAF-16; Supplementary Fig. 3), we conclude that HSE mediated its effects at least in parts, via generation of oxidative stress. However, using paraquat as a redox-cycling agent to produce superoxide radicals continuously or using the mev-1 strain generating higher amounts of ROS, HSE was able to protect against this stressor suggesting also antioxidative properties (Supplementary Fig. 3). Furthermore, HSE was able to decrease the accumulation of the age-pigment lipofuscin (Supplementary Fig. 3). However, the amount of offspring was not changed by HSE (Fig. 1e).
Effects of HSE on lifespan, locomotion and stress resistance in C. elegans. a HSE prolongs lifespan dose-dependently. Wild-type L4 larvae were treated with different concentrations of HSE or the solvent control NGM at 25 °C. Media change was conducted 5 times a week and survival of the nematodes was examined by touch-provoked movement simultaneously, n = 3 (40 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test, ***p ≤ 0.001 vs. control. b HSE prevented age-associated decline in locomotion. Mobility was analysed by prodding the nematodes with a platinum wire at days 8, 14 and 18 of their lifespans. The individuals were classified according to the movement: A—free movement, B—movement after prodding, C—weak movement after prodding, D—dead, n = 3 (40 nematodes per group), two-way ANOVA with Tukey’s multiple comparisons test, **p ≤ 0.01, ***p ≤ 0.001. c HSE did not influence thermal stress resistance. Wild-type L4 C. elegans were pretreated with different concentrations of HSE or the solvent control NGM for 48 h at 20 °C before thermal stress (37 °C) was applied. Individual virtual times of death were determined by measuring the fluorescence of the nucleic acid stain SYTOX®Green. Kaplan–Meier statistics was used for the comparison of the survival curves, n = 3 (8 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test. d HSE increases thermally induced ROS accumulation. The fluorescence of DCF as an indicator for intracellular ROS was measured in the pretreated wild-type nematodes after thermal stress (37 °C) was applied, n = 3 (8 individuals per group), one-way ANOVA with Dunnett‘s multiple comparisons test, **p ≤ 0.01, *p ≤ 0.05 vs. control. e Treatment with HSE does not influence progeny of C. elegans. Left figure: Total progeny, n = 3 (10 individuals/group and trial), one-way ANOVA with Dunnett‘s multiple comparisons test. Right figure: daily progeny, n = 3 (10 individuals/group and trial), two-way ANOVA with Tukey‘s multiple comparisons test
Effects of HCA/ICA on lifespan and stress resistance
Since HSE contains approx. 40% HCA, we investigated if incubation with the respective amount of HCA generates effects comparable to those detected with 1 mg/ml HSE. Similar to HSE, 2 mM HCA increased the median lifespan significantly, but to a lesser extent (6%). ICA, which was used as internal control (comparable structure to HCA, but not a component of HSE) had no effects on the lifespan of the nematodes (Fig. 2a). In contrast to HSE, neither ICA nor HCA affected the locomotion of the animals (Fig. 2b). In analogy to HSE, neither increase in survival (Fig. 2c) nor antioxidative effects (Fig. 2d) were caused by the compounds in response to thermal stress conditions. However, no pro-oxidative effect (increase in ROS accumulation) was detectable as seen after incubation with the highest concentration of HSE.
Effects of HCA/ICA on lifespan, locomotion and stress resistance in C. elegans. a HCA prolongs lifespan. Wild-type L4 larvae were treated with 2 mM HCA/ICA or the solvent control NGM at 25 °C. Media change was conducted 5 times a week and survival of the nematodes was examined by touch-provoked movement simultaneously, n = 3 (40 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test, *p ≤ 0.05 vs. control. b Age-associated decline in locomotion was analysed by prodding the nematodes with a platinum wire at days 8, 14 and 18 of their lifespans. The individuals were classified according to the movement: A—free movement, B—movement after prodding, C—weak movement after prodding, D—dead, n = 3 (40 nematodes per group), two-way ANOVA with Tukey’s multiple comparisons test. c HCA/ICA did not influence thermal stress resistance. Wild-type L4 C. elegans were pretreated with 2 mM HCA/ICA or the solvent control NGM for 48 h at 20 °C before thermal stress (37 °C) was applied. Individual virtual times of death were determined by measuring the fluorescence of the nucleic acid stain SYTOX®Green. Kaplan–Meier statistics was used for the comparison of the survival curves, n = 3 (8 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test. d HCA/ICA did not modulate thermally induced ROS accumulation. The fluorescence of DCF as an indicator for intracellular ROS was measured in the petreated wild-type nematodes after thermal stress (37 °C) was applied, n = 3 (8 individuals per group), one-way ANOVA with Dunnett‘s multiple comparisons test
Lifespan prolongation by HSE and HCA is mediated via intracellular signalling pathways
We analysed which intracellular signalling pathways are involved in the life prolongation caused by HSE/HCA. First, we focused on the insulin-like signalling pathway investigating the effects on the DAF-16 transcription factor. The nuclear localisation of DAF-16 is strongly enhanced by HSE: 0.25 mg/ml HSE more than doubles the number of nematodes with a mainly nuclear phenotype (29% vs. 11%), while 1 mg/ml HSE induced a sixfold increase (60%). A similar effect was caused by an incubation with 2 mM of the main HSE component HCA, while 2 mM of the chemical analogue ICA showed no significant effect (Fig. 3a). We used DAF-16 deficient nematodes to investigate if the life-prolonging effects of HSE/HCA are dependent on functional DAF-16. Repeating the experiment from Fig. 1a using DAF-16 deficient nematodes, no prolongation of lifespan was detectable (0.5 and 1 mg/ml HSE as well as 2 mM HCA) (Fig. 3b; Table 1). Mobility analysis at day 8 of the lifespan showed that 1 mg/ml HSE additionally impaired locomotion (category C) in the DAF-16 mutant animals while HCA shows no effect (congruent with the wild-type nematodes) (Fig. 3c). Next, we investigated the requirement of SKN-1 (Nrf2-orthologue), another pivotal transcription factor for the effects of HSE and HCA. Comparable to the results of DAF-16, incubation of C. elegans with HSE (0.5 and 1 mg/ml) also increases the number of nematodes with a mainly nuclear phenotype of SKN-1 (48% and 49% vs 13%, respectively) (Fig. 4a). However, HCA treatment (2 mM) resulted in no significant change in SKN-1 localisation compared to the control animals (Fig. 4a). Using SKN-1 deficient nematodes, no increase in lifespan was detectable for HSE (0.5 and 1 mg/ml) as well as 2 mM HCA (Fig. 4c, d; Table 1). Furthermore, no increase in locomotion was detectable in the HSE or HCA-incubated animals (Fig. 4e, f).
DAF-16 is essential for lifespan prolongation by HSE and HCA. a HSE (left) and HCA (right) induce nuclear accumulation of DAF-16. L4 nematodes (TJ356) were treated with different concentrations of the compounds or the solvent control NGM for 1 h and subsequently analysed by means of fluorescence microscopy. Nematodes with nuclear localisation of DAF-16 were counted and a short heat stress (5 min 37 °C) was used as a positive control, n = 3 (30 individuals per group), one-way ANOVA with Dunnett‘s multiple comparisons test, ***p ≤ 0.001 **p ≤ 0.01, *p ≤ 0.05 vs. control. b L4 staged daf-16 lof nematodes (CF1038) were treated with 0.5 and 1 mg/ml HSE (left), 2 mM HCA (right) or the solvent control NGM at 25 °C. Media change was conducted 5 times a week and survival of the nematodes was examined by touch-provoked movement simultaneously, n = 3 (40 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test. c HSE (left) and HCA (right) do not modulate locomotion in daf-16 lof nematodes (CF1038). Locomotion was analysed by prodding the nematodes with a platinum wire at day 8 of their lifespans. The individuals were classified according to the movement: A—free movement, B—movement after prodding, C—weak movement after prodding, D—dead, n = 3 (40 nematodes per group), two-way ANOVA with Tukey’s multiple comparisons test
SKN-1 is essential for lifespan prolongation by HSE and HCA. a HSE (left), but not HCA (right), induces nuclear accumulation of SKN-1. L4 nematodes (LD001) were treated with different concentrations of the compounds or the solvent control NGM for 1 h and subsequently analysed by means of fluorescence microscopy. Nematodes with nuclear localisation of SKN-1 in the intestinal cells were counted and 10 mM H2O2 was used as a positive control, n = 3 (30 individuals per group), one-way ANOVA with Dunnett‘s multiple comparisons test, ***p ≤ 0.001 **p ≤ 0.01, *p ≤ 0.05 vs. control. b L4 staged skn-1 lof nematodes (EU1) were treated with 0.5 and 1 mg/ml HSE (left), 2 mM HCA (right) or the solvent control NGM at 25 °C. Media change was conducted 5 times a week and survival of the nematodes was examined by touch-provoked movement simultaneously, n = 3 (40 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test. c HSE (left) and HCA (right) do not modulate locomotion in skn-1 lof nematodes (EU1). Locomotion was analysed by prodding the nematodes with a platinum wire at day 8 of their lifespan. The individuals were classified according to the movement: A—free movement, B—movement after prodding, C—weak movement after prodding, D—dead, n = 3 (40 nematodes per group), two-way ANOVA with Tukey’s multiple comparisons test
HSE protects against amyloid-β toxicity via DAF-16 and SKN-1
Due to some reports showing neuroprotective effects of HSE, we investigated if the extract causes protective effects in a simple C. elegans model of Alzheimer’s disease. Using transgenic nematodes which paralyse after temperature-induced expression of the human amyloid-β peptide, we could show that HSE (0.5 and 1.0 mg/ml) caused a delayed onset of paralysis in comparison to the control nematodes (Fig. 5a) implying a protective effect against amyloid-β-toxicity. The median time span until onset of paralysis was delayed by 2 h in both treatment groups (Table 2). Using RNA-interference technique, we investigated if the pivotal transcription factors DAF-16 and SKN-1 that are involved in the anti-ageing effects of HSE are necessary for this effect. In this modified experimental system, we applied a different E. coli strain (HT115) which expresses dsRNA of the gene of interest. Treatment with 0.5 mg/ml HSE delayed onset of paralysis in the amyloid-β-strain fed with the control vector bacteria while no effect was seen in the nematodes exposed to daf-16 RNAi (Fig. 5b). A similar effect was visible when nematodes were exposed to skn-1 RNAi bacteria indicating that both DAF-16 and SKN-1 are involved in the protection against amyloid-β toxicity (Fig. 5b). Using the highest amount of HSE (1 mg/ml), the protective effect was also abolished in control vector-fed nematodes (Online Resource Suppl. Fig. 3). Additionally, another effect occurred in the nematodes with SKN-1-knock-down: Compared to the nematodes receiving bacteria with control plasmid, the bacteria expressing the SKN-1-interfering RNA were more resistant against toxic amyloid-β, even if no HSE is added (Fig. 5b, Online Resource Suppl. Fig. 3). However, this protection was abolished when using a double knock-down model system (DAF-16 and SKN-1 simultaneously) (Fig. 5b). Additionally, treatment with 0.5 mg/ml of HSE was not able to cause a protective effect in this system reconfirming the requirement of both transcription factors.
HSE protects against Aβ-induced toxicity in C. elegans via DAF-16 and SKN-1. Paralysis curves of pretreated C. elegans strain CL4176 expressing temperature-inducible human Aβ peptide in body wall muscle cells measured every other hour 26 h after temperature up-shift. a Eggs of CL4176 were treated with different concentrations of HSE or the solvent control NGM for 64 h at 16 °C before temperature was shifted to 25 °C, n = 3 (40 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test, **p ≤ 0.01, ***p ≤ 0.001 vs. control. b Eggs of CL4176 nematodes fed with HT115 E. coli expressing daf-16 dsRNA (left), skn-1 dsRNA (right), daf-16 and skn-1 dsRNA (bottom) or empty vector were treated with 0.5 mg/ml HSE or the solvent control NGMk for 40 h at 16 °C before temperature was shifted to 25 °C, n = 3 (40 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test, **p ≤ 0.01, ***p ≤ 0.001 vs. empty vector control
Discussion
H. sabdariffa preparations possess interesting pharmacological properties in mammals, e.g. antidiabetic, antihypertensive and anticancerogenic effects. Due to its common use as tea and food additive, HSE is considered to be safe and well tolerable for humans [4]. Recent in vitro and in vivo studies indicate anti-ageing and neuroprotective effects of HSE [5,6,7,8,9,10]. However, the bioactive compounds as well as the mode of action of HSE are poorly understood.
Here we investigated the effects of HSE on ageing and Aβ-induced toxicity in the model organism C. elegans. HSE treatment resulted in a prominent lifespan extension of C. elegans in a dose-dependent manner. Mobility declines with increasing age as a consequence of sarcopenia and loss of neuromuscular function in the nematode [22]. We categorized the nematodes according to their locomotion states. This behavioural assay has been previously used to analyse the health span (adult lifespan with no physical impairment) of C. elegans [23]. HSE (0.5 and 1 mg/ml) treatment significantly increased the amount of C. elegans without physical impairment indicating a slowed-ageing phenotype. The anti-ageing effect of HSE was not based on caloric restriction since HSE (1 mg/ml) neither influenced pharyngeal pumping as a marker for food intake nor bacterial growth of OP50-1 E.coli as a marker for food availability (Online Resource 1, Fig. 1a). Moreover, developmental growth as well as adult size of C. elegans was not modulated by treatment with HSE (Online resource 1, Fig. 1b, c).
Long-lived mutants often show an increased stress tolerance, an effect that can be simulated by plant compounds like the flavonoids baicalein and myricetin [16, 20]. Fitzenberger et al. showed that HSE ameliorates glucose-impaired thermal stress resistance in C. elegans [18], but no data on effects of HSE without glucose were available in this study. However, we showed that HSE did not influence thermotolerance in C. elegans under basal conditions. During these thermal stress conditions, the extract even induced a prooxidative effect as intracellular ROS levels increased. In contrast to the ROS induced by thermal stress, HSE treatment was able to increase survival under paraquat-induced oxidative stress (Online resource 1, Fig. 3) indicating a protective effect against oxidative insults. However, both kinds of stress (thermal-induced vs. paraquat-induced) are not directly comparable: On the one hand, during paraquat-induced oxidative stress mainly superoxide radical anions are produced over a relatively long time period (days), while on the other hand, during lethal thermal stress conditions (besides other effects like induction of heat-shock proteins, effects on membrane fluidity…) a rapid increase in ROS production occurs and the nematode dies within some hours. Therefore, it is not contradictory that HSE is able to protect against the mild oxidative stress while the extract is not protective during thermal stress conditions. Protective effects of HSE were found in various animal models, e.g. of diabetes, hepatotoxicity, nephrotoxicity and brain injury: Treatment with H. sabdariffa extracts ameliorated oxidative stress in these models by increasing antioxidative enzyme activity [24,25,26,27].
Since H. sabdariffa was reported to possess antioxidative as well as anti-diabetic properties in mammals, we analysed if HSE modulates DAF-16 (orthologue of mammalian FOXO) and SKN-1 (orthologue of mammalian Nrf2), key transcription factors regulating glucose metabolism, oxidative stress and longevity in C. elegans [28, 29]. We demonstrated that the anti-ageing effect of HSE was at least partially mediated by these redox-active transcription factors: Extension of lifespan and health span by HSE was abolished in mutant strains lacking functional DAF-16 or SKN-1. Additionally, HSE induced a nuclear translocation of both transcription factors in C. elegans. Recently, an upregulation of FoxO, the mammalian DAF-16-orthologue by a Hibiscus rosa sinensis extract was demonstrated by Pillai and Mini [30] using pancreatic ß-cells as model.
Flowers of H. sabdariffa consist of multiple bioactive compounds like flavonoids and anthocyanins, which are likely to contribute to these effects. For example, the flavonoids quercetin and myricetin as well as anthocyanin-rich plant extracts were shown to modulate the DAF-16 or the SKN-1-dependent signalling pathways in C. elegans [16, 31,32,33,34]. In addition to the polyphenolic compounds, HSE contains HCA, which is the major organic acid in the water extracts of H. sabdariffa calyces [1]. HCA is thought to induce the anti-obesity and anti-diabetic effects of HSE [35, 36]. Therefore, we investigated if the HSE-mediated effects on longevity are also mediated by the main HSE compound HCA. This was the case: HCA treatment was able to increase lifespan of C. elegans and this effect was dependent on the presence of DAF-16 and SKN-1. However, the anti-ageing effect of HCA alone was weaker than the respective amount of HSE (1 mg/ml) which indicates that HCA may act synergistically with other components of HSE. Organic acids like fumarate, malate and oxaloacetate were shown to increase lifespan of C. elegans in a DAF-16 dependent manner [37, 38]. Therefore, we investigated if the anti-ageing effect of HCA was a more general effect of organic acids rather than a specific effect of HCA. We analysed the effect of the structural derivative ICA, which lacks the C3 hydroxyl moiety compared to HCA. Treatment with ICA neither modulated lifespan nor nuclear DAF-16 translocation in the nematodes suggesting a specific effect for HCA.
Since HSE showed prominent anti-ageing properties, we analysed if HSE also protects against one of the most frequently occurring age-associated diseases. Alzheimer’s disease is the most common form of dementia which affects mostly people exceeding the age of 65 years [39]. A hallmark of Alzheimer’s disease is the accumulation of amyloid-β peptides (Aβ) in the brains of the persons. Overexpression of these Aβ peptides induces neurotoxic effects in different cell lines and animal models [40, 41]. Since extracts of different Hibiscus species showed prominent neuroprotective effects in aged and diabetic mice, we investigated if HSE also protects against Aβ toxicity in vivo [6, 7]. We used a transgenic C. elegans strain expressing Aβ3–42 in the body wall muscle cells resulting in a time-dependent paralysis of the body [42]. Treatment with 0.5 mg/ml HSE resulted in a protection against amyloid-β toxicity in C. elegans, which was dependent on DAF-16 and SKN-1. Surprisingly, the highest concentration of HSE (1 mg/ml) was not effective when nematodes were fed with E. coli strain HT115. Shifting C. elegans from OP50 E. coli to HT115 E. coli results in distinct metabolic changes, e.g. a decrease in triglyceride levels and an increase in lifespan [43]. Therefore, the sort of diet and hence the metabolic state may influence the effect of HSE in vivo. Moreover, skn-1 knock-down resulted in a protection against Aβ toxicity in C. elegans. Slightly lower paralysis rates upon skn-1 knock-down have been observed in C. elegans by several groups [44, 45]. However, downregulation of skn-1 also results in increased Aβ accumulation in the nematode [44]. We think, that knock-down of skn-1 by RNAi may stimulate compensatory mechanisms leading to decreased paralysis rates. Here we could show that double knock-down of skn-1 and daf-16 reversed the protective effects of skn-1 knock-down.
In conclusion, we demonstrated that HSE is able to prolong the lifespan of the model organism Caenorhabditis elegans in a concentration-dependent manner. Since HCA, the main component of HSE, but not its structural derivative ICA, also caused a prolongation of lifespan, this component seems to be, at least in parts, responsible for the effect on longevity. Since the effect is less pronounced, other components of HSE seem to be involved. The effect on lifespan was dependent on the presence of the transcription factors DAF-16 and SKN-1 showing the importance of these pivotal molecules. Moreover, we demonstrated that HSE ameliorates amyloid-β toxicity in C. elegans via DAF-16 and SKN-1 indicating a therapeutic role for HSE in Alzheimer’s disease.
Abbreviations
- daf-16 :
-
Abnormal dauer formation-16 (FoxO orthologue)
- DCF:
-
2′,7′-Dichlorofluorescein
- FoxO:
-
Forkhead box O
- GFP:
-
Green fluorescent protein
- HCA:
-
Hydroxycitric acid
- HSE:
-
Hibiscus sabdariffa L. extract
- ICA:
-
Isocitric acid
- lof:
-
Loss of function
- NGM:
-
Nematode growth medium
- ROS:
-
Reactive oxygen species
- skn-1 :
-
Skinhead-1 (Nrf2-orthologue)
References
Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M (2014) Hibiscus sabdariffa L.—a phytochemical and pharmacological review. Food Chem 165:424–443
Hopkins AL, Lamm MG, Funk JL, Ritenbaugh C (2013) Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia 85:84–94
Serban C, Sahebkar A, Ursoniu S, Andrica F, Banach M (2015) Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: A systematic review and meta-analysis of randomized controlled trials. J Hypertens 33(6):1119–1127
Riaz G, Chopra R (2018) A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharmacother 102:575–586
Addor FAS, Cotta Vieira J, Abreu Melo CS (2018) Improvement of dermal parameters in aged skin after oral use of a nutrient supplement. Clin Cosmet Investig Dermatol 11:195–201
Nade V, Kanhere S, Kawale L, Yadav A (2011) Cognitive enhancing and antioxidant activity of ethyl acetate soluble fraction of the methanol extract of Hibiscus rosa sinensis in scopolamine-induced amnesia. Indian J Pharmacol 43(2):137
Seung TW, Park SK, Kang JY, Kim JM, Park SH, Kwon BS, Lee CJ, Kang JE, Kim DO, Lee U, Heo HJ (2018) Ethyl acetate fraction from Hibiscus sabdariffa L. attenuates diabetes-associated cognitive impairment in mice. Food Res Int 105:589–598
Begum Z, Younus I (2018) Hibiscus rosa sinensis mediate anxiolytic effect via modulation of ionotropic GABA—a receptors: possible mechanism of action. Metab Brain Dis 33:823–827
Hritcu L, Foyet HS, Stefan M, Mihasan M, Asongalem AE, Kamtchouing P (2011) Neuroprotective effect of the methanolic extract of Hibiscus asper leaves in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. J Ethnopharmacol 137:585–591
Strathearn KE, Yousef GG, Grace MH, Roy SL, Tambe MA, Ferruzzi MG, Wu Q-L, Simon JE, Lila MA, Rochet J-C (2014) Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson׳s disease. Brain Res 1555:60–77
Williams RJ, Spencer JPE (2012) Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med 52:35–45
Herranz-López M, Olivares-Vicente M, Encinar JA, Barrajón-Catalán E, Segura-Carretero A, Joven J, Micol V (2017) Multi-targeted molecular effects of Hibiscus sabdariffa polyphenols: an opportunity for a global approach to obesity. Nutrients 9:E907
The C. elegans Sequencing consortium (1998) Genome sequence of the Nematode C. elegans: a platform for investigating biology. Science 282:2012–2018
Hulme SE, Whitesides GM (2011) Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angew Chem Int Ed Engl 50:4774–4807
Griffin EF, Caldwell KA, Caldwell GA (2017) Genetic and pharmacological discovery for Alzheimer’s disease using Caenorhabditis elegans. ACS Chem Neurosci 8:2596–2606
Büchter C, Ackermann D, Havermann S, Honnen S, Chovolou Y, Fritz G, Kampkötter A, Wätjen W (2013) Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int J Mol Sci 14:11895–11914
Fischer N, Büchter C, Koch K, Albert S, Csuk R, Wätjen W (2017) The resveratrol derivatives trans-3,5-dimethoxy-4-fluoro-4′-hydroxystilbene and trans-2,4′,5-trihydroxystilbene decrease oxidative stress and prolong lifespan in Caenorhabditis elegans. J Pharm Pharmacol 69:73–81
Fitzenberger E, Deusing DJ, Wittkop A, Kler A, Kriesl E, Bonnländer B, Wenzel U (2014) Effects of plant extracts on the reversal of glucose-induced impairment of stress-resistance in Caenorhabditis elegans. Plant Foods Hum Nutr 69:78–84
Stiernagle T (2006) Maintenance of C. elegans. In: WormBook (ed) The C. elegans research community. WormBook. https://doi.org/10.1895/wormbook.1.7.1. http://www.wormbook.org
Havermann S, Rohrig R, Chovolou Y, Humpf HU, Wätjen W (2013) Molecular effects of baicalein in Hct116 cells and Caenorhabditis elegans: activation of the Nrf2 signaling pathway and prolongation of lifespan. J Agric Food Chem 61:2158–2164
Havermann S, Chovolou Y, Humpf HU, Wätjen W (2016) Modulation of the Nrf2 signalling pathway in Hct116 colon carcinoma cells by baicalein and its methylated derivative negletein. Pharm Biol 54:1491–1502
Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M (2002) Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419:808–814
Newell Stamper BL, Cypser JR, Kechris K, Kitzenberg DA, Tedesco PM, Johnson TE (2018) Movement decline across lifespan of Caenorhabditis elegans mutants in the insulin/insulin-like signaling pathway. Aging cell 17:1–14
Ademiluyi AO, Oboh G, Agbebi OJ, Akinyemi AJ (2013) Anthocyanin—rich red dye of hibiscus sabdariffa calyx modulates cisplatin-induced nephrotoxicity and oxidative stress in rats. Int J biomed Sci IJBS 9:243–248
Ajiboye TO, Raji HO, Adeleye AO, Adigun NS, Giwa OB, Ojewuyi OB, Oladiji AT (2016) Hibiscus sabdariffa calyx palliates insulin resistance, hyperglycemia, dyslipidemia and oxidative rout in fructose-induced metabolic syndrome rats. J Sci Food Agric 96:1522–1531
Nurkhasanah Nurani LH, Hakim ZR (2017) Effect of rosella (Hibiscus sabdariffa L) extract on glutathione-S-transferase activity in rats. Trop J Pharm Res 16:2411–2416
Owoeye O, Gabriel MO (2017) Evaluation of neuroprotective effect of Hibiscus sabdariffa Linn. Aqueous extract against ischaemic-reperfusion insult by bilateral common carotid artery occlusion in adult male rats. Niger J Physiol Sci 32:97–104
Barbieri M, Bonafè M, Franceschi C, Paolisso G (2003) Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metabol 285:E1064–E1071
Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, Isik M (2015) SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med 88(Pt B):290–301
Pillai SS, Mini S (2018) Attenuation of high glucose induced apoptotic and inflammatory signaling pathways in RIN-m5F pancreatic β cell lines by Hibiscus rosa sinensis L. petals and its phytoconstituents. J Ethnopharmacol 227:8–17
Kampkötter A, Timpel C, Zurawski R, Ruhl S, Chovolou Y, Proksch P, Wätjen W (2008) Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp Biochem Physiol Part B 149:314–323
Chen W, Müller D, Richling E, Wink M (2013) Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J Agric Food Chem 61:3047–3053
Peixoto H, Roxo M, Krstin S, Röhrig T, Richling E, Wink M (2016) An Anthocyanin-rich extract of acai (Euterpe precatoria Mart.) increases stress resistance and retards aging-related markers in Caenorhabditis elegans. J Agric Food Chem 64(6):1283–1290
Yan F, Chen Y, Azat R, Zheng X (2017) Mulberry anthocyanin extract ameliorates oxidative damage in HepG2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 pathways. Oxid Med Cell Longev 2017:7956158
Carvajal-Zarrabal O, Waliszewski SM, Barradas-Dermitz DM, Orta-Flores Z, Hayward-Jones PM, Nolasco-Hipólito C, Angulo-Guerrero O, Sánchez-Ricaño R, Infanzón RM, Trujillo PRL (2005) The consumption of Hibiscus sabdariffa dried calyx ethanolic extract reduced lipid profile in rats. Plant Foods Hum Nutr 60:153–159
Morales-Luna E, Pérez-Ramírez Iza F, Salgado LM, Castaño-Tostado E, Gómez-Aldapa Carlos A, Reynoso-Camacho R (2018) The main beneficial effect of roselle (Hibiscus sabdariffa) on obesity is not only related to its anthocyanins content. J Sci Food Agric. https://doi.org/10.1002/jsfa.9220
Edwards CB, Copes N, Brito AG, Canfield J, Bradshaw PC (2013) Malate and fumarate extend lifespan in Caenorhabditis elegans. PloS one 8:e58345
Williams DS, Cash A, Hamadani L, Diemer T (2009) Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO-dependent pathway. Aging Cell 8:765–768
Kumar K, Kumar A, Keegan RM, Deshmukh R (2018) Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed Pharmacother 98:297–307
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–539
Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ (2006) Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol 572(Pt 2):477–492
McColl G, Roberts BR, Gunn AP, Perez KA, Tew DJ, Masters CL, Barnham KJ, Cherny RA, Bush AI (2009) The Caenorhabditis elegans Aβ 1–42 model of Alzheimer disease predominantly expresses Aβ. J Biol Chem 284:3–42 22697–22702.
Brooks KK, Liang B, Watts JL (2009) The influence of bacterial diet on fat storage in C. elegans. PLoS One 4:1–8
Dostal V, Roberts CM, Link CD (2010) Genetic mechanisms of coffee extract protection in a Caenorhabditis elegans model of β-amyloid peptide toxicity. Genetics 186:857–866
Alavez S, Vantipalli MC, Zucker DJS, Klang IM, Lithgow GJ (2011) Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature 472:226–229
Acknowledgements
The nematode strains used in this work were provided by the Caenorhabditis Genetics Centre, which is funded by the NIH National Centre for Research Resources (NCRR). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. We thank Dr. Sebastian Honnen for helpful discussions.
Author information
Authors and Affiliations
Contributions
NW, KK: performed experiments, KK, CB: supervision of experiments, WW: coordination of experiments, WW, KK: preparation of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1 a
HSE does not influence pharyngeal pumping of C. elegans (left). 10 nematodes per group were incubated with HSE, HCA, ICA or the respective control for 24 h and pharyngeal pumping of every animal was counted for 15 s and repeated three times, n = 3, one-way ANOVA with Dunnett’s multiple comparisons test, *p ≤ 0,05 vs. control; HSE does not influence bacterial growth of E. coli OP50-1 (right). The OD600 of a growing OP50-1 E. coli culture was photometrically measured at different time points. A freshly prepared OP50-1 E. coli solution with an OD600 of 0.2 was mixed with the HSE stock solution to a final concentration of 1 mg/ml HSE or the equivalent amount H2O and 50 µg/ml streptomycin. Bacteria were allowed to grow for 6 h at 37°C while shaking and an aliquot of the mixture was measured every hour (Synergy MX, BioTek Instruments, Inc.). Simultaneously, a mixture with heat-inactivated OP50-1 E. coli (37°C for 60 min) was measured, since we observed a colour change of the HSE after 1 h of incubation. n = 4 for OP50-1 E. coli, n = 1 for inactivated OP50-1 E. coli. b HSE does not influence growth of C. elegans. In order to investigate if HSE affects larvae growth or c adult body size, body area (left) and length (right) of wild-type nematodes (N2) was measured starting from b L1 or c L4 stage on. Synchronisation was performed according to Stiernagle [19] to obtain L4 larvae or arrested L1 larvae. Animals were incubated in liquid media with different HSE concentrations at 20°C. Images of 10 nematodes were taken daily with a camera (Motic Images Plus 2.0) connected to a stereo microscope (Stemi 2000, Zeiss) and the area and length of the body were measured with ImageJ. Adult nematodes were transferred daily to new incubation media to prevent overcrowding. n = 3 (20 individuals per group), one-way ANOVA with Dunnett’s multiple comparisons test (TIF 1003 KB)
Supplemental Fig. 2
Efficiency of daf-16 and skn-1 knock-down in C. elegans via feeding HT115 E. coli expressing dsRNA for the corresponding gene. a Representative images of TJ356 nematodes (left) expressing DAF-16::GFP and LD001 nematodes (right) expressing SKN-1::GFP. b Efficiency of daf-16 knock-down. TJ356 nematodes were fed with HT115 E. coli expressing daf-16 dsRNA or empty vector for 40 h at 16 °C. Images of single nematodes were taken with a camera connected to a fluorescence microscope equipped with a GFP filter. Fluorescence intensities of the single nematodes were measured with ImageJ. n = 1 (30 individuals), Student’s t-test, *** p ≤ 0.001 vs. empty vector control. c Efficiency of skn-1 knock-down. CL4176 nematodes were fed with HT115 E. coli expressing skn-1 dsRNA or empty vector for 40 h at 16 °C. Subsequently nematodes were transferred to RNAi plates and hatched larvae (left) and non-hatched eggs (right) were counted until the end of the reproductive period. n = 2 (10 individuals per group), Student’s t-test, ** p ≤ 0.01 vs. empty vector control (TIF 2254 KB)
Supplemental Fig. 3
A: HSE (1 mg/ml) increases oxidative stress resistance of C. elegans. L4 larvae (N2) were incubated with 1 mg/ml HSE or the respective control at 20°C for 3 days. Afterwards 30 nematodes of every treatment group were transferred to S-media containing 50 mM paraquat, 50 µg/ml streptomycin and 109 OP50-1 E. coli. The survival of the animals was measured daily by touch-provoked movement. Lost or ruptured animals were censored. n = 5 (30 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test, * p ≤ 0.05 vs. control. B: Treatment with HSE increases oxidative stress resistance of Δmev-1 C. elegans (TK22): L4 nematodes of the strain TK22 lacking mev-1 were incubated with 1 mg/ml HSE or the respective control at 20°C for 3 days. Afterwards 40 nematodes of every treatment group were transferred to S-media containing 50 mM paraquat, 50 µg/ml streptomycin and 109 OP50-1 E. coli. The survival of the animals was measured daily by touch-provoked movement. Lost or ruptured animals were censored. n = 3 (40 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test, *** p < 0,001 vs. control. D: HSE treatment decreases lipofuscin accumulation in the upper intestine of C. elegans. L4/young adult nematodes (N2) were treated with 1 mg/ml HSE or the respective control for 3 days at 20°C. Fluorescence intensities of the upper intestine were measured by densitometric analysis, n = 3 (10 individuals/trial), **p ≤ 0.01 one-way ANOVA with Dunnett‘s multiple comparisons test. D: Antioxidant NAC prevents HSE-induced nuclear accumulation of DAF-16 and SKN-1. L4 nematodes of the strain TJ356 (left) or LD001 (right) were incubated with 1 mg/ml HSE, 10 mM NAC, 1 mg/ml HSE and 10 mM NAC or the respective control at 20°C for 1 h and subsequently analysed by means of fluorescence microscopy. Nematodes with nuclear localisation of DAF-16 or SKN-1 in the intestinal cells were counted, n = 3 (30 individuals per group), (TIF 1053 KB)
Supplemental Fig. 4
HSE at a high concentration (1 mg/ml) is not able to protect against Aβ-induced toxicity in C. elegans fed with HT115 E. coli. Eggs of CL4176 nematodes fed with HT115 E. coli expressing daf-16 dsRNA (left), skn-1 dsRNA (right) or empty vector were treated with 1 mg/ml HSE or the solvent control NGMk for 40 h at 16°C before temperature was shifted to 25°C. Paralysis curve of pretreated C. elegans strain CL4176 was measured 26 h after temperature up-shift every other hour. n = 3 (40 individuals per group), Kaplan–Meier survival analysis with Log-Rank (Mantel-Cox)-test, *** p ≤ 0.001 vs. empty vector control (TIF 673 KB)
Rights and permissions
About this article
Cite this article
Koch, K., Weldle, N., Baier, S. et al. Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1. Eur J Nutr 59, 137–150 (2020). https://doi.org/10.1007/s00394-019-01894-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-01894-w