Abstract
Purpose
Obesity is the main feature of a complex illness known as metabolic syndrome. Anti-obesogenic therapies are often associated with side effects and represent a high cost in conventional pharmacological approaches. New strategies based on natural remedies are under continuous investigation. Leopoldia comosa (L.) Parl. (L. comosa) is a spontaneous plant with diuretic, anti-inflammatory and antioxidant properties. Recently, a hypoglycemic activity mediated by inhibition of carbohydrate digestion has been identified. The aim of this study was to evaluate the effects of a diet supplemented with L. comosa extracts on a rat model of diet-induced obesity.
Methods
Leopoldia comosa bulb extracts were obtained using a dynamic extractor. Phytochemical properties and in vitro determination of the antioxidant activity and of the inhibitory effects on lipase and pancreatic amylase were performed. Rats were fed (12 weeks) a standard diet, or a high-fat diet (HFD), or an HFD plus L. comosa (20 or 60 mg/die) extracts. The metabolic and anthropometric parameters were recorded.
Results
Results indicated that L. comosa inhibited lipase and pancreatic amylase activities. In vivo data showed that the supplementation with both doses of L. comosa extracts counteracted the HFD-dependent effects. It reduced body weight, abdominal obesity and dyslipidemia, and improved glucose tolerance with a reduction of lipidic tissue hypertrophy and liver steatosis, as compared to HFD-fed rat. In liver, L. comosa reduced protein expression levels of PEPCK and G6Pase.
Conclusion
We suggest that L. comosa extracts prevent obesity-dependent metabolic disorders. This paves the way for their therapeutic application as a natural anti-obesity drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last two decades, obesity-induced mortality has increased significantly. In fact, obesity is able to cause or exacerbate several health problems such as coronary heart disease, cancer and diabetes [1]. Obesity depends on the interaction between genetic, environmental and psychosocial factors [2]. Physiologically, the main cause of obesity is an imbalance between energy intake and consumption that determines an excessive accumulation of fat in adipose tissue [3]. A World Health Organization committee (WHO Tech Rep Ser. no. 854 1995) has proposed the classification of overweight and obesity according to the body mass index (BMI) [2]. When the body fat increases, important changes in physiological functions happen. The total blood volume and the cardiac function can be altered, the intra-abdominal fat deposition contributes to the development of hypertension and, among plasma metabolic parameters, an increase in insulin and lipid levels can be observed; all these factors can lead to insulin resistance and diabetes [2]. In particular, hyperinsulinemia and insulin resistance contribute to determine the plasma lipid profile associated with obesity: elevated fasting plasma triglyceride concentration, reduced HDL-cholesterol, elevation of total cholesterol and LDL-cholesterol concentrations [4]. Moreover, an increase of total body oxygen consumption and of total blood volume induces an enhancement of the cardiac work that often results in a compensatory eccentric hypertrophy of the left ventricle [5]. Even if the current anti-obesogenic drugs work in a satisfactory manner on diet-induced obesity, new strategies, based on the administration of natural remedies to overcome the side effects and high costs of the conventional pharmacological therapies, are currently under investigation. A strict interconnection between nutrition and health care has always been proposed [6, 7]. Many bitter plants have important biological activities [8]. In fact, several studies show a close relationship between the bitter taste of plants and their medicinal properties [8]. Mediterranean lifestyle contemplates the utilization of non-cultivated plants and aromatic bitter species, for which an increasing number of experimental evidences describe unexpected health benefits [9]. A popular endemic plant used as ingredient in the traditional gastronomy of Southern Italy is Leopoldia comosa (L.) Parl., previously named Muscari comosum (L.) Mill., a species belonging to the Hyacinthaceae family and growing in all the Mediterranean areas [10]. L. comosa bulbs were traditionally used for the treatment of skin spots and toothache [11]. A previous study provided evidence for its diuretic, anti-inflammatory and antioxidant properties [9]. In addition, L. comosa bulbs showed promising antioxidant and hypoglycemic activity via the inhibition of carbohydrate digestive enzymes [12]. Very recently, enzyme-inhibitory effects and in vitro antitumoral activities have also been identified [13]. Different phytochemicals have been previously isolated from L. comosa [14,15,16,17]. Borgonovo and collaborators [8] isolated several 3-benzyl-4-chromanones and one stilbene derivative that seem to be responsible for the typical bitter taste of this plant.
The present study aimed to evaluate the effects of chronic administration of L. comosa bulb extracts on a rat model of high-fat diet-induced obesity.
Materials and methods
Plant material and extraction
Bulbs of L. comosa (L.) Parl. were collected in the fields of the Sila Massif (39°40′27.34″N, 16°46′79.24″E–39°40′9.20″N, 16°46′84.91″E) (Calabria, Southern Italy) during August 2015. The bulbs were stored in a cool and dry environment and subsequently separated from roots and cleaned of soil residues. The bulbs (580 g) were extracted with a water/ethanol mixture (1:1 v/v) using a Naviglio® extractor (Atlas Filtri S.r.L., Limena, PD, Italy): the complete process required two extraction cycles (4 L of solvent) and each cycle was completed at 3.20 h with a working temperature of 20 °C and a pressure of 8 bar. The solvent was evaporated under reduced pressure using Rotavapor R-220 SE (BÜCHI Labortechnik AG, Flawil, Switzerland). The average extraction yield was 18.5%. An aliquot was submitted to further processing for its phytochemical profiling, while the remaining part was used for biological testing in vitro and in vivo. A suitable volume of test solution was evaporated, the dry residue was re-suspended in 100% methanol, centrifuged (6000 rpm, d = 10 cm) and subjected to subsequent analysis (test solution).
Total phenols and total flavonoids
The total phenol content of the total extracts was determined using Folin–Ciocalteu reagent and chlorogenic acid used as standard, modified according to [13]. The total flavonoid content of the crude extract was determined by the AlCl3 colorimetric method on the same extracts used for total phenol determination [18]. The samples were heated to 60 °C (water bath) for 1 h, allowed to cool to room temperature and finally homogenized. An amount of 200 µL (three replicates) was introduced into screw cap test tubes. Then, 1.0 mL of Folin–Ciocalteu reagent and, after 3 min, 1.0 mL of Na2CO3 (7.5%) were added. The tubes were vortexed and heated at 40 °C (water bath) for 30 min. The calibration curve was determined with seven standards with concentrations ranging from 50 to 1200 µg/mL. The absorption at 726 nm was measured (Perkin-Elmer Lambda 40 UV/VIS spectrophotometer) and the total phenol content expressed as mg of chlorogenic acid equivalents (CAE) per g of fresh material (FW).
Phytochemical profiling
The phytochemical composition of the bulbs’ hydroalcoholic extract was investigated by high-performance liquid chromatography coupled to ultraviolet detection (HPLC–UV–DAD) and by gas chromatography coupled to mass spectrometric detection (GC–MS) after derivatization (silanization) of the sample. For HPLC–UV–DAD, a suitable amount of hydroalcoholic extract was dried under reduced pressure; the dry residue was extracted with 1 mL of HPLC-grade methanol, the mixture centrifuged and the resulting clear solution analysed. Analysis was done using a Varian LC-940 semi-preparative HPLC system (Varian, Leinì, Turin, Italy) equipped with a binary pump system, an autosampler, a fraction collector, a UV-DAD detector operating at λ = 290 nm and an analytical/semi-preparative scale-up module (pump heads volume 10 mL). Analytical separations were carried out using a Kinetex PFP column (100 mm × 3 mm, pore size 100 Å, particle size 5 µm) (Phenomenex, Castelmaggiore, Bologna, Italy). Solvent system: (A) 0.05% aqueous formic acid, (B) 0.05% in 100% acetonitrile. Gradient program: 0–5 min, B = 10%; 5–30 min B = 10–90%, 30–40 min B = 90%. Flow 1.6 mL/min (XRs column). Volume of injection: 10 µl.
For GC–MS analysis, a suitable amount of the hydroalcoholic extract was evaporated, and the dry residue was subjected to silanization using 70 µL of BTSFA 30 µL of pyridine and 100 µL of ethyl acetate (T = 70 C, 4 h). 1 µL of this solution was injected into the GC–MS system. GC–MS analysis was performed on a Bruker Scion SQ equipped with a single quadrupole and a FactorFour Varian column (VF-5 ms, 30 m; 0.25 mm i.d., film thickness 0.25 µm). The oven temperature was initially set at 60 °C (hold time 3 min), with a gradient from 60 to 120 °C (8.0 °C/min, hold 1 min), then from 120 to 280 °C (4°C/min, hold 1.5 min) and from 280 to 330 °C (10 °C/min, hold 2 min). Injector temperature 250 °C, column flow at 1.00 mL/min. Carrier gas was helium 5.5; ionization energy 70 eV; the split/splitless ratio was set to 1:30 after 40 s. Data acquisition was performed in full scan (m/z 50–1200) in electron impact (EI) from 3 to 60 min. Injection volume: 1 µl.
Antioxidant activity
Free radical scavenging assay
The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay was adapted according to [19] as previously described. An ethanol solution of DPPH radical (final concentration = 1.0 × 10− 4 mol/L), extracts at different concentrations was added. The absorbance of the resulting solutions was measured at 517 nm, against a blank with DPPH. The DPPH solution without the sample solution was used as control. Ascorbic acid was used as the positive control. Decreasing absorbance values of the DPPH solutions indicated an increase of DPPH radical scavenging activity. This activity was given as % DPPH radical scavenging, calculated by the following equation: % DPPH radical scavenging = (absorbance control − absorbance sample)/absorbance control × 100.
Antilipoperoxidation activity
The antilipoperoxidation activity was measured using the β-carotene bleaching (BCB) test method [19]. The test solutions were obtained by adding 0.04 mL of linoleic acid and 0.4 mL of Tween 20–2 mL of a β-carotene solution in chloroform (0.5 mg/mL). The solvent was then evaporated under reduced pressure at 40 °C for 10 min through a rotary evaporator and the dry residue was immediately diluted with 150 mL of distilled water. Water was slowly added to the mixture and vigorously stirred to form an emulsion. Then, 0.2 mL of sample solutions at different concentrations (120, 60, 30, 15, 8, 4, 2, 0.5 mg/mL) were added to 5 mL of the emulsion. Ascorbic acid was used as positive control. The tubes were stirred slowly and kept at 45 °C in a water bath. The absorbance was read at λ = 470 nm at the starting incubation time (t0) and at t = 30 min. The antioxidant activity (AA) was calculated using the following equation: AA = [1 − (A0 − At)/(A0° − At°)] × 100, where A0 and A0° are the absorbance values measured at t = 0 min for samples/standard and control, respectively, and At and At° are the absorbance values measured in the samples/standard and control at t = 30 min, respectively.
In vitro enzymatic assays
Pancreatic lipase inhibition assay
The porcine pancreatic lipase activity was assayed using p-nitrophenyl-octanoate (NPO) as substrate and spectrophotometrically recorded at λ = 412 nm.
A water solution (3 mg/mL) was prepared from type II crude porcine pancreatic cells [20]. Then, a 7.5 mmol/L solution of NPO in dimethyl sulfoxide (DMSO) was prepared. The composition of the reaction mixture was the following: 100 µL of 7.5 mmol/L NPO, 4 mL of Tris–HCl buffer (pH = 8.5), 100 µL of extract (concentration 400, 250, 100, 55, 30, 15, 5, 2, 0.8, 0.20, 0.05 mg/mL) and 100 µL of enzyme solution. The mixture was incubated at 37 °C. In the control, the extract was replaced with the same volume of DMSO. A blank sample without the enzyme was prepared for each extract. Orlistat was used for comparison. The pancreatic inhibitory activity was calculated using the following formula: I (%) = [1 − (Ac − As)/A0] × 100.
Ac is defined as the absorbance of the substrate and of the porcine enzyme with the sample, As the absorbance of the porcine enzyme with the sample without substrate and A0 the absorbance of 100% enzyme activity on the substrate (without sample).
Pancreatic α-amylase inhibition assay
The inhibition of α-amylase enzyme was evaluated using a modified version of the method used by Kwon et al. [21]. 100 µL of sample solutions (concentration 400, 250, 100, 55, 30, 15, 5, 2, 0.8, 0.20, 0.05 mg/mL) were added to 500 µL of a 0.5 mg/mL enzyme solution in cold distilled water and to 500 µL of a 1% (w/v) starch solution in 0.01 mol/L phosphate buffer at pH 7.0. The reaction mixture was incubated at 37 °C for 5 min; the reaction was stopped after the addition of 1 mL of the reagent dye DNS (3,5-dinitrosalicylic acid and 1% potassium sodium tartrate in 2% NaOH 0.4 mol/L). The reaction mixture was incubated at 100 °C for 5 min and the absorbance measured at 540 nm. The α-amylase inhibitory activity was calculated using the following formula: I (%) = [(A1 − A0)/A1] × 100. A0 is defined as the absorbance of 100% enzyme activity (without sample) and A1 as the absorbance of a test sample (with enzyme). The concentration of acarbose and of plant extracts required to inhibit 50% of α-amylase activity under these conditions was defined as the IC50 value. The α-amylase inhibitory activity of the plant extracts and of acarbose was calculated, and its IC50 values were determined.
In vivo study design
The hydroalcoholic extract of L. comosa bulbs was orally administered at two different doses: 20 or 60 mg/die. Doses were estimated on the base of IC50 values determined using the enzymatic activity assays with orlistat as positive control. The dose of 20 mg/die is similar to that orlistat dose administration [22].
The study was performed on Wistar rats (Envigo—Udine, Italy). The mean animals’ weight, at the beginning of treatment, was 250 ± 2 g. Animals were individually housed in cages, under controlled light and temperature conditions, and fed by free access to food and water. All protocols were conducted in accordance with the Declaration of Helsinki, the Italian law (D.L. 26/2014), the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (2011) and the Directive 2010/63/EU of the European Parliament on the protection of animals used for science. The project was approved by the Italian Ministry of Health, Rome, and by the ethics review board.
Diets used for the study were purchased by Envigo (Udine, Italy). Diet 2018 was used as normolipidic diet (SD, standard diet: 6.2% kcal fat, 18.6% kcal protein, and 44.2% kcal carbohydrate), while Teklad Diet TD 06414 was used as hypercaloric diet (HFD, high-fat diet: 60% kcal fat, 18.3% kcal protein and 21.7% kcal carbohydrate) able to induce obesity as previously described by [23].
Rats were randomly assigned to four different experimental groups (n = 5 for each group):
-
1.
Group SD (fed with SD).
-
2.
Group HFD (fed with HFD).
-
3.
Group HFD + Lc (20 mg) (fed with HFD plus 20 mg of L. comosa extract).
-
4.
Group HFD + Lc (60 mg) (fed with HFD plus 60 mg of L. comosa extract).
The experimental groups were subjected to their specific diet for 12 weeks; the L. comosa extract solutions were administered orally every day for 12 weeks. At the end of each treatment, plasma analysis was performed.
“Anthropometric” variables
The body weight, waist circumference and body length of each rat were weekly measured; food and water intake was recorded daily. Rat length was evaluated by measuring the nose–anus distance according to Okamura et al. [24]. BMI was calculated as reported by Panchal et al. [25]. After the animal was killed, the heart, the liver and different types of adipose tissue (abdominal, perirenal, epididymal, retroperitoneal) were removed and separately weighted. The cardiac somatic index (CSI) was calculated as the ratio between the heart weight and the animal weight, multiplied by 100.
Tissue histology
Abdominal fat samples from SD, HFD, HFD + Lc (20 mg) and HFD + Lc (60 mg)-treated rats were excised and fixed in M.A.W. (methanol:acetone:water = 2:2:1 v/v) solution, dehydrated in graded ethanol, cleared in xylol, embedded in paraplast (Sherwood, St. Louis, USA) and serially sectioned at 8 µm [26]. Sections were placed onto Superfrost Plus slides (Menzel-Gläser, Braunschweig, Germany), dewaxed and stained with hematoxylin and eosin (EE) [26] for a general assessment of tissues. The adipocyte’s area of the abdominal fat was calculated on eight randomly selected nine images for each group, using ZEN Lite 2012-ZEISS. The values represent the mean ± SEM of measurements for the area of each adipocyte present in the image.
In parallel, liver samples from SD, HFD, HFD + Lc (20 mg) and HFD + Lc (60 mg)-treated rats were embedded in OCT, fixed in liquid nitrogen and stored at − 80 °C until use. The sections (7 µm), obtained using a cryostat (Microm HM505E) were postfixed with 4% formalin and put in distillated water. Sections were stained with OIL RED O (Bio-Optica) to detect fat vacuoles and counterstained with hematoxylin to localize nuclei.
Blood biochemical measurements
Blood for plasma analysis was collected after the killing and centrifuged at 4 °C for 15 min at 4000g within 30 min of collection. A plasma aliquot was refrigerated at 4 °C for glucose analysis, while the remaining plasma was frozen (− 80 °C) for the subsequent biochemical analyses. Glucose levels were determined using a glucometer (ACCUCHEK, Roche Diagnostics, Germany). The ROS levels were evaluated by a commercial assay kit (Sunred Biological Technology, Shanghai, China); total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides were determined using a kit from PKL® POKLER ITALY; LDL cholesterol-Ox was determined using a kit from Elabscience Biotechnology (WuHan, PRC); insulin levels were evaluated through a specific Millipore kit (St. Charles, MO, USA).
HOMA-IR: homeostatic model assessment-estimated insulin resistance
The homeostatic model assessment (HOMA) is an index of basal insulin sensitivity and therefore of insulin resistance (HOMA-IR). It was calculated according to Matthews et al. [27]. Control rats (SD group) were assumed to have an average HOMA-IR of 1. This index was computed from the product of fasting plasma glucose (FPG) and fasting plasma insulin (FPI) levels, divided by a constant as follows: HOMA-IR = [FPG (mUI/mL) × FPI (mg/dL)]/2.430 [28].
Western blot analysis: gluconeogenic/gluconeogenesis enzymes
Livers from each experimental group were dissected and homogenized in ice-cold RIPA lysis buffer (Sigma-Aldrich, Saint Louis, USA) containing a mixture of protease and phosphatase inhibitors (1 mmol/L aprotinin, 20 mmol/L phenylmethylsulfonyl fluoride, and 200 mmol/L sodium orthovanadate). Samples were centrifuged at 15,000g at 4 °C for 25 min and the supernatants were collected for immunoblotting analysis. The protein concentration was determined using the Bradford method according to the manufacturer’s instructions (Sigma-Aldrich, Saint Louis, USA). Equal amounts of proteins (30 µg) were separated on 10% SDS-PAGE, transferred to a PVDF membrane (RPN303F, GE Healthcare), blocked with nonfat dry milk and incubated overnight at 4 °C with anti-phosphoenolpyruvate carboxykinase (PEPCK) (1:1000) or anti-glucose-6-phosphatase-α (G6Pase-α)(1:250) (Santa Cruz Biotecnology, Inc., USA), used as primary antibodies. An anti-mouse IgG peroxidase-linked secondary antibody (1:1000) was used (Santa Cruz Biotecnology, Inc., USA). An anti-β-actin primary antibody (1:1000) was used as loading control (Santa Cruz Biotechnology, Inc., USA). The chemiluminescent signal of the blots was detected by the enhanced chemiluminescence system (GE Healthcare, Milan, Italy). After immunoblots digitalization, densitometric analysis of the bands was performed. The areas and the pixel intensity, represented by 256 gray values (0 = white; 256 = black), were evaluated. The background was subtracted. NIH IMAGE 1.6 was used (National Institutes of Health, Bethesda, Maryland, USA).
Statistics
Statistical analysis was performed using the GraphPad Prism Software® (version 5.0; Graph Pad Software, San Diego, CA, USA). Data were expressed as the mean ± SEM. One-way ANOVA (analysis of variance) Newman Keuls multiple comparison test was used. Statistically significant differences: *p = < 0.05, **p = < 0.01, ***p = < 0.001.
Results
Total phenols and flavonoids, and antioxidant activity
Total phenolics are expressed as chlorogenic acid equivalents (CAE) per gram of fresh material (FW) (mg CAE/g FW). The total phenol content in the bulbs of L. comosa was 102.89 ± 0.21 mgCAE/g of FW (n = 5). Total flavonoids are expressed as quercetin equivalents (QE) per gram of fresh material (FW) (mg QE/g FW). The total flavonoid content was 28.07 ± 0.88 mg QE/g of FW (n = 5). The IC50 free radical scavenging activity (DPPH, used as positive control) was 10.2 ± 0.2 µg/mL (n = 5). The IC50 of antilipoperoxidant activity, measured by BCB assay, for ascorbic acid was: 1.50 ± 0.10 µg/mL. The IC50 of bulb extracts was: 10.80 ± 0.74 µg/mL (β-carotene 30 min) and 81.4 ± 1.28 µg/mL (β-carotene 60 min) (n = 5 for each test).
Phytochemical profiling
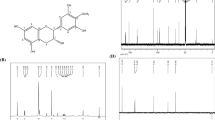
Previous investigations on the bulbs of L. comosa identified two different homoisoflavanones classes: those with a 3-benzyl-4-chromones structure and those belonging to the scillascillin type (Fig. 1) [29]. However, no data regarding the distribution of these unusual derivatives have been reported in the literature. For this reason, in this study, the phytochemical profile of the hydroalcoholic extract from L. comosa bulbs was preliminarily investigated using a combination of different analytical techniques.
Basic structures of homoisoflavonoids belonging to the a 3-benzyl-4-chromones and to the b scillascillin type previously identified in L. comosa bulbs. R1–R6 = H–, OH–, or CH3O– (see ref. [29] for details)
The HPLC–UV–DAD profile (Fig. 2) presented several chromatographic peaks generated by substances with UV spectra with absorption maxima at around λmax = 290 nm, consistent with the presence of poly-hydroxylated aromatic rings as major chromophores. The absence of peaks with absorption maxima at higher λ values indicated the minimal contribution of flavone and flavonol structures (λmax = 330–390 nm) to the bulbs’ total composition.
These results were consistent with those from the GC–MS analysis (Fig. 3a), in which a series of peaks is generated by substances sharing a diagnostic fragment ion at m/z 179 [C10H15OSi]+, detected in the RT range 49.7 < RT < 45 min. This fragment ion is typically generated from species bearing O-silylated cresoyl moieties (Fig. 3b), such as those present in the 3-benzyl-4-chromones homoisoflavones previously reported in L. comosa bulbs.
The peak at RT = 49.94 min showed the presence of the fragment ion at m/z 179 (base peak) with additional minor, but diagnostic fragment ions at m/z 307 and m/z 337 (− 30 u.m.a, neutral loss of CH2O). These results, merged with those previously reported in literature, were consistent with the proposed structure of compound 1 (see Fig. 3c).
The fragmentogram of compound 2, generating the major peak at RT = 51.53 min, showed the presence of a diagnostic fragment at m/z 209 [C11H17O2Si]+ consistent with the presence of one additional methoxyl group of the above-reported trimethyl-silylated moieties (see Fig. 3c).
In vitro enzymatic assays
Pancreatic lipase inhibition assay
L. comosa bulb extracts showed a significant inhibitory action on pancreatic lipase. The lipase inhibitory effects are indicated by the IC50 values of 70.5 ± 0.89 µg/mL (R2 = 0.997). The activity is comparable to the inhibitory action of orlistat used as control drug (IC50 = 57.20 ± 0.19 µg/mL) (n = 5 for each test).
Pancreatic α-amylase inhibition assay
The in vitro inhibitory effect of L. comosa bulb extracts was evaluated on porcine pancreatic amylase activity. The IC50 value was 46.3 ± 0.23 µg/mL (R2 = 0.989) and comparable to the inhibitory activity of acarbose, used as control drug (IC50 = 36.50 ± 0.32 µg/mL) (n = 5 for each test).
Anthropometric and metabolic variables
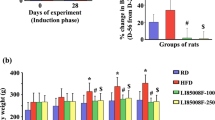
The body weight of all animals at the beginning of the treatment was 250 ± 2 g. After 12 weeks of treatment with the different diets, the final mean body weight of rats fed HFD was higher than SD, but significantly decreased when Lc (20 mg) or Lc (60 mg) were given together with the HFD (Fig. 4a). The waist circumference of all animals at the beginning of the experimental protocol was 16.6 ± 0.2 cm. After 12 weeks, the waist circumference of the HFD group was significantly higher than in Lc (20 mg) and in Lc (60 mg) groups (Fig. 4b).
a Body weight of rats fed SD, or HFD, or HFD + Lc (20 mg), or HFD + Lc (60 mg) at the end of the treatment. n = 5 for each group; significance of difference: *p < 0.05, **p <0.01. b Waist circumference of rats fed SD, or HFD, or HFD + Lc (20 mg), or HFD + Lc (60 mg) at the end of the treatment. n = 5 for each group; significance of difference: *p < 0.05, **p <0.01
Abdominal (Fig. 5a), perirenal (Fig. 5b), epididymal (Fig. 5c) and retroperitoneal (Fig. 5d) fat deposition was significantly elevated in HFD compared to SD, Lc (20 mg) and Lc (60 mg) groups (Fig. 5).
Heart (Fig. 6a) and liver (Fig. 6b) weights and CSI values (Fig. 6c) were increased in HFD animals with respect to SD; the administration of Lc (20 mg) and Lc (60 mg) reversed these alterations (Fig. 6).
No statistical differences were observed in all experimental groups in water intake and body length during the 12 weeks of treatment (Table 1), while food intake significantly decreased in HFD compared to SD and in both HFD + Lc (20 mg) and HFD + Lc (60 mg) with respect to the HFD group (Table 1). BMI significantly increased in the HFD group with respect to the SD, while it was reduced in both HFD + Lc (20 mg) and HFD + Lc (60 mg) groups (Table 1).
Total cholesterol (Fig. 7a), LDL-cholesterol (Fig. 7c), LDL-cholesterol-ox (Fig. 7d), triglycerides (Fig. 7e) and ROS production (Fig. 7f) were higher in HFD with respect to SD, while in Lc (20 mg) and Lc (60 mg) groups they were significantly reduced. On the contrary, HDL-cholesterol levels presented a reverse trend (Fig. 7b).
Plasma determination of: a total cholesterol, b HDL-cholesterol, c LDL-cholesterol, d LDL-cholesterol-ox, e triglycerides, f ROS in rats fed with SD (standard diet) or HFD (high fat diet) or HFD + Lc (20 mg) or HFD + Lc (60 mg) for 12 weeks. n = 5 for each group; significance of difference: *p < 0.05, **p < 0.01, ***p < 0.001
After 12 weeks, the HFD group showed a significant increase in basal glycemia, plasma insulin levels and HOMA-IR index compared to the SD group. The treatment with both Lc (20 mg) and Lc (60 mg) reduced the basal glycemia, the plasma insulin levels and the HOMA-IR index compared to the HFD group (Table 2).
Adipose and liver tissue histology
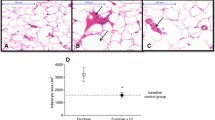
Abdominal fat-stained sections showed, in all conditions, the typical aspect of adipocytes which appear as big cells with peripheral nuclei and a plasma membrane surrounding an empty/clear space (Fig. 8a). Some crown-like structures in HFD adipose tissues were present. Comparison between areas of adipocytes from the different groups showed relevant differences. In particular, an increase in the area in HFD with respect to SD was observed (Fig. 8a). This increment persisted also in the HFD + Lc (20 mg) diet, but a significant decrement after HFD + Lc (60 mg) diet (Fig. 8a) was detected. The liver samples from SD, HFD, HFD + Lc (20 mg) and HFD + Lc (60 mg) rats showed differences in the presence of fat vacuoles (red droplets) (Fig. 8b). In particular, in HFD a strong increase of fat vacuoles was observed (Fig. 8b). The steatosis persisted also in the Lc (20 mg)-treated animals without differences with respect to HFD (Fig. 8b). However, after administration of Lc (60 mg), the fat vacuoles appeared strongly reduced (Fig. 8b). Hepatic triglyceride content was higher in HFD with respect to SD, while in Lc (20 mg) and Lc (60 mg) groups it was significantly reduced (Fig. 8b).
a Sections showing the basic histological features in the SD or HFD, or HFD + Lc (20 mg), or HFD + Lc (60 mg) of the abdominal rat fat stained with hematoxylin–eosin. In the graph, the statistical differences in the adipocyte’s area between different diets have been shown (n = 3 for each group). b Sections illustrating steatosis in the SD or HFD, or HFD + Lc (20 mg) or HFD + Lc (60 mg) of the rat liver stained with hematoxylin–eosin (n = 3 for each group). Liver determination of triglycerides in rats of the SD, or HFD, or HFD + Lc (20 mg), or HFD + Lc (60 mg) groups; significance of difference: *p < 0.05, **p < 0.01, ***p < 0.001
Expression of enzymes of gluconeogenesis: PEPCK and G6Pase
In liver, the expression levels of PEPCK and G6Pase-α, key regulatory enzymes of gluconeogenesis, indicate that in the HFD group the expression of these enzymes significantly increased compared with the SD group. In the HFD + Lc (60 mg) group, the expression of PEPCK and GPase-α was significantly reduced compared with the HFD group (Fig. 9).
Western blots and relative densitometric analysis expressed by the ratio of PEPCK/β-actin and G6Pase-α/β-actin on liver extracts of SD, or HFD, or HFD + Lc (20 mg), or HFD + Lc (60 mg). Changes were evaluated as mean ± SE (n = 3). Significant difference from control values (one-way ANOVA, Newman–Keuls test): *p < 0.05
Discussion
Leopoldia comosa (L.) Parl. is a popular endemic plant growing in all the Mediterranean areas and used in the traditional gastronomy of Southern Italy [10, 12]. For the first time, this work has evaluated the long-term metabolic and anthropometric effects of L. comosa extracts on a high-fat diet-induced obesity rat model, demonstrating the important biological effects of this plant on obesity.
Obesity, together with a high cardiovascular disease risk, is the main feature of a complex illness known as metabolic syndrome (MetS). This pathology is characterized by the combination of altered glucose homeostasis, dyslipidemia and hypertension [30]. An appropriate nutrition is a major determinant for the prevention and the treatment of MetS and it becomes even more important in the presence of obesity, a severe problem in the Western society, often associated with fatal diseases [31]. In fact, a strict interconnection between nutrition and health care has always been proposed. In particular, traditional nutrition of many Mediterranean areas contemplates the consumption of wild plants not only as food, but also as therapeutic agents [32]. On the basis of this information, L. comosa extracts (20 or 60 mg) were orally administered in addition to a high-fat diet to assess whether and to what extent they could counteract the disorders related to MetS. First of all, the present data show a significant inhibitory action of the extract from L. comosa on pancreatic lipase enzymatic activity in vitro.
The inhibitors of nutrients digestion, in an attempt to reduce energy intake through pancreatic lipase and amylase inhibition, represent one of the most important strategies in the treatment of obesity [33, 34]. The inhibition of the lipase enzyme can be exerted in the lumen of the stomach and of the small intestine by forming a reversible/irreversible bond with the active residue site of pancreatic lipases. The inactivation of the lipase inhibits the hydrolyzation of dietary fat into triglycerides, absorbable free fatty acids and monoglycerides [13]. As undigested triglycerides are not absorbed, the resulting caloric deficit may have a positive effect on weight control. For this reason, pancreatic lipase inhibitory properties have been extensively examined for the determination of the potential effect of natural products as antiobesity agents [35].
Previously, Casacchia et al. [13] studied this inhibitory activity by comparing the extracts from raw bulbs and from traditionally cooked bulbs harvested in Calabria (Italy). It was demonstrated that raw bulb extracts exhibit significantly higher activity in the inhibition of lipase and amylase and that this action is lost during technological treatments. Moreover, their phytochemical profile changes depend on the pedoclimatic conditions and the harvesting period.
Of note, the results from the present study provide a strong indication about L. comosa bulb extracts’ ability to inhibit also α-amylase action. α-Amylase is a key enzyme in the digestive system that catalyzes the initial step in the hydrolysis of starch to a mixture of smaller oligosaccharides [36]. It is known that the activity of the human pancreatic α-amylase in the small intestine correlates to an increase in postprandial glucose levels, whose control could represent an important aspect in treatment of type 2 diabetes [36]. The inhibition of pancreatic α-amylase delays carbohydrate digestion, causing a reduction in the rate of glucose absorption and lowering the postprandial serum glucose levels [37]. In this work, a phytochemical screening showed that the main phytochemicals of L. comosa bulb extract activity seem to be generated by homoisoflavonoids of the 3-benzyl-4-chromones type that, according to the results obtained from the evaluation of the antioxidant activity of the extract, confer a strong antioxidant capacity. Two classes of homoisoflavanones have been identified from the bulbs of L. comosa, the 3-benzyl-4-chromones and the scillascillin type.
The wide range of activities observed in vitro for L. comosa bulb extracts may account to their effects on metabolic and “anthropometric” parameters evaluated in HFD rats supplemented with Lc (20 mg) or Lc (60 mg). The HFD is able to alter the balance between introduced and utilized calories, causing firstly overweight and then obesity, responsible for MetS. In the present study, HFD induced an increase of body weight without modifying the body length, resulting in an augmented BMI. Actually, BMI represents a common and simple way to estimate body fat, starting from the assumption that in subjects with the same height, variations in the weight are due to fat mass [2]. According to this consideration, HFD-treated rats showed an increase of fat mass. Increased BMI, fat mass and waist circumference represent strong predictors of pathophysiological conditions such as type 2 diabetes, hypertension and dyslipidemia [2, 38]. The increase of body fat implies notable changes in important physiological functions, dependent on the regional distribution of adipose tissue. For example, generalized obesity alters the total blood volume and then the cardiac function, while adipose distribution around the thoracic cage and the abdomen alters the respiratory function [2]. On the other hand, the intra-abdominal visceral deposition of adipose tissue, as visible in the HFD group, is mainly responsible for the development of hypertension, elevated plasma insulin levels and insulin resistance, diabetes mellitus and hyperlipidemia [2]. In particular, the preliminary evaluation of insulin resistance in the present study was performed using simple parameters, derived from fasting glucose and insulin levels, generating the HOMA-IR index, although tolerance tests for glucose and insulin sensitivity are more appropriate. An increased HOMA-IR index, as recorded in HFD rats, could indicate insulin resistance in the early stage of diabetes and steatohepatitis [27]. This data match with both the increased plasma levels of insulin and glucose of the HFD group as well as with the strong increase in fat vacuoles and adipocyte areas observed by light microscopy, indicative of steatosis and also responsible for the augmented liver weight. In this study, the hepatic changes of two key regulatory enzymes of gluconeogenesis, such as PEPCK and G6Pase-α, were also evaluated [39]. It was found that an impairment of gluconeogenesis promotes hepatic glucose production, aggravating insulin resistance [39] and strongly contributing to the pathogenesis of hyperglycemia and glucose intolerance [40]. In our data, L. comosa reduced the protein expression levels of PEPCK and G6Pase-α. These may reinforce the results, indicating the capacity of L. comosa to improve insulin sensitivity and glucose tolerance modulating hepatic gluconeogenesis.
The increase of total blood volume typical of MetS causes an increase of left ventricular preload and of the resting cardiac output [41]. The heart responds with structural changes, increasing the left ventricular filling, the left ventricular cavity dimension and the wall stress. As detected in the present study, left ventricular mass and CSI augment proportionally the BMI, as myocardial dilatation causes hypertrophy [42]. Taken together, all the effects observed in HFD rats are particularly relevant compared to those obtained in the experimental groups where HFD was supplemented with Lc (20 mg) or Lc (60 mg) bulb extracts.
In this study, both Lc (20 mg) and Lc (60 mg) were able to abolish the adverse effects on the metabolic and anthropometric parameters of HFD animals. A key role of the positive effects elicited by the extracts is certainly played by its antioxidant ability, being able to counteract both ROS and LDL-cholesterol-ox production induced by HFD. Cellular oxidative stress and subsequent oxidative damage are associated with an oversupply of reducing equivalents (i.e., electrons) to metabolic pathways [43]. In fact, high levels of circulating glucose and lipids can result in an excessive supply of energy substrates to metabolic pathways in adipose and non-adipose cells, which in turn can increase the production of reactive oxygen species (ROS) [43]. ROS are essential signaling molecules, but if not well controlled can damage proteins, lipids and DNA. In particular, the subsequent oxidation of LDL-cholesterol and its deposition within the walls of blood vessels lead to a proliferation of inflammatory cells within the arterial wall and formation of atheroma and plaques that gradually narrow the vessel lumen and hamper blood flow, giving rise to atherosclerosis [44].
In conclusion, the main beneficial actions observed for L. comosa bulb extracts, in addition to its antioxidant ability, are its inhibitory effects on lipase and amylase activities.
Actually, all negative side effects of MetS are linked to insulin resistance and subsequent dyslipidemia, caused by an altered balance between energy intake and energy consumption, which, in turn, is caused by wrong nutritional behavior. So, the upstream action of L. comosa bulb extracts, in reducing fat and carbohydrates digestion and absorption in the intestine lumen, could determine, indirectly, all the other beneficial effects.
The assumption that both Lc (20 mg) and Lc (60 mg) are able to counteract the negative effects of HFD on the metabolic and anthropometric parameters paves the way for the use of L. comosa as a natural drug in obesity and MetS therapy. This allows to obtain a natural therapeutic drug without the typical side effects of traditional synthetic medicines.
References
González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA (2017) Obesity. Nat Rev Dis Primers 3:17034. https://doi.org/10.1038/nrdp.2017.34
Kopelman PG (2000) Obesity as a medical problem. Nature 404:635–643. https://doi.org/10.1038/35007508
Mopuri R, Islam MS (2017) Medicinal plants and phytochemicals with anti-obesogenic potentials: a review. Biomed Pharmacother 89:1442–1452. https://doi.org/10.1016/j.biopha.2017.02.108
Sniderman A, Cainflone K (1995) Metabolic disruptions in the adipocyte–hepatocyte fatty acid axis as causes of HyperapoB. Int J Obesity 19:S27–S33
Alpert MA (2001) Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci 321(4):225–236. https://doi.org/10.1097/00000441-200104000-00003
de la Iglesia R, Loria-Kohen V, Zulet MA, Martinez JA, Reglero G, Ramirez de Molina A (2016) Dietary strategies implicated in the prevention and treatment of metabolic syndrome. Int J Mol Sci. https://doi.org/10.3390/ijms17111877
de Freitas Junior LM, de Almeida EB Jr (2017) Medicinal plants for the treatment of obesity: ethnopharmacological approach and chemical and biological studies. Am J Transl Res 9(5):2050–2064
Borgonovo G, Caimi S, Morini G, Scaglioni L, Bassoli A (2008) Taste-active compounds in a traditional Italian food: Lampascioni. Chem Biodivers 5(6):1184–1194. https://doi.org/10.1002/cbdv.200890095
Pieroni A, Janiak V, Dürr CM, Lüdeke S, Trachsel E, Heinrich M (2002) In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytother Res 16(5):467–473. https://doi.org/10.1002/ptr.1243
Pieroni A, Nebel S, Quave C, Münz H, Heinrich M (2002) Ethnopharmacology of liakra: traditional weedy vegetables of the Arbëreshë of the Vulture area in southern Italy. J Ethnopharmacol 81(2):165–185. https://doi.org/10.1016/S0378-8741(02)00052-1
Motti R, Antignani V, Idolo M (2009) Traditional plant use in the Phlegraean Fields Regional Park (Campania, southern Italy). Hum Ecol 37:775–782. https://doi.org/10.1007/s10745-009-9254-1
Loizzo MR, Tundis R, Menichini F, Pugliese A, Bonesi M, Solimene U, Menichini F (2010) Chelating, antioxidant and hypoglycaemic potential of Muscari comosum (L.) Mill. (L.) Mill. bulb extracts. Int J Food Sci Nutr 61(8):780–791. https://doi.org/10.3109/09637486.2010
Casacchia T, Sofo A, Casaburi I, Marrelli M, Conforti F, Statti GA (2017) Antioxidant, enzyme-inhibitory and antitumor activity of the wild dietary plant Muscari comosum (L.). Mill Int J Plant Biol 8:6895. https://doi.org/10.4081/pb.2017.6895
Adinolfi M, Barone G, Belardini M, Lanzetta R, Laonigro G, Parilli M (1984) Homoisoflavanones from Muscari comosum (L.) Mill. Bulbs Phytochem 24:2423–2426. https://doi.org/10.1016/S0031-9422(00)83055-1
Adinolfi M, Barone G, Belardin M, Lanzetta R, Laonigro G, Mangoni L, Parilli M (1985) Three 3-benzyl-4-chromanones from Muscari comosum (L.) Mill. Phytochemistry 24:624–626. https://doi.org/10.1016/S0031-9422(00)84989-4
Adinolfi M, Barone G, Corsaro MM, Lanzetta R, Mangoni L (1987) Glycosides from Muscari comosum (L.) Mill. 7. Structure of three novel muscarosides. Can J Chem 65:2317–2326. https://doi.org/10.1139/v87-387
Adinolfi M, Barone G, Corsaro MM, Mangoni L, Lanzetta R, Parrilli M (1988) Absolute configuration of homoisoflavanones from Muscari species. Tetrahedron 44:4981–4988. https://doi.org/10.1016/S0040-4020(01)86202-5
Marrelli M, Cristaldi B, Menichini F, Conforti F (2015) Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem Toxicol 86:16–24. https://doi.org/10.1016/j.fct.2015.09.011
Marrelli M, Conforti F, Toniolo C, Nicoletti M, Statti G, Menichini F (2014) Hypericum perforatum: influences of the habitat on chemical composition, photo-induced cytotoxicity, and antirad-ical activity. Pharm Biol 52(7):909–918. https://doi.org/10.3109/13880209.2013.872675
Conforti F, Perri V, Menichini F, Marrelli M, Uzunov D, Statti GA, Menichini F (2012) Wild Mediterranean dietary plants as inhibitors of pancreatic lipase. Phytother Res 26(4):600–604. https://doi.org/10.1002/ptr.3603
Kwon YI, Apostolidis E, Kim YC, Shetty K (2007) Health benefits of traditional corn, beans and pumpkin: in vitro studies for hyperglycemia and hypertension management. J Med Food 10:266–275. https://doi.org/10.1089/jmf.2006.234
Ferraz RR, Tiselius HG, Heiberg IP (2004) Fat malabsorption induced by gastrointestinal lipase inhibitor leads to an increase in urinary oxalate excretion. Kidney Int 66:676–682. https://doi.org/10.1111/j.1523-1755.2004.00790.x
Kim JH, Kim OK, Yoon HG, Park J, You Y, Kim K, Lee YH, Choi KC, Lee J, Jun W (2016) Anti-obesity effect of extract from fermented Curcuma longa L. through regulation of adipogenesis and lipolysis pathway in high-fat diet-induced obese rats. Food Nutr Res 60:30428. https://doi.org/10.3402/fnr.v60.30428
Okamura T, Pei XY, Miyoshi I, Shimizu Y, Takanashi-Yanobu R, Mototani Y, Kanai T, Satoh J, Kimura N, Kasai N (2013) Phenotypic characterization of LEA rat: a new rat model of non obese type 2 diabetes. J Diabetes Res. 2013:986462. https://doi.org/10.1155/2013/986462
Panchal SK, Poudyal H, Brown L (2012) Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J Nutr 142(6):1026–1032. https://doi.org/10.3945/jn.111.157263
Garofalo F, Imbrogno S, Tota B, Amelio D (2012) Morpho-functional characterization of the goldfish (Carassius auratus L.) heart. Comp Biochem Physiol A Mol Integr Physiol 163(2):215–222. https://doi.org/10.1016/j.cbpa.2012.05.206
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP (2008) Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab 295(5):E1269–E1276. https://doi.org/10.1152/ajpendo.90207.2008
Mulholland DA, Schwikkard SL, Crouch NR (2013) The chemistry and biological activity of the Hyacinthaceae. Nat Prod Rep 30(9):1165–1210. https://doi.org/10.1039/c3np70008a
Vera Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S (2007) Gender aspects of the role of the MetS as a risk factor for cardiovascular disease. Gend Med 4:162–167. https://doi.org/10.1016/S1550-8579(07)80056-8
Tolpin DA, Collard CD, Lee VV, Elayda MA, Pan W (2009) Obesity is associated with increased morbidity after coronary artery bypass graft surgery in patients with renal insufficiency. J Thorac Cardiovasc Surg 138:873–879. https://doi.org/10.1016/j.jtcvs.2009.02.019
Etkin NL (1996) Medicinal cuisines: diet and ethnopharmacology. Int J Pharmacognosy 34:313–326. https://doi.org/10.1076/phbi.34.5.313.13246
Marrelli M, Loizzo MR, Nicoletti M, Menechini F, Conforti F (2014) In vitro investigation of the potential health benefits of wild Mediterranean dietary plants as anti-obesity agents with α-amylase and pancreatic lipase inhibitory activities. J Sci Food Agric 94(11):2217–2224. https://doi.org/10.1002/jsfa.6544
Pare D, Hilou A, Ouedraogo N, Guenne S (2016) Ethnobotanical study of medicinal plants used as anti-obesity remedies in the nomad and hunter communities of Burkina Faso. Medicines 3:9. https://doi.org/10.3390/medicines3020009
Seyedan A, Alshawsh MA, Alshagga MA, Koosha S, Mohamed Z (2015) Medicinal plants and their inhibitory activities against pancreatic lipase: a review. Evid Based Complement Altern Med. 2015:973143. https://doi.org/10.1155/2015/973143
Eichler HG, Korn A, Gasic S, Prison W, Businger J (1984) The effect of new specific α-amylase inhibitor on post-prandial glucose and insulin excursions in normal subjects and Type 2 (non-insulin dependent) diabetic patients. Diabetologia 26(4):278–281. https://doi.org/10.1007/BF00283650
Tarling CA, Woods K, Zhang R, Brastianos HC, Brayer GD, Andersen RJ, Withers SG (2008) The search for novel human pancreatic α-amylase inhibitors: high-throughput screening of terrestrial and marine natural product extracts. Chem Bio Chem 9:433–438. https://doi.org/10.1002/cbic.200700470
Han TS, van Leer EM, Seidell JC, Lean ME (1995) Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ 311(7017):1401–1405. https://doi.org/10.1136/bmj.311.7017.1401
Sharabi K, Tavares CD, Rines AK, Puigserver P (2015) Molecular pathophysiology of hepatic glucose production. Mol Aspects Med 46:21–33. https://doi.org/10.1016/j.mam.2015.09.003
Barthel A, Schmoll D (2003) Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 285(4):E685–E692. https://doi.org/10.1152/ajpendo.00253.2003
de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M (1981) Obesity and cardiac function. Circulation 64(3):477–482. https://doi.org/10.1161/01.CIR.64.3.477
Lauer MS, Anderson KM, Kannel WB, Levy D (1991) The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA 266(2):231–236. https://doi.org/10.1001/jama.1991.03470020057032
McMurray F, Patten DA, Harper ME (2016) Reactive oxygen species and oxidative stress in obesity-recent findings and empirical approaches. Obesity 24(11):2301–2310. https://doi.org/10.1002/oby.21654
Huang H, Mai W, Liu D, Hao Y, Tao J, Dong Y (2008) The oxidation ratio of LDL: a predictor for coronary artery disease. Dis Markers 24(6):341–349. https://doi.org/10.1155/2008/371314
Funding
This research was supported by “Dottorato di Ricerca in Medicina Traslazionale” (C.T.), “Dottorato di Ricerca in Scienze della Vita” (S.F., R.C., G.M.C., D.A., R.M., T.A) and MIUR of Italy (ex 60%).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Casacchia, T., Scavello, F., Rocca, C. et al. Leopoldia comosa prevents metabolic disorders in rats with high-fat diet-induced obesity. Eur J Nutr 58, 965–979 (2019). https://doi.org/10.1007/s00394-018-1609-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1609-1