Abstract
Purpose
The association between dietary protein intake and type 2 diabetes risk has been inconsistent in the previous epidemiological studies. We aimed to quantitatively assess whether dietary total, animal, and plant protein would be associated with type 2 diabetes risk.
Methods
A comprehensive literature review was conducted to identify related articles by searching PubMed, Embase, Web of Science, and Wiley Online Library through 20th March 2018. Generalized least squares for trend estimation and restricted cubic spline regression model were applied in the dose–response analysis.
Results
Eight publications with ten prospective cohorts of 34,221 type 2 diabetes cases were included. After adjustment of potential confounders, a 5% of energy increment from dietary total and animal protein intake was related to a 9% (1.04, 1.13; I2 = 42.0%) and 12% (95% CI 1.08, 1.17; I2 = 14.0%) higher risk of type 2 diabetes respectively. However, for plant protein, a significant U-shaped curve was observed with the most risk reduction at intake of about 6% of energy intake from plant protein intake (Pnonlinearity = 0.001). The results were robust in sensitivity analysis and no publication bias was detected.
Conclusions
These findings indicate that the consumption of protein particularly animal protein may be associated with an increased risk of type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of type 2 diabetes (T2D) is increasing rapidly worldwide. The number of people living with T2D has almost quadrupled from 108 million in 1980 to 422 million in 2014 [1]. Individuals with diabetes may develop serious complications including cardiovascular diseases, nerve damage, kidney damage, and eye problems [2]. To determine the causes of T2D, numerous studies have been conducted to explore the potential linkages between diet and T2D.
Short-term trials have shown that diets high in protein were beneficial for weight loss and glucose homeostasis [3,4,5], which may play an important role in the development of T2D. Therefore, increasing dietary protein intake seems to be a promising strategy for preventing T2D. However, long-term prospective cohort studies suggested that animal-sourced foods with high protein, such as red and/or processed meat, were positively related to diabetes mellitus risk [6, 7], whereas, plant-based high-protein foods, such as nuts and legumes, were associated with lower risk of diabetes mellitus [8, 9]. Based on such evidence, a hypothesis was postulated that whether dietary protein per se is independently associated with risk of T2D given the evidence that protein theoretically could influence the development of T2D through the mechanism of insulin secretion and resistance [3, 4].
Recently, several prospective studies have focused on the effect of dietary protein and risk of T2D [10,11,12,13,14]. Nonetheless, results were inconsistent and not yet fully elucidated. To our knowledge, the dose–response associations of total protein and protein subtypes (animal and plant protein) with T2D risks have not been systematically synthesized, thus, we performed a meta-analysis using prospective studies to quantify the dose–response relationship.
Methods
Search strategy
We conducted and reported the present study according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. Comprehensive literature search was performed by searching databases of PubMed, Embase, Web of Science, and Wiley Online Library on 20th March 2018 using the following keywords: (protein intake or dietary protein or protein consumption) and (diabetes or diabetes mellitus) and (cohort studies or follow-up studies or longitudinal studies or prospective studies or nested case–control studies or case–cohort studies). Detailed search terms on each specific database were presented in online supplementary materials, Table S1. No restrictions on language or year of publication were applied. Articles included had to: (1) assess dietary protein as exposure of interest; (2) report T2D incidence as outcome; (3) use a prospective design in general population and a quality score ≥ 6; (4) provide estimates of relative risks (RR) or hazard ratios (HR) and corresponding 95% confidence intervals (CI) or data necessary to calculate these estimates. We furthermore manually searched the reference lists of included studies, all relevant reviews, and meta-analysis to identify additional articles that might be missed in the primary literature review. If duplicate publications in the same population were found, the most recent or the one with most applicable information was selected.
Data extraction and quality assessment
Two investigators (L.-G.Z and Q.-L.Z) independently reviewed the identified publications and evaluated their relevance to the research topic based on the prespecified inclusion criteria. For eligible studies, both of the two authors carried out the data extraction and quality assessment. Discrepancies were resolved through consensus of the two authors. A standard data extraction table was developed to obtain the following information: first author’s last name, year of publication, country in which the study was conducted, study name, baseline years, years of follow-up, overall number of participants, number of T2D cases, gender, age range of study population at recruitment, protein assessment methods, methods of identification of T2D cases, type of dietary protein, median or mean dietary protein intake in each category and the RR and 95% CI of T2D incidence related to those categories of protein intake, and covariates included in multivariable models. If more than one estimate was provided, priority was given to the one with the most adjusted potential confounding factors. One study [16] provided results with biomarker-calibrated or uncalibrated. To be consistent with other studies, we chose uncalibrated HRs in the current analyses. The Newcastle–Ottawa Scale (NOS) for cohort study based on selection, comparability, and outcome assessment with a full score of 9-star was used to assess the study quality [16].
Statistical analyses
To investigate the association between protein intake and T2D risk, we not only calculated pooled RR for the highest versus the lowest categories of protein intake, but also investigated the dose–response association between protein intake and T2D risk. We used random-effects models proposed by DerSimonian and Laird [17], which incorporated both within- and between-study variability to combine the study-specific risk estimates.
In dose–response analysis, we both estimated the RR and 95% CI for each increment of 5% energy from protein intake and explored potential nonlinear associations. For each study, the trend from the correlated log relative risks across categories of protein intake was calculated using the method proposed by Greenland et al. [18] and Orsini et al. [19]. The method requires more than two exposure categories and the following information for each category should be available: (1) the number of cases and total number of participants or person-years, (2) the RR and corresponding 95% CI, and (3) the mean or median protein consumption. When protein intake was presented in g/day, we transformed it into percent of energy using the energy 1 g of protein provides (4 kcal/g) and the average daily energy intake of the population. We assigned the median or mean protein intake of each category to the corresponding risk estimates of each study. For nonlinear associations, we used a two-stage, random-effect dose–response meta-analysis by modeling protein consumption using restricted cubic splines with three knots at fixed percentiles (10, 50, and 90%) of the distribution [19, 20]. We first fitted a restricted cubic spline model into each set of relative risks within the specific study [19, 20] and then combined the two regression coefficients and the variance/covariance matrices for each study using multivariate random-effects model [21]. A P value for nonlinearity was calculated by testing whether the coefficient of the second spline was equal to zero [22].
Heterogeneity among studies was assessed using the Q test and I2 statistic. A P < 0.10 for Q test or an I2 > 50% for I2 statistic was used to define heterogeneity [23]. We evaluated small study bias, such as potential publication bias by visual inspection of funnel plots and using Egger’s test [24]. In addition, a sensitivity analysis was conducted by reanalyzing the pooled estimates after excluding one study at a time to test the robustness of the result. We also explored whether the gender, specific adjusted covariates, and FFQ types had impacts on the main results. All statistical analyses were conducted using Stata, version 13.0 (Stata Corp, College Station, TX, United States). Two-sided test with P value of less than 0.05 was considered to be statistically significant if not specified.
Results
Literature search
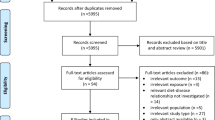
A flowchart (Fig. 1) presents the process of study selection. Briefly, we identified a total of 368 records in PubMed, 436 in Embase, 269 in Web of Science, and 101 in Wiley Online Library, among which 389 records were excluded, because they are duplicates. After a review of title and abstract, we further removed 745 records because of the violation of prescribed inclusion criteria. Four articles were identified through manual search of the reference lists. Among the remaining 44 articles for full-text review, 36 were excluded due to the reasons listed in Table S2. Finally, eight articles with ten studies (one article provided results of three separate studies) including 440,418 participants and 34,221 T2D cases were included in the current meta-analysis.
Included study characteristics
Table 1 shows the characteristics of included studies. Of the included ten prospective studies, five were conducted in the United States [14, 25, 26], two in Australia [27, 28], two in Europe [13, 29], and one in Japan [30]. Nine studies analyzed the effect of total protein and eight studies analyzed the effect of protein subtype (animal protein and plant protein). Four studies used interview-based food-frequency questionnaires [25, 26, 28, 31], and one used 4-day food record [32] and another five used self-administrated food-frequency questionnaires [14, 27, 30] to obtain information on the protein intake. T2D case ascertainment was through self-report in six studies, of which five studies with combination of registry information and another one with physician-confirmed diagnoses. All studies controlled age, sex, body mass index (BMI), physical activity, smoking, and alcohol use. Furthermore, most studies additionally provided results that did not adjust BMI. Most studies adjusted energy intake (n = 9) and family history of T2D (n = 7). As displayed in Table S3, all studies included in the current analysis were of high quality (All quality scores ≥ 6).
Highest versus lowest category
Figure S1 shows the relative risks of T2D related to protein intake by comparing participants in the highest category with those in the lowest category. Total protein and animal protein but not plant protein were significantly associated with higher T2D risk no matter whether BMI was adjusted (Figure S1). Apart from plant protein when BMI was not adjusted, no significant between-study heterogeneity was found for other exposures. We did not find evidence of publication bias in above associations (Fig. S3). In sensitivity analysis, the results were similar to the overall estimate when we excluded one study at a time (Fig. S5).
Dose–response meta-analysis
All included studies provided sufficient information for dose–response analyses. In models that adjusted for BMI, total protein and animal protein consumption showed a significantly positive association, while plant protein presented a borderline inverse correlation with T2D incidence in a linear fashion (Fig. 2a). For a 5% of energy increment from protein, the relative risk of T2D was 1.09 (95% CI 1.04, 1.13) for total protein, 1.12 (95% CI 1.08, 1.17) for animal protein, and 0.86 (95% CI 0.75, 1.00) for plant protein. Variances in heterogeneity between studies were 42.0% (P = 0.078), 14.0% (P = 0.317), and 34.2% (P = 0.144) for total, animal, and plant protein accordingly. No evidence of publication bias was detected (Fig. S2). Sensitivity analysis with one study at a time was excluded which showed that the pooled relative risks were not materially influenced by a single study (Fig. S4). When we excluded Tinker’s study which failed to adjust energy intake, the results remain stable and robust (Pooled RRper 5% of Energy from protein = 1.07, 95% CI 1.02, 1.12; I2 = 29.8%, P = 0.180). When we restricted our analysis in studies with use of semi-FFQ, the results did not change materially. The sex-specific results were also consistent with our main analysis. To determine whether animal or plant protein per se can exert its effect on T2DM, we analyzed studies with models in which animal or plant protein was mutually adjusted. However, the results seem to be in consistence with main analyses. These sensitivity results are provided in Table S4.
By combining studies that provided results before adjustment for BMI (seven studies for total protein; six studies for animal protein and plant protein), an increased intake of 5% of energy was related to a 36% (95% CI 1.23, 1.52) higher risk of T2D for total protein and a 32% (95% CI 1.27, 1.36) higher risk for animal protein (Fig. 2b).
In models failed to adjust BMI, we observed statistically significant nonlinear associations of T2D risk with total and animal protein intake (Fig. 3b, d). However, these nonlinear relationships became insignificant when adjusted for BMI (Fig. 3a, c). In addition, we observed a significant nonlinear association of T2D risk with plant protein intake in both models with BMI adjusted and BMI not adjusted (Fig. 3e, f). With the increase of dietary plant protein intake, a U-shaped relationship was observed for risk of T2D with the maximum reduction occurred at about 6% of energy from plant protein intake. However, with further increase in consumption, the relative risk tended to be closer to the null.
Discussion
To our knowledge, the present study is the first time to quantitatively assess the T2D risks related to total protein and protein type from prospective studies. In models that adjusted for BMI, we found that a 5% of energy increase in consumption of total and animal protein was related to a 9 and 12% higher risk of T2D, respectively. For plant protein, we observed a statistically significant nonlinear association with the largest risk reduction at intake of about 6% of energy from plant protein. These associations attenuated but remained statistically significant compared to the results without BMI adjusted.
Results from the current study were in agreement with the previous studies on T2D risk related to diets high in protein. A meta-analysis showed each 100 g unprocessed red meat per day and each 50 g processed red meat per day increase were associated with 19% (95% CI 1.04, 1.37) and 51% (95% CI 1.25, 1.83) higher risk of T2D, respectively [6]. Summary results including six eligible observational studies on nuts and T2D suggested that nuts were inversely associated with T2D (RR 0.87; 95%CI 0.81, 0.94) [8]. In addition, another meta-analysis on dietary patterns found that the Mediterranean diet characterized by high plant-based food was associated with 23% (95% CI 0.66, 0.89) lower risk of developing T2D by comparing the upper and the lowest available centile [33]. For western diet high in animal food, the relative risk was 1.41 (95% CI 1.32–1.52) for people in the highest category compared with the lowest category [34]. Although we cannot rule out the possibility that these observed associations were related to other components in foods, our results indicated these relationships were attributed at least partly if not all to the protein consumption.
The biological mechanism of dietary protein impact on T2D risk was largely unknown. There are several potential explanations for the observed associations of total protein and protein sources with T2D development. Studies suggested that dietary protein may increase glucagon, which is a contributor to high blood glucose level [35]. In addition, dietary proteins are also known to promote the secretion of insulin, while hyperinsulinemia is a risk factor for insulin resistance [4, 36]. The discrepancy in effect between animal protein and plant protein may be determined by the difference of amino acid composition. In metabolomics studies, branched-chain and aromatic amino acids, such as leucine, tyrosine, and phenylalanine, were found to be positively related to incident T2D [37, 38]. Although these amino acids exist in all high-protein contents food, they mainly present in animal food like meat and dairy [39]. All the above-mentioned biological effect might explain the positive association of total and animal protein with T2D risk as well as the different impact between animal protein and plant protein.
Recommendations of dietary intake of protein for diabetics should not exceed 20% of energy intake in U.S. [40], but the true relationship between protein consumption and T2D remains unknown. The current study improved the evidence that total and animal protein should be considered for T2D prevention in general population and provided the ideal daily intake amount of plant protein for T2D prevention according to its dose–response association with T2D risk. In addition, studies have previously suggested that high intake of total and animal protein was positively related to mortality risk, while plant protein inversely was associated with risk of deaths, which was also partly supported our findings [41, 42]. As all except one studies included in the present meta-analysis were conducted in countries with a Westernized lifestyle and food source and consumption level of protein varied among people in different places, future studies should explore the relationship between dietary protein intake and health outcomes in other geographical locations. Furthermore, studies investigating its associations with other diseases such as cardiovascular diseases and cancer are needed to assess the effect of protein comprehensively.
Strengths of the meta-analysis included the prospective cohort design of original studies, which greatly reduced the possibility of recall bias. A large number of cases and participants provided sufficient statistical power to figure out the association between dietary protein and incident T2D. Though two studies have summarized the associations between dietary proteins and T2D, they just provided results from comparisons of high- and low-protein consumers [28, 43]. In the current analysis, we provided a comprehensive and quantitative analysis and broadened the evidence on this topic. Finally, studies included in this meta-analysis adjusted most of the known potential confounding factors to rule out substantial amount of confounding bias. For total protein intake, all included studies except for Tinker’s [26] adjusted energy and fat intake in the multivariable model, which means that the impact of dietary protein can be interpreted as the effects of substituting dietary protein for dietary carbohydrate. When we only included studies using substitution models, results did not change materially compared with our main analysis.
Some limitations also should be considered. First, residual confounding still might exist though most confounders were taken into consideration in original studies. For example, the observed association between protein and T2D risk might be caused by other factors related to protein intake such as iron and nitrates in protein-rich food. Second, the potential role of obesity as a potential mediator or confounding factor is unclear, so we presented the associations adjusting with and without adjustment for BMI in the current analysis to elucidate the possible difference. However, unfavorable effects of total and animal protein independent of obesity may exist as the direction and significance of the association unchanged with adjustment for BMI. Third, some degree of heterogeneity was detected. Differences in study locations, sex, various sources and types of protein, cohort size, and follow-up time could lead to the heterogeneity. We provided sex-specific results with no significant differences observed. When excluded Tinker’s study [16], we observed the I2 changed from 42.0 to 29.8%. Another limitation is the underlying publication bias. Although, in the primary analysis, we did not detect publication bias using a statistical method, since test power for publication bias was limited, especially when the number of studies was not many.
In conclusion, we found total protein was associated with a higher risk of T2D and this association was largely due to the protein of animal origin. Plant protein has a modest nonlinear dose–response association with T2D risk. Therefore, public health recommendations should consider the protein sources for T2D prevention.
Abbreviations
- T2D:
-
Type 2 diabetes
- RR:
-
Relative risks
- CIs:
-
Confidence intervals
- BMI:
-
Body mass index
References
World Health Organization (2016) Global report on diabetes. World Health Organization, Geneva
Gregg EW, Sattar N, Ali MK (2016) The changing face of diabetes complications. Lancet Diabetes Endocrinol 4:537–547. https://doi.org/10.1016/s2213-8587(16)30010-9
Promintzer M, Krebs M (2006) Effects of dietary protein on glucose homeostasis. Curr Opin Clin Nutr Metab Care 9:463–468. https://doi.org/10.1097/01.mco.0000232909.84483.a9
Rietman A, Schwarz J, Tome D, Kok FJ, Mensink M (2014) High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr 68:973–979. https://doi.org/10.1038/ejcn.2014.123
Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR (2009) Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr 29:21–41. https://doi.org/10.1146/annurev-nutr-080508-141056
Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB (2011) Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 94:1088–1096. https://doi.org/10.3945/ajcn.111.018978
Bendinelli B, Palli D, Masala G, Sharp SJ, Schulze MB, Guevara M, van der AD, Sera F, Amiano P, Balkau B, Barricarte A, Boeing H, Crowe FL, Dahm CC, Dalmeijer G, de Lauzon-Guillain B, Egeberg R, Fagherazzi G, Franks PW, Krogh V, Huerta JM, Jakszyn P, Khaw KT, Li K, Mattiello A, Nilsson PM, Overvad K, Ricceri F, Rolandsson O, Sanchez MJ, Slimani N, Sluijs I, Spijkerman AM, Teucher B, Tjonneland A, Tumino R, van den Berg SW, Forouhi NG, Langeberg C, Feskens EJ, Riboli E, Wareham NJ (2013) Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia 56:47–59. https://doi.org/10.1007/s00125-012-2718-7
Afshin A, Micha R, Khatibzadeh S, Mozaffarian D (2014) Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr 100:278–288. https://doi.org/10.3945/ajcn.113.076901
Nanri A, Mizoue T, Takahashi Y, Kirii K, Inoue M, Noda M, Tsugane S (2010) Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J Nutr 140:580–586. https://doi.org/10.3945/jn.109.116020
Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, van der Schouw YT (2010) Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 33:43–48. https://doi.org/10.2337/dc09-1321
Wang ET, De Koning L, Kanaya AM (2010) Higher protein intake is associated with diabetes risk in South Asian Indians: the metabolic syndrome and atherosclerosis in South Asians Living in America (MASALA) study. J Am Coll Nutr 29:130–135
Ericson U, Sonestedt E, Gullberg B, Hellstrand S, Hindy G, Wirfalt E, Orho-Melander M (2013) High intakes of protein and processed meat associate with increased incidence of type 2 diabetes. Br J Nutr 109:1143–1153. https://doi.org/10.1017/S0007114512003017
Nielen M, Feskens EJM, Mensink M, Sluijs I, Molina E, Amiano P, Ardanaz E, Balkau B, Beulens JWJ, Boeing H, Clavel-Chapelon F, Fagherazzi G, Franks PW, Halkjaer J, Huerta JM, Katzke V, Key TJ, Khaw KT, Krogh V, Kuhn T, Menendez VVM, Nilsson P, Overvad K, Palli D, Panico S, Rolandsson O, Romieu I, Sacerdote C, Sanchez MJ, Schulze MB, Spijkerman AMW, Tjonneland A, Tumino R, A DL, Wurtz AML, Zamora-Ros R, Langenberg C, Sharp SJ, Forouhi NG, Riboli E, Wareham NJ (2014) Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. Diabetes Care 37:1854–1862. https://doi.org/10.2337/dc13-2627
Malik VS, Li Y, Tobias DK, Pan A, Hu FB (2016) Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol 183:715–728. https://doi.org/10.1093/aje/kwv268
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349:g7647. https://doi.org/10.1136/bmj.g7647 (Clinical research ed.)
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 11 Nov 2017
DerSimonian R, Laird N (2015) Meta-analysis in clinical trials revisited. Contemp Clin Trials 45:139–145. https://doi.org/10.1016/j.cct.2015.09.002
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175:66–73. https://doi.org/10.1093/aje/kwr265
Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose–response data. Stata J 6:40–57
Jackson D, White IR, Thompson SG (2010) Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 29:1282–1297. https://doi.org/10.1002/sim.3602
Desquilbet L, Mariotti F (2010) Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 29:1037–1057. https://doi.org/10.1002/sim.3841
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Song Y, Buring JE, Manson JE, Liu SM (2004) A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women. Diabetes Care 27:2108–2115. https://doi.org/10.2337/diacare.27.9.2108
Tinker LF, Sarto GE, Howard BV, Huang Y, Neuhouser ML, Mossavar-Rahmani Y, Beasley JM, Margolis KL, Eaton CB, Phillips LS, Prentice RL (2011) Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women’s Health Initiative. Am J Clin Nutr 94:1600–1606. https://doi.org/10.3945/ajcn.111.018648
Alhazmi A, Stojanovski E, McEvoy M, Garg ML (2014) Macronutrient intake and type 2 diabetes risk in middle-aged Australian women. Results from the Australian Longitudinal Study on Women’s Health. Public health Nutr 17:1587–1594. https://doi.org/10.1017/s1368980013001870
Shang X, Scott D, Hodge AM, English DR, Giles GG, Ebeling PR, Sanders KM (2016) Dietary protein intake and risk of type 2 diabetes: results from the Melbourne Collaborative Cohort Study and a meta-analysis of prospective studies. Am J Clin Nutr 104:1352–1365. https://doi.org/10.3945/ajcn.116.140954
Virtanen HEK, Koskinen TT, Voutilainen S, Mursu J, Tuomainen TP, Kokko P, Virtanen JK (2017) Intake of different dietary proteins and risk of type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr 117:882–893. https://doi.org/10.1017/S0007114517000745
Nanri A, Mizoue T, Kurotani K, Goto A, Oba S, Noda M, Sawada N, Tsugane S (2015) Low-carbohydrate diet and type 2 diabetes risk in Japanese men and women: the Japan Public Health Center-Based Prospective Study. PLoS One 10:e0118377. https://doi.org/10.1371/journal.pone.0118377
Van Nielen M, Feskens E, Mensink M, Forouhi N, Wareham N (2013) Dietary protein intake and the incidence of type 2 diabetes in Europe: the epic-interact case-cohort study. Ann Nutr Metab 63:201
Virtanen HEK, Koskinen TT, Voutilainen S, Mursu J, Tuomainen T-P, Kokko P, Virtanen JK (2017) Intake of different dietary proteins and risk of type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. https://doi.org/10.1017/s0007114517000745
Koloverou E, Esposito K, Giugliano D, Panagiotakos D (2014) The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metab Clin Exp 63:903–911. https://doi.org/10.1016/j.metabol.2014.04.010
McEvoy CT, Cardwell CR, Woodside JV, Young IS, Hunter SJ, McKinley MC (2014) A posteriori dietary patterns are related to risk of type 2 diabetes: findings from a systematic review and meta-analysis. J Acad Nutr Diet 114:1759–1775 e1754. https://doi.org/10.1016/j.jand.2014.05.001
Neu A, Behret F, Braun R, Herrlich S, Liebrich F, Loesch-Binder M, Schneider A, Schweizer R (2015) Higher glucose concentrations following protein- and fat-rich meals—the Tuebingen Grill Study: a pilot study in adolescents with type 1 diabetes. Pediatr Diabetes 16:587–591. https://doi.org/10.1111/pedi.12224
Tremblay F, Lavigne C, Jacques H, Marette A (2007) Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr 27:293–310. https://doi.org/10.1146/annurev.nutr.25.050304.092545
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17:448–453. https://doi.org/10.1038/nm.2307
Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG, Fritsche A, Haring HU, Hrabe de Angelis M, Peters A, Roden M, Prehn C, Wang-Sattler R, Illig T, Schulze MB, Adamski J, Boeing H, Pischon T (2013) Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 62:639–648. https://doi.org/10.2337/db12-0495
Adeva MM, Calvino J, Souto G, Donapetry C (2012) Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 43:171–181. https://doi.org/10.1007/s00726-011-1088-7
Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML (2008) Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 31(Suppl 1):S61–78. https://doi.org/10.2337/dc08-S061
Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, Giovannucci EL (2016) Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2016.4182
Chen X, Wei G, Jalili T, Metos J, Giri A, Cho ME, Boucher R, Greene T, Beddhu S (2016) The associations of plant protein intake with all-cause mortality in CKD. Am J Kidney Dis Off J Natl Kidney Found 67:423–430. https://doi.org/10.1053/j.ajkd.2015.10.018
Alhazmi A, Stojanovski E, McEvoy M, Garg ML (2012) Macronutrient intakes and development of type 2 diabetes: a systematic review and meta-analysis of cohort studies. J Am Coll Nutr 31:243–258
Acknowledgements
We would like to thank the original studies for the contribution to conduct our meta-analysis.
Funding
This work was supported by funds from the State Key Laboratory of Oncogenes and Related Genes (#91-15-10).
Author information
Authors and Affiliations
Contributions
Y-BX obtained the funding, conducted the research design, interpreted the results, and also had primary responsibility for the final content. L-GZ and Q-LZ analyzed the data and interpreted the results. L-GZ and Q-LZ drafted first manuscript. All authors critically reviewed and approved the manuscript. No authors have any conflicts of interest to declare.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, LG., Zhang, QL., Liu, XL. et al. Dietary protein intake and risk of type 2 diabetes: a dose–response meta-analysis of prospective studies. Eur J Nutr 58, 1351–1367 (2019). https://doi.org/10.1007/s00394-018-1737-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1737-7