Abstract
Purpose
Barley is a low-glycemic index grain that can help diabetic and obese patients. The effect of barley intake depends on the host and the associated gut microbiota. This study investigated the effect of barley intake on the fecal microbiota, caecal biochemistry, and key biomarkers of obesity and inflammation.
Methods
Obese db/db mice were fed diets with and without barley during 8 weeks; lean mice were used as lean controls. Fecal microbiota was evaluated using 16S marker gene sequencing in a MiSeq instrument; several markers of caecal biochemistry, obesity, and inflammation were also evaluated using standard techniques.
Results
Bacterial richness (i.e., Operational Taxonomic Units) and Shannon diversity indexes were similar in all obese mice (with and without barley) and higher compared to lean controls. Barley intake was associated with increased abundances of Prevotella, Lactobacillus, and the fiber-degraders S24-7 (Candidatus Homeothermaceae) compared to both lean and obese controls. The analysis of unweighted UniFrac distances showed a separate clustering of samples for each experimental group, suggesting that consumption of barley contributed to a phylogenetically unique microbiota distinct from both obese and lean controls. Caecal butyrate concentrations were similar in all obese mice, while succinic acid was lower in the barley group compared to obese controls. Barley intake was also associated with lower plasma insulin and resistin levels compared to obese controls.
Conclusions
This study shows that barley intake is associated with a different fecal microbiota, caecal biochemistry, and obesity biomarkers in db/db mice that tend to be more similar to lean controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammals and other animals are made of tissues that have evolved in the presence of trillions of different microorganisms over vast periods of time, a phenomenon that has shaped the characteristics of both forms of life [1]. This microbiota mostly inhabits the mucosal surfaces that are in contact with the exterior world such as the respiratory, reproductive, and digestive systems as well as the skin. The metabolic activity of the microbiota, especially the gut microbiota, is crucial to health but the normal microbe–host homeostasis can be disrupted during the course of disease (e.g., obesity, Inflammatory Bowel Disease) in a complex phenomenon known as microbial dysbiosis [2].
Obesity is a growing health concern that has promoted collaborative interdisciplinary efforts to study the disease in order to help patients ameliorate the clinical signs. Several alternatives do exist for helping patients with obesity-related disorders (e.g., exercise, surgical procedures) but dietary manipulation is an easy and feasible strategy to help relieve symptoms and improve quality of life in these patients [3]. While dietary changes alone are not necessarily a remedy for everyone in all circumstances [4], accumulating evidence shows a potential of dietary interventions to influence health in obese and overweighed patients [5]. Research in this area is valuable for the millions of patients worldwide with body weight disorders and related health problems in both developed and developing countries [6].
Several research groups have examined the efficacy of dietary supplements to prevent and/or modify body weight and metabolism. Examples include prebiotics and probiotics [7] and anti-oxidants such as polyphenols [3, 8]. Since the gut microbiota is closely involved in the digestion and absorption of nutrients, dietary disturbances consequently lead to responses of gut microbial communities [9] that have a direct impact on the immune system [10]. Recent advances in these areas have allowed the emergence of key findings about the behavior of the gut microbial ecosystem under different dietary regimens [11].
Barley is an interesting food that has one of the lowest glycemic indexes, meaning it is one of the food ingredients with the lowest effect of blood glucose levels, while at the same time providing nutrients such as fiber, protein, vitamins, and minerals [12]. Several mechanisms have been proposed to explain the physiological effects of barley, including a delay of intestinal absorption of glucose and lipids and increased excretion of bile acids [13, 14]. Interestingly, barley has also been shown to exert an effect on the gut microbiota in humans and various animal species [15, 16], thus suggesting a potential link between the health benefits associated with consumption of barley and the gut microbial ecosystem. However, additional studies are needed to investigate this phenomenon more deeply especially in a context of obesity-related disorders. Therefore, the objective of this research was to investigate the effect of barley supplementation on fecal microbiota structure, caecal biochemistry, and key obesity biomarkers using an in vivo model of obesity.
Materials and methods
Ethical approval
This study has been approved by the Institutional Animal Care and Use Committee at Washington State University (WSU, IACUC# 04436-001).
Experimental design
Mice (all male, 4–5 weeks of age) were located in a controlled constant environment, visually inspected every day, and received food and water ad libitum throughout the whole study period. Leptin receptor-deficient obese db/db mice (BKS.Cg-+Leprdb/+Leprdb/OlaHsd—fat, black, homozygous) were used as the in vivo obesity model due to its usefulness to study obesity-associated disorders [17]. After 1 week of acclimatization, obese db/db mice were randomly assigned to obese-control group (n = 10) fed with AIN-93G standard diet or barley group (n = 10) fed with a modified AIN-93G diet containing barley as a source of carbohydrates and proteins (barley group, Table 1). Control (i.e., AIN-93G) and experimental diets were offered for a period of 8 weeks. The AIN-93G diet was also used during acclimatization. The approach to formulate the barley diet was to normalize as much as possible carbohydrates, proteins, and caloric density to those contents in the standard diet (AIN-93G) using barley as a source of these nutrients (Table 1). However, both diets (with and without barley supplementation) did not have the exact amount of protein and carbohydrates (Table 1) and such small differences may be sufficient to exert an effect on gut physiology and microbial activity. The BKS.Cg-Dock7m+/+Leprdb/OlaHsd—lean, black, heterozygous mice were used as lean controls (n = 11) for the obese db/db control because they have the closely linked recombinant genes Dock7m+/+Leprdb instead of the +Leprdb/+Leprdb that makes the obese counterparts leptin receptor deficient. Lean controls were fed the standard AIN-93G diet used to feed obese-control mice (Table 1). All mice were purchased from Harlan Laboratories (Kent, WA, USA) and diets were formulated and prepared by Dyets Inc. (Bethlehem, PA, USA), shipped overnight to WSU and maintained at 4 °C for use in animal feeding. In this study, we used the barley variety ‘Havener’ (two-row, hulless, spring food barley variety with the following seed characteristics: protein 13.8%, moisture 11%, β-glucan 6%). Please note that up to 10% of barley β-glucans was well tolerated by Wistar rats without any signs of toxicity [18]. Barley was conventionally grown at Spillman Farm in Pullman, WA, USA. Compared to the more common hulled barley types, which require a pearling process to remove the hull, hulless barley is the whole grain form of barley, in which the hull naturally separates during harvest. Barley grains were ground with a cyclone mill (Udy, Boulder, CO, USA) through 0.5-mm sieving rings to obtain barley flour which was used for diet preparation.
Body weight was recorded once a week and food consumption was recorded every other day. Two mice from the barley-supplemented group died during the last week of the study for unknown reasons (from these animals we were only able to collect feces for analysis of microbiota). At the end of the study (8 weeks), mice were fasted overnight, exposed to inhalation with CO2 overdose until the animal become unconscious, blood was obtained by cardiac puncture, and animals were terminated by cervical dislocation. Blood (~100 to ~500 µL) was collected from n = 8 (barley group), n = 10 (obese control), and n = 11 (lean control), transferred into a tube containing 10 µL of ethylenediaminetetraacetic acid (EDTA) (Sommerville, NJ, USA), and centrifuged at 5000g at 4 °C for 10 min to separate plasma from erythrocytes. The erythrocytes were stored at −80 °C for reactive oxygen species (ROS) determination, and plasma was aliquoted and stored at −80 °C for future use. The liver, heart, kidney, subcutaneous fat, epididymal fat, heart fat, and abdominal fat were dissected and weighed. Liver tissues were flash-frozen in liquid nitrogen, and stored at −80 °C for future analysis. The weight of the cecum and its contents were also recorded for analysis.
Body mass index and adiposity index
Body mass indexes (BMI) were determined by dividing body weight (kg) by body length (m2) [19]. Adiposity index was determined by the sum of weights of abdominal, epididymal, heart, and subcutaneous fat divided by body weight × 100 and expressed as adiposity index [19]. Caloric intake was calculated by multiplying the food intake (g) by caloric density (calories/g) in each diet (Table 1).
Fecal collection and DNA extraction
Fecal samples were collected from terminal colon and rectum in the moment of euthanasia at the end of the study (8 weeks). Total genomic DNA was extracted and purified using bead-beating followed by a commercial DNA extraction kit (QIAamp DNA Stool Mini Kit, Qiagen Inc., Valencia, CA, USA) as shown elsewhere [20, 21]. The concentration and purity of all DNA samples was determined using a NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
PCR and high-throughput sequencing of 16S rRNA genes
The 16S rRNA gene contains both conserved and variable regions that allow for differentiation of bacterial groups. We used primers F515 (5′-GTGCCAGCMGCCGCGGTAA-3′) and R806 (5′-GGACTACHVGGGTWTCTAAT-3′) that can amplify the V4 semi-conserved region (~250 bp) as described elsewhere [22]. Sequencing was performed using an Illumina MiSeq instrument at the Genome Center DNA Technologies Core at the University of California Davis as described previously [20, 21]. All raw 16S sequences are available at the Sequence Read Archive (SRA, NCBI) under accession numbers: PRJNA281761 and PRJNA314690. The trim.seqs command in MOTHUR [23] was utilized for splitting raw fastq files for uploading data to SRA.
Bioinformatics
Raw fastq files were demultiplexed (i.e., assignment of reads to samples), quality filtered, and analyzed using default scripts and parameters in Quantitative Insights into Microbial Ecology (QIIME) v. 1.8.0 [24, 25]. Operational Taxonomic Units (OTUs) were assigned using two approaches. First, using the open-reference algorithm described by Rideout et al. [26] for alpha and beta diversity analyses. This open approach is very useful at assigning sequences to groups because it does not necessarily discard sequence data when there is no match with databases. UCLUST [27] was used to classify sequences into groups and also to assign taxonomy (this is the default method in the pick_open_reference_otus.py QIIME script). Second, using a closed reference algorithm as currently required by PICRUSt (see Prediction of metabolic profiles below). The Greengenes 13_5 97% OTU representative 16S rRNA gene sequences was used as the reference sequence collection [28].
Prediction of metabolic profiles
The Galaxy platform of Phylogenetic Investigation of Communities by Reconstruction of Unobserved states (PICRUSt, [29]) was used to predict the functional profile of the microbial communities using the OTU table from the closed reference algorithm described above. The current galaxy version of PICRUSt supports several types of functional predictions; in this study, we used the Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthologs [30].
Measurement of short-chain fatty acids (SCFAs) in caecal contents
Fresh distal caecal contents were collected from all mice at the end of the study (8 weeks) and SCFAs were quantified using a High-Performance Liquid Chromatography(HPLC)-Photo-diode array (PDA) analysis system as reported elsewhere [20, 21, 31].
Obesity biomarkers in plasma
Analysis of plasma was performed in a subset of samples (n = 4 to n = 11) due to limitations in collected blood volumes; therefore, each specific analysis was carried out in a different subset of samples depending on several factors including the relevance of each analysis for the study and the amount of material (plasma in μL) needed to perform the analysis. Plasma glucose, triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), and total cholesterol concentrations (Total-c) were measured using commercial kits according to manufacturer’s protocol (Wako Diagnostics, Richmond, VA, USA). Low-density lipoprotein (LDL-c) and very-low-density lipoprotein (VLDL-c) were determined according to [32] (LDL-c = Total-c − HDL-c − TG/5; VLDL-c = TG/5). Plasma levels of lipid peroxidation were measured using the thiobarbituric acid reactive substance (TBARS) assay (Cayman Chemical Co. Ann Arbor, MI, USA), according to the manufacturer’s protocol. The level of lipid peroxidation was expressed as μM malondialdehyde (MDA). Protein carbonyls in plasma were determined using the 2,4-dinitrophenylhydrazine (DNPH) reagent as reported previously [33] and adapted to microplate. Briefly, 25 µL of plasma were mixed with 25 µL 10 mM of DNPH in 2.5 M HCl, incubated at room temperature, and vortexed every 5 min. Protein carbonyls were precipitated with 12.5 µL of 10% w/v trichloroacetic acid (TCA) on ice-bath for 30 min followed by centrifugation at 9000g for 15 min (4 °C). Pellets were washed three times with cold ethanol/ethyl acetate solution (1:1 v/v) to eliminate the excess of DNPH and dissolved in 100 µL of 6 M guanidine-HCl. Absorbance was measured at 370 nm against a blank without DNPH. Protein carbonyls were determined using extinction coefficient of DNPH (ε = 22,000 mol/L/cm) and expressed as nmol/mg protein quantified by Bradford assay (Bio-Rad, Hercules, CA, USA). Plasma levels of interleukin (IL)-6, plasminogen activator inhibitor-1 (PAI-1), leptin, insulin, and resistin were quantified with the multiplex magnetic bead-based immunoassay Luminex system using the Milliplex mouse adipokine kit (Millipore, Billerica, MA, USA), according to the manufacturer’s protocol. Data were analyzed using Luminex xPonent 3.0 software (Austin, TX, USA).
ROS in erythrocytes
Blood erythrocytes were analyzed for production of ROS using a fluorometric assay that is based on the oxidation of the fluorochrome, 2′,7′-dichlorofluoroscein-diacetate (DCFH-DA; Sigma-Aldrich, St Louis, MO, USA) as previously reported [34]. Briefly, 900 μL of phosphate-buffered saline (PBS), pH 7.4 was used to wash the erythrocytes (100 µL), followed by centrifugation at 1500g for 5 min. The supernatant was discarded and condensed erythrocytes were diluted with PBS to a final content of 5% erythrocytes. Aliquots of 50 μL of erythrocyte suspension were added to a 96-well plate followed by 50 μL of 100 mM of DCFH-DA. The rate at which DCFH-DA was oxidized by intracellular ROS to DCF was determined fluorometrically. Fluorescence at 484 nm extinction and 535 nm emission was determined after 60-min reaction. Results were expressed in arbitrary relative fluorescence units (RFU).
Liver cholesterol
Liver cholesterol was extracted as previously reported [35] with minor modifications. Briefly, frozen liver samples were weighed and ground with liquid nitrogen into a homogenous powder in a mortar. Approximately 100 mg of ground tissue was added to 2 mL of chloroform/methanol (2:1 v/v) solution and homogenized in high speed homogenizer (Tekmar TR-10, Vernon, BC, Canada) for 2 min at 60% output. The mixture was centrifuged at 3000 rpm at 4 °C for 20 min. Supernatants were collected into 500-µL Eppendorf tube and mixed with 100 µL deionized water followed by centrifugation for 30 s at 3000 rpm at room temperature. The supernatant (methanol-water) was discarded and the chloroform phase was evaporated in a vacuum evaporator. The dry residue was resuspended in 100 μL isopropanol to measure total cholesterol using a commercial kit (Wako Diagnostics, Richmond, VA, USA). Liver weight values were used to calculate total liver cholesterol levels (mg cholesterol/liver).
Statistical analysis
Unless otherwise noted, the non-parametric alternative Kruskal–Wallis test was used to compare our dependent variables (e.g., final body weights, obesity biomarkers) in PAST [36]. Multiple comparisons were performed using Mann–Whitney tests and adjustment for multiple comparisons was performed using the Bonferroni method. In an effort to investigate possible differences in body weight gain over time, the MIXED procedure in SAS University Edition was used to analyze the dependent variable body weight with the independent variables “treatment” as a discrete fixed effect and “week” as a continuous explanatory covariate in a mixed model. This additional statistical analysis is very important because dietary ingredients may not be necessarily related with differences in final body weights (i.e., at the end of the study) but instead with delays in body weight gain over time, as recently shown by our research group [21]. An Analysis of Similarities (ANOSIM) and the Adonis test were used to test for clustering of microbial communities using both weighted and unweighted UniFrac distance matrices in QIIME. The difference between weighted and unweighted UniFrac is that the former uses information about the number of sequences as well as the phylogenetic information contained in the sequences, while the unweighted UniFrac does not take into account the number of sequences. Using both metrics is very useful to inform about the factors that structure microbial communities [20, 21, 37, 38]. The Linear Discriminant Analysis (LDA) Effect Size (LEfSe) method was used to investigate differences in microbial communities using a LDA score threshold of three [39]. Principal Coordinate Analysis (PCoA) was performed in PAST using the weighted and unweighted distance matrices obtained from QIIME. STAMP [40] was used to analyze the PICRUSt data with ANOVA and False Discovery Rate. An alpha of 0.05 was used to reject null hypotheses.
Results
Physical and dietary parameters of mice
Body weight of all obese mice (with and without barley) was similar (median = 47.3 g for supplemented mice; median = 45.6 g for obese controls) at the end of the study (8 weeks) and both were significantly higher compared to lean mice (median = 30 g, P < 0.0001, Kruskal–Wallis test). While food consumption was higher in barley group (average 5.9 ± 0.87 g food/animal/day) compared to obese-control group (4.4 ± 0.4 g food/animal/day) and to lean control (3.0 ± 0.1 g food/animal/day) (P < 0.001, Kruskal–Wallis test), the body weight gain was similar between obese control and barley throughout the study period (Fig. 1). Interestingly, the BMI in barley group was higher compared to obese control and lean control but this phenomenon was not due to body fat accumulation since barley group and obese control had similar adiposity indexes (Supplementary Table S1). The higher BMIs in barley-supplemented mice (compared to obese controls) was due to a higher body weight (although not statistically significantly, P = 0.3922 at the end of the study, Mann–Whitney test) and a relatively lower body length (also not statistically significant when compared to obese controls, P = 0.2166, Mann–Whitney test), which may be related to the difference in caloric intake (Table 1; Table S1). Consumption of barley was associated with higher cecum weight and cecum content compared to obese-control group (Table S1). Liver, heart, and kidney weights were not different among treatment groups (Table S1).
Body weight (g) in lean and obese mice with (barley group) and without (obese-control group) barley supplementation. Despite a significant difference in slopes between the obese groups with and without barley, there was no statistically significant difference in body weight between the barley group and the obese-control group in all time points
Microbial membership composition
All quality-filtered reads were assigned to 5366 OTUs using an open OTU picking algorithm that does not discard sequences for not matching reference databases [26]. As shown in other studies, the fecal microbiota of all mice was comprised mostly by Firmicutes and Bacteroidetes (median >90% of both phyla together) (Fig. 2). Lean mice had the highest abundance of Firmicutes and the lowest Bacteroidetes, while barley-supplemented mice showed opposite abundances of these two major phyla: barley group had ~55% of Bacteroidetes compared to ~25% in lean mice and only ~40% of Firmicutes compared to ~65% in lean mice (Fig. 2). Interestingly, the microbiota associated with barley supplementation did not resemble the obese microbiota either (Fig. 2). The significance of these results for the abundant phyla Firmicutes and Bacteroidetes is at best modest due to the many—often divergent—bacterial groups that constitute each of these phyla at lower taxonomic levels. Other phyla showed low abundances including Actinobacteria, Proteobacteria, and others (Fig. 2).
Composition of fecal microbiota at the phylum level. Please note that the y axis (percentage of sequences) was modified to allow visualization of low abundant groups (e.g., Proteobacteria). Bacteroidetes and Proteobacteria are highlighted using different border styles for better visualization. Asterisks denote groups that were significantly different among treatment groups based on a Kruskal–Wallis test (P < 0.05)
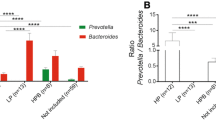
The LEfSe method is useful at finding potentially relevant bacterial groups that are significantly enriched in each treatment group [39]. In this study, each treatment group was associated with higher abundances of distinctive subsets of taxa (Fig. 3; Supplementary Figure S1), thus confirming that barley helped output a unique microbial membership composition that is different from both lean and obese controls. For example, barley intake was associated with increased relative abundances of Prevotella, Lactobacillus, the fiber-degraders S24-7 (Candidatus Homeothermaceae) as well as Mogibacteriaceae (Fig. 3; Fig. S1). The finding that barley intake is associated with a distinctive microbiota is further supported by the separate clustering of bacterial relative abundances for each experimental group (Fig. 4).
Bar plots showing relative abundance of taxa that showed statistical significance according to the LEfSe method. The LEfSe method offers results accordingly to which treatment group showed the highest abundances of a particular bacterial group, and therefore the bacterial groups in A were higher in barley-supplemented mice, the bacterial groups in B were higher in lean mice, and the bacterial groups in C were higher in obese-control mice. Dotted lines represent medians; straight lines represent averages
Diversity analyses
Alpha and beta diversity (see below) were calculated using 1925 sequences per sample because this was the lowest number of sequences obtained in a given sample (minimum = 1925; maximum = 16,869 sequences). There was a significant difference in species richness (i.e., number of OTUs) and the Shannon–Weaver diversity index among treatment groups, with barley group having the highest median numbers of OTUs and lean group the lowest (Table 2). Similarly, PD Whole Tree metrics were higher in the barley group compared to the lean group (Table 2).
UniFrac is a phylogenetic method that allows for determination of differences in microbial communities [37] and has been widely used in Microbial Ecology to date. In this study, PCoA plots of weighted UniFrac distances showed a distinctive cluster of all samples from lean mice independently from obese-control and barley groups (in other words all obese samples clustered together, Fig. 5a), thus suggesting that the abundance of the different bacterial groups are an important determinant to separate microbial communities based on genetic background primordially (in this case obesity). Strikingly, unweighted UniFrac distances showed a distinctive clustering of samples from each one of the three treatment groups (Fig. 5b), strongly suggesting that barley supplementation favored the emergence of microbial communities that are phylogenetically distinctive from both lean and obese. This difference between weighted and unweighted UniFrac is important to highlight because other studies from our research group have shown a clustering of all control samples (lean and obese) separated from whole-wheat-supplemented obese mice using unweighted UniFrac distances [20]. In this study, both ANOSIM and Adonis tests confirmed this clustering of samples for both weighted and unweighted UniFrac distances (P < 0.005), but the variation explained by this grouping was higher in the weighted analysis (52%) compared to the unweighted analysis (only 17% of the variation was explained by diet grouping). This current study and other studies [20, 21, 38] shows that both UniFrac leads to different conclusions about the effect of diet on the gut microbiota, thus highlighting the need for using both versions of UniFrac for explaining the factors associated with different microbial communities.
Predictive metabolic profile
A predictive approach of functional profiles using PICRUSt revealed that obese mice with and without barley supplementation had similar relative abundances of most gene groups compared to lean mice (over 300 gene families are usually detected by this approach). Interesting exceptions include genes related to vitamin B6 metabolism and one carbon pool by folate (higher in barley), bacterial chemotaxis and motility proteins (higher in obese), and biosynthesis of ansamycins (lower in barley) (Fig. 6).
Boxplots showing proportion of sequences (percentages) of PICRUSt predictive features. The comparison among groups of these four features showed statistically significant differences (P < 0.05, ANOVA with False Discovery Rate in STAMP). Medians are denoted by the horizontal line within the boxplot, averages are shown as white stars, and outliers are defined with the symbol + by STAMP
SCFAs in caecal contents
Butyrate and other SCFAs are important for colonic health and integrity [41] and regulation of immune system [42]. In this study, barley was not associated with lean-like butyrate concentrations in caecal contents; in fact, caecal butyrate concentrations were very similar between obese-control and barley groups and both were about 3 times higher compared to lean mice (Table 3). In contrast, the less studied succinic acid showed in this study a much lower concentration in caecal contents of barley-supplemented mice compared to all obese mice (Table 3).
Biomarkers of obesity and inflammation
Using a subset of samples, results from plasma biomarkers of obesity and inflammation showed that the levels of fasting glucose, triglycerides, total-CHL, and LDL-CHL were similar between all obese mice (Supplementary Table S2), even though barley group showed higher BMI compared to obese control (Table S1). Interestingly, barley supplementation was associated with lower levels of insulin in plasma and total cholesterol in liver compared to obese controls (Table S2).
Chronic inflammation is closely linked to obesity-related chronic diseases and dietary interventions have shown to decrease inflammation, with consequences in delaying or preventing the onset of such diseases [43]. In obesity, the overproduction of ROS promotes cell injury and production of pro-inflammatory cytokines, thus contributing to pro-inflammatory signaling pathways [44]. However, plasma levels of inflammatory cytokine IL-6 were similar between the obese groups with and without barley supplementation (Supplementary Table S3), while MCP-1 were below the range of detection (data not shown). In contrast, barley diet decreased significantly ROS levels in blood erythrocytes compared to both lean and obese controls and these results were accompanied with lower levels of protein carbonyls in plasma (Table S3). Barley diet was also associated with lower TBARS levels compared to obese controls (Table S3). This biomarker is linked to oxidative stress and adipose tissue inflammation, plays a role in obesity pathologies, and is associated with disease severity [45]. However, these results should be interpreted cautiously due to the limited plasma volumes that prevented us from analyzing TBARS and other obesity biomarkers in all blood samples collected.
The adipose tissue hormone signaling molecule leptin plays a role in energy homeostasis [46]. The absence of leptin receptor in the Lepr db/db mouse strain used in our study leads to obesity, hyperphagia, neuroendocrine dysfunction, and severe hyperglycemia and insulin resistance. Levels of leptin in the obese (db/db) mice were similar, as related to their genetic mutation, and higher than lean control (Supplementary Table S4). This was consistent with the similar body weight and food intake among obese animals. However, barley supplementation decreased resistin to levels similar to lean animals (Table S4). Resistin links obesity to diabetes due to its action in impairing glucose tolerance and insulin action [47].
Discussion
Barley is a nutritious, heart-healthy whole grain that can reduce blood glucose levels and the risk of diabetes [48], while at the same time providing nutrients such as fiber, protein, vitamins, and minerals [12]. The beneficial effects of barley and other cereals are due to different (often intertwined) mechanisms including a delayed intestinal absorption of glucose and lipids, inhibition of absorption–reabsorption of cholesterol and bile acids, and increased excretion of bile acids [13, 14, 49]. It is also possible that the high viscosity of β-glucan (very high in some varieties of barley) solutions increases the viscosity of the intestinal contents thus reducing absorption of cholesterol [50, 51], and that β-glucan is preferentially fermented in the large intestine by specific microorganisms that produce SCFAs thus impeding cholesterol biosynthesis [13].
This study shows that body weight was not affected by consumption of barley in db/db mice. Interestingly, the BMI in barley group was higher compared to obese control and lean control but this phenomenon was not due to body fat accumulation since all obese mice (with and without barley) had similar adiposity indexes. This is important considering the role of body fat in obesity-related chronic inflammation and the development of insulin resistance [52]. Consumption of barley was also associated with higher cecum weight and cecum content compared to obese-control group, which may be related to the content of dietary fiber in barley (hulled barley contains 17.3% of dietary fiber according to the National Nutrient Database, USDA 2016) [53]. The biological effects of dietary fiber include inducement of caecal fermentation and increase in cecum and stool weights [54]. The increase in cecum contents has been related to β-glucans in barley. Barley diets with 3% β-glucan increased significantly caecal digesta mass and individual and total short-chain fatty acids compared to 0% β-glucan barley [55]. In addition, β-glucans have been shown to cause changes in caecal microbiota and profile of SCFA in rats fed high-fat diets [56].
The metabolism of barley and other food ingredients inside the digestive tract is closely associated with the gut microbiota. Interestingly, several publications have demonstrated an effect of barley on the fecal microbiota of healthy [15] and mildly hypercholesterolemic human subjects [57]. Barley has also been shown to modify the gut microbiota of horses [16], growing pigs [58], and rats fed high-fat diets [56]. To our knowledge, however, there are no studies that have investigated the effect of barley on the gut microbiota of genetically obese mice.
LEfSe analysis revealed interesting differences in bacterial membership in barley-supplemented mice. For example, the poorly studied group of bacteria S24-7 (Candidatus Homeothermaceae) within the Bacteroidetes was higher in barley-supplemented mice. This group is particularly interesting because a recent study also showed that whole wheat was associated with more S24-7 [20], suggesting that this group is susceptible to exposure to whole-grain cereals (and probably other nutrients too) irrespective of the source. In support of this hypothesis, Serino et al. [59] and Shen et al. [60] showed that the abundance of S24-7 is susceptible to diets enriched in gluco-oligosaccharides and dietary protein, respectively. Interestingly, a recent genomic analysis of S24-7 revealed that this group is actually composed by three trophic guilds each roughly defined by increased amounts of enzymes involved in the degradation of specific carbohydrates [61]. Another important group that showed increased abundance in barley-supplemented mice was Prevotella, a relatively well-studied group capable of fermenting β-glucans [62] and other carbohydrates [63]. In fact, one seminal study showed that Prevotella was the bacterial group most closely associated with long-term diets consisting of carbohydrates [11]. It is important to recall that S24-7 and Prevotella are only two members of Bacteroidetes, a vast heterogeneous group of microorganisms [64] that, as a whole, have been associated with body weight conditions. For example, one study showed that Bacteroidetes was lower in obese compared to lean mice [65] although conflicting reports where obese individuals have more Bacteroidetes have been published [66, 67]. These conflicting results are due in part to the heterogeneity within the phylum [64]. Another group of interest that showed higher abundances in barley-supplemented mice is the family Mogibacteriaceae, a member of the Clostridiales. Interestingly, this group has been associated with a phenotype characterized by a high frequency of bowel movements and also a lean body type [68]. Another study showed increases in this group in feces of mice upon cold exposure [69].
Different members of the gut microbiota have historically been associated with beneficial properties (e.g., Lactobacillus and Bifidobacterium spp.). In this study, lean mice had higher fecal Bifidobacterium compared to obese mice with and without barley supplementation, while Lactobacillus was lower in lean mice and higher with barley. The genus Allobaculum was also found to be higher in lean compared to both obese groups. Allobaculum is a poorly studied group of microorganisms that correlate negatively with adiposity [70]. The finding of similar bacterial populations (e.g., Bifidobacterium and Allobaculum) among all obese mice (irrespective of diet) may suggest resilience upon dietary modification and that the population control of these groups is mostly related to host genetics. However, currently we know little about the heritability of gut microbes [71] and therefore these are only speculations that need further evaluation. Diversity analyses revealed that all obese mice (with and without barley intake) showed similar values with regard to alpha diversity measurements (numbers and types of bacteria) and that these values were relatively higher compared to lean controls. This is interesting because a more diverse ecosystem is usually associated with more stability and health in adult animal hosts including humans [72].
In this study, caecal butyrate concentrations were similar between obese-control and barley groups and both were about three times higher compared to lean mice. In high contrast, two recent studies from our research group showed higher and lower concentrations of caecal butyrate during supplementation of whole wheat and quinoa, respectively, compared to obese controls [20, 21]. The fact that different food ingredients have distinct effects on the gut microbiota has also been shown in pigs fed wheat and barley [73]. While our current study did not aim to identify the specific microbial groups responsible for production of butyrate in the gut, it does offer valuable information for future studies of health benefits. In particular, butyrate is important because it has been shown to improve insulin sensitivity and energy expenditure in mice [74] and its close connection with the microbiota–gut–brain axis [75].
Other SCFAs aside butyrate are also of interest for the biomedical community. For example, in this study the less studied succinic acid showed a much lower concentration in caecal contents of barley-supplemented mice compared to obese-control mice. Similarly, this compound was also lower in caecal contents of whole-wheat- [20] and quinoa-supplemented obese mice [21]. Succinic acid can have deleterious effects such as inhibition of large intestine motility and depression of the proliferation rate of colonic epithelial cells [76]. Interestingly, Jakobsdottir et al. [77] showed an increase of this compound in rats fed a high-fat diet and a positive effect of dietary fiber at lowering its concentrations. Based on these observations, the fact that barley helped lower the concentration of this compound in this current study (compared to obese controls) could be considered a positive effect; however, the question remains as to why metabolically healthy lean mice had similar (in fact higher although not statistically) concentrations of succinic acid compared to obese (Zhong et al. [56] showed that the concentrations of succinic acid were correlated with the abundance of Clostridium and Akkermansia, thus suggesting that the concentrations of succinic acid may depend on a consortium of different organisms, a well-known phenomenon for other SCFAs such as butyrate [78]). This is important because high concentrations of succinic acid have been shown in other microbial ecosystems such as the human vagina in the presence of harmful bacterial vaginosis [79]. Interestingly, different studies suggest that the abundance of succinate increases during disruption of normal gut microbial homeostasis, thereby promoting infection by bacterial pathogens [80]. Our results show that barley consumption is associated with much lower concentrations of caecal succinic acid and this deserves scrutiny in further studies.
Barley has one of the lowest glycemic indexes thus offering a good dietary alternative for patients with diabetes, obesity, and associated disorders. In this study, consumption of barley supplementation was associated with lower levels of insulin and total-CHL in liver compared to the obese-control mice. This result may be due to the high viscosity of β-glucan solutions which increases the viscosity of the intestinal contents thus reducing absorption of cholesterol [50]. These results are consistent with a study in which a diet containing ~6% β-glucan from barley flour decreased liver lipids in Zucker diabetic fatty rats [81]. In contrast, another study reported that barley diet (independent of the β-glucan contents) failed to improve the levels of liver lipids in rats fed high-fat diet [82]. This suggests an important difference between genetically obese and diet-induced obese rodent models as shown elsewhere [83].
In this study, barley supplementation decreased plasma resistin, insulin, and protein carbonyls compared to obese controls. This is consistent with reports linking resistin to obesity, inflammation, diabetes, and cardiometabolic diseases due to the effect of resistin in impairing glucose tolerance and insulin action [44, 84]. However, even though resistin plays an important regulatory role in the inflammatory response [84], our results showed that IL-6 was similar in all obese mice. This might suggest that, by the end of the study, mice were not old enough for diabetes progression to raise the inflammatory cytokines as it has been shown in older db/db mice [85, 86]. The effect of barley supplementation on circulating resistin levels might be in part related to its content of folic acid (19–23 µg/100 g) (USDA nutrient database [53]). One study reported that plasma resistin was reduced in obese/diabetic mice by 25% after 4 weeks of supplementing with 71 µg folic acid/kg [87]. In addition, resistin and resistin-like molecules are also produced in the intestinal tract and secreted into circulation [88]. This leads to a relatively unexplored field of research on gut microbiota–intestinal tract–resistin secretion axis. Likewise, the lower levels of oxidative stress in barley-supplemented group quantified through ROS in erythrocytes might indicate changes in gut pathogenic bacteria [89]. The production of ROS by the mitochondria has been recognized as an important target of pathogenic bacteria. An imbalance in microbiota can regulate the mitochondria interaction with the host and may result in a pathogenic state in which excessive mitochondria ROS production regulate gut epithelial barrier acting as feedback loop that aggravates the microbiota–host interaction [89]. Therefore, dietary strategies that decrease ROS might be the cause or consequence of an improved microbiota balance.
Limitations
Barley is an interesting option as a functional food for obese and diabetic patients [13, 14] but there are differences among varieties of barley and processing regimes, thus affecting nutrient composition and consequently any potential health effects on the consumer [90]. Second, each meal and dietary ingredient (e.g., wheat, barley) possess their own unique complex microbiota [91] that when consumed could exert changes on the native gut microbiota of the host. In this study, the barley-associated microbiota was not assessed (i.e., diets were offered without irradiation in an effort to simulate a scenario that reflects barley consumption in real life). Third, we acknowledge that diet supplementation with approximately 88% barley is not relevant to human nutrition. Our study can nonetheless lead to another study in which different doses of barley can be compared to find out the minimum doses in which barley intake is able to promote changes in the gut microbiota. Finally, in this study we used a genetic model of obesity, which often diverges from systems of diet-induced obesity as shown elsewhere [83]. In this regard, diet-induced models of obesity are useful mainly because diet plays an important role in human obesity. However, diet-induced obesity has the disadvantage of altering feeding patterns [92]. Also, different protocols have been published regarding the length of feeding time and the type of diet (i.e., constituent ingredients) to make the animals obese, without clear guidelines [93,94,95]. Moreover, there are cases of resistance to diet-induced obesity, a poorly studied phenomenon [96]. Finally, there are already studies that have investigated the effect of barley on diet-induced obesity animal models [56].
Conclusions
In this study, barley intake was associated with higher abundances of Prevotella, Lactobacillus, and S24-7 as well as divergent proportions of genes related to vitamin B6 metabolism and biosynthesis of ansamycins. Barley supplementation did not have an effect on caecal butyrate compared to obese controls but succinic acid was much lower compared to lean and obese controls. The potential health effects and physiological benefits of barley incorporation in diet are supported by a reduced concentration of plasma insulin, resistin, and decreased oxidative stress measured through levels of ROS in erythrocytes compared to obese controls receiving a standard diet. Thus, the relationship between these important biomarkers to changes in gut microbiota and microbiota metabolites modulated by barley are worth investigating in future studies to aid prevent or treat obesity-related disorders.
References
Shapira M (2016) Gut microbiotas and host evolution: scaling up symbiosis. Trends Ecol Evol 31(7):539–549. doi:10.1016/j.tree.2016.03.006
Clemente JC, Ursell LK, Wegener Parfrey L, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270. doi:10.1016/j.cell.2012.01.035
Rangel-Huerta OD, Aguilera CM, Martin MV, Soto MJ, Rico MC, Vallejo F et al (2015) Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J Nutr 145(8):1808–1816. doi:10.3945/jn.115.213660
Carson TL, Hidalgo B, Ard JD, Affuso O (2014) Dietary interventions and quality of life: a systematic review of the literature. J Nutr Educ Behav 46(2):90–101. doi:10.1016/j.jneb.2013.09.005
Janssen AW, Kersten S (2015) The role of the gut microbiota in metabolic health. FASEB J 29(8):3111–3123. doi:10.1096/fj.14-269514
NCD Risk Factor Collaboration (NCD-RisC) (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurements studies with 19.2 million participants. Lancet 387:1377–1396. doi:10.1016/S0140-6736(16)30054-X
Ojeda P, Bobe A, Dolan K, Leone V, Martinez K (2016) Nutritional modulation of gut microbiota—the impact on metabolic disease pathophysiology. J Nutr Biochem 28:191–200. doi:10.1016/j.jnutbio.2015.08.013
Noratto GD, Garcia-Mazcorro JF, Markel M, Martino HS, Minamoto Y, Steiner JM et al (2014) Carbohydrate-free peach (Prunus persica) and plum (Prunus salicina) juice affects fecal microbial ecology in an obese animal model. PLoS One 9(7):e101723. doi:10.1371/journal.pone.0101723
Howe A, Ringus DL, Williams RJ, Choo Z-N, Greenwald SM, Owens SM et al (2015) Divergent responses of viral and bacterial communities in the gut microbiome to dietary disturbances in mice. ISMEJ 10:1217–1227. doi:10.1038/ismej.2015.183
Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12(1):5–9. doi:10.1038/ni0111-5
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334(6052):105–108. doi:10.1126/science.1208344
Baik BK, Ullrich SE (2008) Barley for food: characteristics, improvement, and renewed interest. J Cereal Sci 48:233–242. doi:10.1016/j.jcs.2008.02.002
Wilson TA, Nicolosi RJ, Delaney B, Chadwell K, Moolchandani V, Kotyla T et al (2004) Reduced and high molecular weight barley β-glucans decrease plasma total and non-HDL cholesterol in hypercholesterolemic Syrian golden hamsters. J Nutr 134(10):2617–2622
Pins JJ, Kaur H, Dodds E, Keenan JM (2007) The effects of cereal fibers and barley foods rich in beta-glucan on cardiovascular disease and diabetes risk. In: Marquart L, Jacobs DR Jr, McIntosh GH, Poutanen K, Reicks M (eds) Whole grains and health. Blckwell, London, pp 75–85
DeAngelis M, Montemurno E, Vannini L, Cosola C, Cavallo N, Gozzi G et al (2015) Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl Environ Microbiol 81:7945–7956. doi:10.1128/AEM.02507-15
Philippeau C, Sadet-Bourgeteau S, Varloud M, Julliand V (2015) Impact of barley form on equine total tract fibre digestibility and colonic microbiota. Animal 9:1943–1948. doi:10.1017/S1751731115001524
Wang B, Chandrasekera PC, Pippin JJ (2014) Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 10(2):131–145. doi:10.2174/1573399810666140508121012
Jonker D, Hasselwander O, Tervilä-Wilo A, Tenning PP (2010) 28-Day oral toxicity study in rats with high purity barley beta-glucan (Glucagel). Food Chem Toxicol 48(1):422–428. doi:10.1016/j.fct.2009.10.034
Jeyakumar SM, Vajreswari A, Giridharan NV (2006) Chronic dietary vitamin A supplementation regulates obesity in an obese mutant WNIN/Ob rat model. Obesity 14(1):52–59. doi:10.1038/oby.2006.7
Garcia-Mazcorro JF, Ivanov I, Mills DA, Noratto G (2016) Influence of whole-wheat consumption on fecal microbial structure of obese diabetic mice. PeerJ 4:e1702. doi:10.7717/peerj.1702
Garcia-Mazcorro JF, Mills D, Noratto G (2016) Molecular exploration of fecal microbiome in quinoa-supplemented obese mice. FEMS Microbiol Ecol 92(7):fiw089. doi:10.1093/femsec/fiw089
Bokulich NA, Thorngate JH, Richardson PM, Mills DA (2014) Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc Natl Acad Sci USA 111(1):E139–E148. doi:10.1073/pnas.1317377110
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB et al (2009) Introducing mothur: open source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Envion Microbiol 75:7537–7541. doi:10.1128/AEM.01541-09
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi:10.1038/nmeth.f.303
Navas-Molina JA, Peralta-Sanchez JM, Gonzalez A, McMurdie PJ, Vazquez-Baeza Y, Xu Z et al (2013) Advancing our understanding of the human microbiome using QIIME. Methods Enzymol 531:371–444. doi:10.1016/B978-0-12-407863-5.00019-8
Rideout JR, He Y, Navas-Molina JA, Walters WA, Ursell LK, Gibbons SM et al (2014) Subsampled open-reference clustering creates consistent, comprehensive OTU definitions and scales to billions of sequences. PeerJ 2:e545. doi:10.7717/peerj.545
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi:10.1093/bioinformatics/btq461
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K et al (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi:10.1128/AEM.03006-05
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. doi:10.1038/nbt.2676
Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M (2012) KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40:D109–D114. doi:10.1093/nar/gkr988
Campos D, Betalleluz-Pallardel I, Chirinos R, Aguilar-Galvez A, Noratto G, Pedreschi R (2012) Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem 135:1592–1599. doi:10.1016/j.foodchem.2012.05.088
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Hawkins CL, Morgan PE, Davies MJ (2009) Quantification of protein modification by oxidants. Free Radic Biol Med 46:965–988. doi:10.1016/j.freeradbiomed.2009.01.007
Alam MA, Sernia C, Brown L (2013) Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J Cardiovasc Pharmacol 61:240–249. doi:10.1097/FJC.0b013e31827cb600
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:1–9
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi:10.1128/AEM.71.12.8228-8235.2005
Lozupone CA, Hamady M, Kelley ST, Knight R (2007) Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol 73:1576–1585. doi:10.1128/AEM.01996-06
Segata N, Izard J, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. doi:10.1186/gb-2011-12-6-r60
Parks DH, Beiko RG (2010) Identifying biologically relevant differences between metagenomic communities. Bioinformatics 26:715–721. doi:10.1093/bioinformatics/btq041
Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA (2006) Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243
Corrêa-Oliveira R, Fachi JL, Vieria A, Sato FT, Vinolo MA (2016) Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol 5:e73. doi:10.1038/cti.2016.17
Egger G, Dixon J (2009) Should obesity be the main game? Or do we need an environmental makeover to combat the inflammatory and chronic disease epidemics? Obes Rev 10(2):237–249. doi:10.1111/j.1467-789X.2008.00542.x
Fresno M, Alvarez R, Cuesta N (2011) Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem 117(3):151–164. doi:10.3109/13813455.2011.562514
Venkatesan V, Madhira SL, Malakapalli VM, Chalasani M, Shaik SN, Seshadri V et al (2013) Obesity, insulin resistance, and metabolic syndrome: a study in WNIN/Ob rats from a pancreatic perspective. Biomed Res Int. doi:10.1155/2013/617569
Triantafyllou GA, Paschou SA, Mantzoros CS (2016) Leptin and hormones: energy homeostasis. Endocrinol Metab Clin N Am 45:633–645. doi:10.1016/j.ecl.2016.04.012
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM et al (2001) The hormone resistin links obesity to diabetes. Nature 409:307–312. doi:10.1038/35053000
Nilsson AC, Johansson-Boll EV, Björck IME (2015) Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: a randomised cross-over study in healthy middle-aged subjects. Br J Nutr 114(06):899. doi:10.1017/S0007114515002524
Anderson JW, Bridges SR (1993) Hypocholesterolemic effects of oat bran in humans. In: Wood PJ (ed) oat bran. American Association of Cereal Chemists, St. Paul, pp 139–157
Tong LT, Zhong K, Liu L, Zhou X, Qiu J, Zhou S (2015) Effects of dietary hull-less barley β-glucan in the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem 169:344–349. doi:10.1016/j.foodchem.2014.07.157
Wursch P, Pi-Sunyer FX (1997) The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereal rich in beta-glucan. Diabetes Care 20:1774–1780
Bluher M (2016) Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci 130:1603–1614. doi:10.1042/CS20160005
USDA Food Composition Databases. National Nutrient Database for Standard Reference Release 28, released September 2015, slightly revised May 2016. https://ndb.nal.usda.gov/ndb/ (retrieved on Nov 2016)
McRorie JW, McKeown NM (2017) Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet 117(2):251–264. doi:10.1016/j.jand.2016.09.021
Belobrajdic DP, Jobling SA, Morell MK, Taketa S, Bird AR (2015) Wholegrain barley beta-glucan fermentation does not improve glucose tolerance in rats fed a high-fat diet. Nutr Res 35(2):162–168. doi:10.1016/j.nutres.2014.12.006
Zhong Y, Nyman M, Fåk F (2015) Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res 59:2066–2076. doi:10.1002/mnfr.201500187
Wang Y, Ames NP, Tun HM, Tosh SM, Jones PJ, Khafipour E (2016) High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol 7:129. doi:10.3389/fmicb.2016.00129
Moen B, Berget I, Rud I, Hole AS, Kjos NP, Sahlstrøm S (2016) Extrusion of barley and oat influence the fecal microbiota and SCFA profile of growing pigs. Food Funct 7:1024–1032. doi:10.1039/c5fo01452b
Serino M, Luche E, Gres S, Baylac A, Bergé M, Cenac C et al (2012) Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 61:543–553. doi:10.1136/gutjnl-2011-301012
Shen TC, Chehoud C, Ni J, Hsu E, Chen YY, Bailey A et al (2016) Dietary regulation of the gut microbiota engineered by a minimal defined bacterial consortium. PLoS One 11:e0155620. doi:10.1371/journal.pone.0155620
Ormerod KL, Wood DL, Lachner N, Gellatly SL, Daly JN, Parsons JD et al (2016) Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 4:36. doi:10.1186/s40168-016-0181-2
Hughes SA, Shewry PR, Gibson GR, McCleary BV, Rastall RA (2008) In vitro fermentation of oat and barley derived beta-glucans by human faecal microbiota. FEMS Microbiol Ecol 64:482–493. doi:10.1111/j.1574-6941.2008.00478.x
Kovatcheva-Datchary P, Egert M, Maathuis A, Rajilić-Stojanović M, de Graaf AA, Smidt H et al (2009) Linking phylogenetic identities of bacteria to starch fermentation in an in vitro model of the large intestine by RNA-based stable isotope probing. Environ Microbiol 11:914–926. doi:10.1111/j.1462-2920.2008.01815.x
Hoyles L, McCartney AL (2009) What do we mean when we refer to Bacteroidetes populations in the human gastrointestinal microbiota? FEMS Microbiol Lett 299:175–183. doi:10.1111/j.1574-6968.2009.01741.x
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2015) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102:11070–11075. doi:10.1073/pnas.0504978102
Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P et al (2008) Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 32:1720–1724. doi:10.1038/ijo.2008.155
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al (2009) Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA 106(7):2365–2370. doi:10.1073/pnas.0812600106
Oki K, Toyama M, Banno T, Chonan O, Benno Y, Watanabe K (2016) Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol 16:284. doi:10.1186/s12866-016-0898-x
Ziętak M, Kovatcheva-Datchary P, Markiewicz LH, Ståhlman M, Kozak LP, Bäckhed F (2016) Altered microbiota contributes to reduced diet-induced obesity upon cold exposure. Cell Metab 23:1216–1223. doi:10.1016/j.cmet.2016.05.001
Baldwin J, Collins B, Wolf PG, Martinez K, Shen W, Chuang CC et al (2016) Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J Nutr Biochem 27:123–135. doi:10.1016/j.jnutbio.2015.08.027
Moeller AH, Caro-Quintero A, Mjungu D, Georgiev AV, Lonsdorf EV, Muller MN et al (2016) Cospeciation of gut microbiota with hominids. Science 353:380–382. doi:10.1126/science.aaf3951
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human microbiota. Nature 489:220–230. doi:10.1038/nature11550
Weiss E, Aumiller T, Spindler HK, Rosenfelder P, Eklund M, Witzig M et al (2016) Wheat and barley differently affect porcine intestinal microbiota. J Sci Food Agric 96:2230–2239. doi:10.1002/jsfa.7340
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M et al (2009) Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58:1509–1517. doi:10.2337/db08-1637
Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF (2016) The neuropharmacology of butyrate: the bread and butter of the microbiota–gut–brain axis? Neurochem Int 99:110–132. doi:10.1016/j.neuint.2016.06.011
Inagaki A, Ichikawa H, Sakata T (2007) Inhibitory effect of succinic acid on epithelial cell proliferation of colonic mucosa in rats. J Nutr Sci Vitaminol (Tokyo) 53:377–379. doi:10.3177/jnsv.53.377
Jakobsdottir G, Xu J, Molin G, Ahrné S, Nyman M (2013) High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, live fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One 8:e80476. doi:10.1371/journal.pone.0080476
Louis P, Flint HJ (2009) Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294:1–8. doi:10.1111/j.1574-6968.2009.01514.x
Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R et al (2015) Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 6:164. doi:10.3389/fphys.2015.00164
Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL (2014) Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 16(6):770–777. doi:10.1016/j.chom.2014.11.003
Brockman DA, Chen X, Gallaher DD (2013) Consumption of a high beta-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur J Nutr 52:1743–1753. doi:10.1007/s00394-012-0478-2
Zhong Y, Marungruang N, Fak F, Nyman M (2015) Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low- and high-fat diets. Br J Nutr 113:1558–1570. doi:10.1017/S0007114515000793
Pfalzer AC, Nesbeth PDC, Parnell LD, Iyer LK, Liu Z, Kane AV et al (2015) Diet- and genetically-induced obesity differentially affect the fecal microbiome and metabolome in Apc1638N mice. PLoS One 10:e0135758. doi:10.1371/journal.pone.0135758
Park HK, Kwak MK, Kim HJ, Ahima RS (2017) Linking resistin, inflammation, and cardiometabolic diseases. Koren J Intern Med 32(2):239–247. doi:10.3904/kjim.2016.229
Fujimoto S, Mochizuki K, Goda T (2010) Gene expression of inflammatory cytokines in peripheral leukocytes in db/db mice rose with progression of diabetes. Biosci Biotechnol Biochem 74(7):1488–1490. doi:10.1271/bbb.100149
Ladefoged M, Buschard K, Hansen AM (2013) Increased expression of toll-like receptor 4 and inflammatory cytokines, interleukin-6 in particular, in islets from a mouse model of obesity and type 2 diabetes. APMIS 121(6):531–538. doi:10.1111/apm.12018
Seto SW, Lam TY, Or PM, Lee WY, Au AL, Poon CC et al (2010) Folic acid consumption reduces resistin level and restores blunted acetylcholine-induced aortic relaxation in obese/diabetic mice. J Nutr Biochem 21(9):872–880. doi:10.1016/j.jnutbio.2009.06.015
Shojima N, Ogihara T, Inukai K, Fujishiro M, Sakoda H, Kushiyama A et al (2005) Serum concentrations of resistin-like molecules beta and gamma are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia 48(5):984–992. doi:10.1007/s00125-005-1735-1
Saint-Georgees-Chaumet Y, Edeas M (2016) Microbiota–mitochondria inter-talk: consequence for microbiota–host interaction. Pathog Dis 74(1):ftv096. doi:10.1093/femspd/ftv096
Ames NP, Rhymer CR (2008) Issues surrounding health claims for barley. J Nutr 138:1237S–1243S
Lang JM, Eisen JA, Zivkovic AM (2014) The microbes we eat: abundance and taxonomy of microbes consumed in a day’s worth for three diet types. PeerJ 2:3659. doi:10.7717/peerj.659
Blancas-Velazquez A, Mendoza J, Garcia AN, la Fleur SE (2017) Diet-induced obesity and circadian disruption of feeding behavior. Front Neurosci 11:23. doi:10.3389/fnins.2017.00023
Hariri N, Thibault L (2010) High-fat diet-induced obesity in animal models. Nutr Res Rev 23(2):270–299. doi:10.1017/S0954422410000168
Mercer JG, Archer ZA (2008) Putting the diet back into diet-induced obesity: diet-induced hypothalamic gene expression. Eur J Pharmacol 585(1):31–37. doi:10.1016/j.ejphar.2007.11.077
Mobbs CV, Mastaitis J, Yen K, Schwartz J, Mohan V, Poplawski M et al (2007) Low-carbohydrate diets cause obesity, low-carbohydrate diets reverse obesity: a metabolic mechanism resolving the paradox. Appetite 48(2):135–138. doi:10.1016/j.appet.2006.06.007
Ding C, Guo J, Su Z (2015) The status of research into resistance to diet-induced obesity. Horm Metab Res 47(6):404–410. doi:10.1055/s-0034-1395584
Acknowledgements
DAM acknowledges the Peter J. Shields Endowed Chair. The authors would like to express their deepest gratitude to the QIIME and PICRUSt Help Forums for all the support provided. The authors would also like to thank Alejandra Mencia for her technical assistance in the analysis of blood parameters.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garcia-Mazcorro, J.F., Mills, D.A., Murphy, K. et al. Effect of barley supplementation on the fecal microbiota, caecal biochemistry, and key biomarkers of obesity and inflammation in obese db/db mice. Eur J Nutr 57, 2513–2528 (2018). https://doi.org/10.1007/s00394-017-1523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1523-y