Abstract
Introduction
Long-chain polyunsaturated fatty acids, the most abundant fatty acids in the brain, are essential for the growth and development of the brain and the retina.
Objective
To evaluate the effect of fish oil supplementation on the development (primary outcome) and growth of 4- and 6-month-old infants.
Methods
In this triple-blind randomized controlled trial, 150 pregnant women aged 18–35 years, who were referred to healthcare centres of Tabriz-Iran, were randomly allocated into two groups. One group of women consumed fish oil supplementation (containing 120 mg docosahexaenoic acid and 180 mg eicosapentaenoic acid) daily, while the other consumed a placebo from the 20th week of pregnancy till 30 days after childbirth in a parallel design by a computer-generated block randomization scheme. The neurodevelopment of infants was the primary outcome; it was assessed using the ages and stages questionnaire (ASQ) at 4- and a-6 months of age. The growth of these infants was measured using weight, length and head circumference. The participants, the caregivers, and those assessing the outcomes were blind to the group assignment.
Results
Only one woman in the placebo group discontinued the intervention because of persistent severe nausea. All 75 neonates aged 4- and a-6 months in the fish oil supplementation group, along with 73 and 71 neonates aged 4 and 6 months, respectively in the placebo group, were followed and analysed. Although the mean scores of neurodevelopment at the end of 4 and 6 months were higher in the supplemented group than in the placebo group in each ASQ domain, a statistically significant difference was observed only in the communication domain at the 4th month (adjusted mean difference 2.63; 95% confidence interval 0.36–4.89). There was no significant difference in weight, length, or head circumference between the two groups of infants aged 4 and 6 months (P ≥ 0.05).
Conclusion
Based on the results, perinatal fish oil supplementation is beneficial for the communication domain of neurodevelopment of 4-month-old infants. The study results relating to the supplementation effect on other domains are inconclusive. There ought to be further studies with up-to-date lipidomic analysis to find biochemical correlate compared to an intervention and developmental finding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growth of the foetus and small infants, especially their brains during the last trimester of pregnancy and the first year of life, is exceptionally fast and depends on the quality of their environment and the mother’s nutrition [1].
Fish oil contains a long chain of omega-3 fatty acids of docosahexaenoic acid (DHA) (22:6 n-3) and eicosapentaenoic acid (EPA) (20:5 n-3) [2]. Long-chain polyunsaturated fatty acids (LCPUFAs) particularly DHA, are the most abundant fatty acids in the brain; they are essential for the growth and development of the brain and the retina [3]. The known functions of DHA are neurogenesis, neurotransmission, and protection of the brain and the retina from oxidative agents [4]. DHA, during the second half of pregnancy and during the first 2 years of life, accumulates in the brain [5]. DHA is transferred from the mother during pregnancy through the placenta and after birth via breast milk. The maternal serum concentration of DHA is reduced after childbirth significantly; it is dependent on the mother’s intake. Therefore, a dietary source rich in DHA guarantees higher levels of DHA in breast milk [6, 7]. DHA can also be synthesized in the body from the essential fatty acid (EFA) alpha-linolenic acid (ALA) in very limited amounts [8, 9]. Most studies estimate that the conversion of ALA to EPA is about 5% with less than 1% into DHA [10]. The omega-3 precursor (ALA) is found in some plant sources such as walnuts and flax seeds [11]. However, fish oil (FO) and marine oils derived from algae contain a longer chain omega-3 of DHA and EPA [2, 12].
The European Commission and the International Society of the Study of Fatty Acids and Lipids specifically suggest that pregnant and breastfeeding mothers should receive at least 200 mg of DHA daily [13]. During pregnancy, the required amount of omega-3 to support the normal development of foetal brain and eye probably increased [14]. Those infants whose mothers were supplemented with 200 mg of DHA daily for 4 months after childbirth obtained significantly higher Bayley psychomotor development index score after 30 months [6]. Some of the trials related to the intake of FO during pregnancy have shown that it helps subsequent mental development of infants and children, but the results were not convincing and also inconsistent [2]. Currently, there is insufficient evidence to support or refute LCPUFA supplementation for lactating mothers to promote child growth and development [4]. A cochrane systematic review concluded that the most randomized controlled trials (RCTs) on prenatal FO supplementation have been performed in the high-income countries. Thus, the findings might not be generalized to the population of low- and middle-income countries [2]. Therefore, this study aimed to evaluate the effect of perinatal FO supplementation on the growth and neurodevelopment of 4- and 6-month-old infants in Tabriz, Iran.
Materials and methods
Study design and participants
This was a triple-blind randomized placebo-controlled trial carried out in Tabriz, Iran. The study population included 4- and 6-month-old infants whose mothers participated in the trial. The mothers’ inclusion criteria were the following: being pregnant within the age of 18–35 years in first to fifth gravidity, being at the first half of gestation with singleton, low-risk pregnancy, having a household health record in recruitment healthcare centres, consent to participate in the study, ability to read and write, and having a fixed cell phone number. The exclusion criteria were: history of allergy to FO or other fish products, consumption of fish more than two times a week, taking anticoagulants, and pre-pregnancy body mass index of more than 30 kg/m2.
The eligibility criteria for the infants were the following: alive infants at 4 and 6 months of age, absence of major congenital anomalies, and obvious gastrointestinal or metabolic disorders.
The primary outcome measure was the infants’ neurodevelopment status at the end of 4 and 6 months; the major secondary outcome parameter was the absence of neonates in the treatment group. The other secondary outcome parameters were weight, height, and head circumference at birth, 4 and 6 months.
The sample size has been estimated as 75 for each group, considering the results of a study in Iran [15]. It was calculated based on the mean scores for all domains at 4 and 6 months, regarding m1 = 50.26 (mean development score in fine motor domain at 6 months of age with highest SD), m2 = 57.79 (considering at least 15% increase through this intervention), SD1 = SD2 = 12.08, α = 0.05, β = 0.05, two-tailed test, and account for the possibility of up to 10% study drop out, the total sample size was calculated as 150 infants. The other domains in the study [15] resulted in smaller sample size.
Sampling
After obtaining the approval of the Ethics Committee of Research and the vice-chancellor of the Tabriz University of Medical Sciences (92141), and also after registering the study in the Iranian registry of clinical trials (IRCT2013100914957 N1), 5 out of 27 public healthcare centres of Tabriz which had the most referrals for pregnant women were selected. The written informed consent form was signed by all the participants.
Randomization and intervention
The recruited women were randomly assigned into the following two groups: (1) FO daily supplementation capsule about 1000 mg fish oil containing 120 mg of DHA, 180 mg of EPA; (2) placebo (each capsule contained 1000 mg liquid paraffin) with similar shape, size, and weight. The allocation sequence was determined by a computer-generated randomization scheme with the block sizes of four and six and the allocation ratio 1:1. The stratification was done based on gravidity (first, second, or more) so that 1–75 numbers were allocated to nulliparous women and 76–150 to multiparous women. As many as 30 packages were allocated to each centre with sequential numbering (15 for nulliparous and 15 for multiparous women). The FO and placebo capsules were produced by the Zahravi pharmaceutical company in Tabriz, Iran. The daily dose of the FO or placebo capsules was 1000 mg once a day, from the end of the 20th week of pregnancy till 30 days after birth (about 24 weeks, 168 capsules).
The analysis of fish oil capsules’ composition has been given in the following: DHA: 12.15%, EPA: 18.21%, ARA: 1.43%, myristate (14:0): 0.76%, palmitate (16:0): 28.90, trans palmitate (16:1t): 1.18%, cis palmitoleate (16:1c): 9.69%, stearate (18:0): 6.72%. trans oleate (18:1t): 0.04%, oleate (18:1C): 16.95%, linoleate (18:2): 2.03%, arachidate (20:0): 1.56%, linolenate (18:3): 0.09%, CLA 9–11: 0.08%, CLA 10–12: 0.21%.
The cold vapour atomic absorption spectrometry (CV-AAS) method was used to ensure the absence of mercury in the FO capsules; after measuring and examining three capsules three times, no mercury was found in the FO capsules.
To conceal allocation and to preserve blinding, a person not involved in the recruitment and data collection determined the allocation sequence and placed the FO and placebo capsules inside the packages according to the allocation sequence. The capsules were placed into two packets for 10 and 14 weeks of use (containing 70 and 98 capsules). These two packets were placed in opaque, identical, and sequentially numbered packages.
The participants were taught how to take the capsules and cautioned not to forget their medication. The first packet was given in the second pregnancy visit (weeks 16–20), while the second packet was given in the third pregnancy visit (weeks 26–30), after ensuring that the capsules from the previous packet had been consumed. Non-involved staff at the five healthcare centres (Shahidan Ebrahimi; Hafez; Emamieh; Seid Bavafa; Abouzar; Tabriz, Iran) checked out the consumed capsules sheets and daily recording checklist. Additionally, to follow the medication consumption, phone calls were made to the participants in 24 and 34 weeks of pregnancy, and through 3–7 days after birth. The participants, healthcare providers, data collectors, and those assessing the outcomes were blind in this study.
Assessment of study variables
A self-reporting questionnaire was used to collect data about maternal socio-demographic and fertility characteristics. The validity of this tool that is content validity was confirmed by ten academic faculty members. The mothers’ pre-pregnancy height and weight, the newborns’ data such as anthropometric indices at birth, sex, gestational age, and mode of delivery, and the infants’ data regarding anthropometric indices and breastfeeding at the end of 4 and 6 months were obtained from healthcare records.
The neurodevelopment status of 4- and 6-month-old infants were evaluated by means of age and stages questionnaire (ASQ-2). The ASQ second edition is a 30-item, parent-completed developmental screening instrument with age-specific versions from 4 months to 5 years. The versions are organized by age into domains of communication, gross motor, fine motor, problem solving, and personal-social. Each domain was evaluated by six items. The parents report development of specific skills by responding ‘not yet’, ‘sometimes’, or ‘yes’ and are asked open-ended questions regarding general concerns. The ASQ is considered to have failed when the score is two standard deviations below the mean in each area (P < 0.05). In these cases, the infant should be referred for more detailed follow-up and evaluation [16, 17]. The sensitivity and specificity of the ASQ measured in different studies are 75 and 95%, respectively [18]. The questionnaire was translated into Persian, and its validity and reliability was approved [19].
For measuring the serum levels of DHA, EPA, and ARA (Arachidonic acid) at the baseline and 35–37 weeks of pregnancy, 3 cc of fasting blood was taken from the participants. The samples were immediately centrifuged (Hettich, Universal 320, Germany) at 3500 rpm for 10 min and sent to the laboratory at the Faculty of Medicine, Tabriz, Iran for fatty acid analysis. The gas chromatography equipment (Buck Scientific, Model 610) with a 60 m × 0.25 mm × 0.2 μm column (Teknokroma TR-CN 100) was applied, and the area under the curve peak was calculated for measurements. Data were analysed with the Peak Simple Chromatography Software from SRI Instruments. The results were displayed as a percentage of total FAs.
The infants’ weight and height were assessed using a lever scale with a precision of 0.1 kg (Seca, Germany) and a stadiometer table accurate to 0.1 cm in the supine position without shoes and hats (Laica, Italy). The head circumference (HC) was measured using a 2 m long measuring tape (Laica, Italy).
Statistical analysis
The Kolmogorov–Smirnov test was used to confirm the normal distribution of continuous variables. General characteristics were compared between two groups using the independent t test for continuous variables and Chi square and Fisher’s exact test for categorical variables. Analysis of covariance, repeated measures ANOVA, and binary logistic regression were used for testing the differences between the two groups in terms of ASQ scores and anthropometric indices and in terms of the frequency of subnormal neurodevelopmental test, respectively. Assumptions related to model fit consisted of independence of observations, linearity, outliers, homogeneity of variances, homoscedasticity, and normality were assessed.
All analyses were done using the Statistical Package for Social Sciences version 21 and were based on the intention-to-treat approach. P < 0.05 was considered as statistically significant.
Results
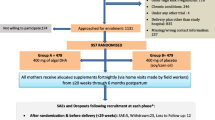
In this study, 652 women were assessed for eligibility; 502 were excluded for not meeting the eligibility criteria (n = 192) or for declining to participate in the study (n = 310). Finally, 150 women were randomly allocated into FO supplementation or placebo groups (75 per group). Two neonatal losses to follow-up occurred at the end of the 4th month in the placebo group: one was because of neonatal death on the third day due to congenital abnormality and the other one was related to family relocation. Additionally, two more infant losses happened at the end of the 6th month in the placebo group (one due to relocation and the other because of the inability to come to the centre). There was no loss of follow-up in the FO supplementation group (Fig. 1). There were no significant differences between the two groups in terms of baseline characteristics. Low birth weight (LBW) as a major secondary outcome was significantly less prevalent in the supplemented group compared to the same in the control group [0 versus 5 (6.7%)]. The two study groups were similar in terms of other characteristics and measurements at birth. The mean gestational age of infants at birth was 39.3 (SD:1.2) weeks (Table 1).
About two-thirds of the women in both groups (67% in fish oil, 73% in placebo group) reported not consuming any fish in the last month. The others consumed fish or its products only once/twice in a week or one to two times per month (P = 0.68).
Maternal serum DHA, EPA, and ARA fatty acid levels
The mean (SD) maternal serum proportion of DHA fatty acid at 35–37 weeks significantly increased in the intervention group [0.57 (0.2) versus 0.39 ± 0.2 placebo group] (adjusted MD = 0.15 [95% CI 0.08–0.23]). The mean (SD) serum proportion of EPA and ARA fatty acids at 35–37 weeks was not different (P = 0.12) (Table 2), as published in previous studies [20].
Neurodevelopmental status
We examined five domains at two ages (ten endpoints) and found a statistically significant association at one age in one domain. Although the mean scores of neurodevelopment were higher in the supplemented group compared to that in the placebo group at 4 and 6 months of age, a statistically significant difference was observed only in the communication domain at 4 months (adjusted MD 2.63; 95% CI 0.36–4.89) (Table 3).
The neurodevelopmental mean (SD) score of 4 out of 5 LBW infants (one infant died on the first day of life) in communication, gross motor, fine motor, problem solving, and personal–social domains were 52.5 (8.6), 51.2 (14.3), 52.5 (6.4), 54.8 (8.2), and 57.5 (2.9), respectively, at 4 months of age. They were 52.5 (6.4), 35.0 (12.9), 53.7 (6.3), 54.7 (7.1), and 40.0 (12.9), respectively, at 6 months.
Three (3.9%) subnormal neurodevelopment tests (below 2 SD less than mean scores) were observed in the FO-supplemented group, as opposed to the eight (11%) observed in the placebo group at the end of the 4th month, across the different domains (adjusted odds ratio 0.33; 95% CI 0.08–1.32).
At the end of the 6th month, four (5.2%) subnormal neurodevelopment tests (below 2 SD less than mean scores) were observed in the FO-supplemented group, while seven (9.8%) were observed in the placebo group across the different domains (adjusted odds ratio 0.33; 95% CI 0.08–1.32). However, these differences were not statistically significant (Table 4).
Anthropometric measures
There were no significant differences between the supplemented and the placebo groups in terms of mean infant weight (adjusted difference, 0.11; 95% CI, −0.07 to 0.28), infant length (adjusted difference; 0.12 95% CI, −0.52 to 0.76), and infant head circumference (adjusted difference, −0.03; 95% CI, −0.38 to 0.30) (Table 5).
Exclusively breastfed infants
There were no significant differences between the supplemented and placebo groups in terms of the frequency of exclusively breastfeeding at 4 months (91% versus 99%, P = 0.17) and at 6 months (85 versus 94%; P = 0.22).
Side events
The reported side events were nausea in 7 (9.3%) participants and unpleasant taste in 6 (8%) women in the fish oil group. These frequencies were 10 (13.3%) for nausea and 10 (13.3%) for unpleasant taste in the placebo group. Vomiting and mild diarrhoea was reported only in 1 (1.33%) participant in the intervention group and stomach pain in 1 (1.33%) in the placebo group. These events occurred mostly in the early use and did not prevent taking the capsules in the fish oil group.
Discussion
Supplementation with fish oil significantly increased the serum levels of DHA. However, the increment of EPA serum levels was not significant.
LCPUFAs, specifically ARA and DHA, accrue rapidly in the grey matter of the brain during development [21,22,23]. DHA is the main component of the nervous system and accumulates quickly in the brain during late pregnancy and early postpartum period [24, 25]. ARA is involved in cell-signalling pathways and cell division; it acts as an inflammatory precursor for eicosanoids [26]. Among 20 edible fatty acids, only omega-3 and omega-6 fatty acids cannot be synthesized in body [27]. Unlike omega-6, omega-3 intake is at a less than favourable level and their ratio is undesirable [27,28,29]—this resembles the case of a previous study on our population where the ratio was 60:1 [20].
DHA is either taken from the diet or may be converted into small amounts from EPA via docosapentaenoic acid (DPA, 22:5 ω-3) as an intermediate [30]. Using higher doses of fish oil supplements may increase significantly the serum EPA and decrease its conversion into DHA. EPA competes with ARA for responsible enzymes of eicosanoid production (cyclooxygenase, lipoxygenase) [31].
In the present study, the consumption of FO supplements (1000 mg/day) from week 20 of pregnancy to 30 days after childbirth significantly improved only the mean score of the communication domain in 4-month-old infants. Although supplementation reduced significantly the rate of LBW [0 versus 5 (6.7%)], it is impossible to statistically compare the development status of LBW infants among groups in this study.
Fat is stored in adipose tissue during early pregnancy, but it is broken later in pregnancy [32], leading to higher levels of free fatty acids in the blood and actively transfer them to the foetus [33]. Stopping this mechanism after delivery, accompanied with cessation of supplementation, may down DHA serum levels and return the newborn to its normal development within 2 months (month 4–6).
Only cross-sectional studies in Iran have used the ASQ questionnaire for screening of children’s neurodevelopment. Delayed development was reported in 11.8% of the normal children aged 4–60 months [34]. It was reported to be 3.69–4.31% in other studies in the developmental domains—the high frequency was related to fine motor and personal-social skills [35], which is consistent with our findings in the placebo group.
Data from observational studies has indicated that omega-3 fatty acid consumption during pregnancy—either in the diet or through supplements—is correlated to enhanced neurodevelopmental outcomes in the children [36,37,38].
However, the results of RCTs on the effect of supplementation with n-3 LCPUFAs on the infant neurodevelopment are contradictory.
Considering the fact that 50–60% of the dry weight of the adult brain is fatty acids (FAs) [39] and a large percentage of these are LCPUFAs, it is likely that the accessibility to specific FAs during development are important for neurodevelopment [40]. High levels of LCPUFAs have been found in the basal ganglia, thalamus, hippocampus, and pre- and post-central cortices in neonates of rats and baboons, suggesting that LCPUFAs affect sensorimotor integration and memory [39, 41]. Recently, studies have suggested that relatively small reductions of DHA could lead to subtle performance defects that are difficult to recognize [5].
Makrides et al., in an RCT conducted in Australia, have concluded that the use of 800 mg/day DHA-rich FO capsules during pregnancy compared to taking three 500-mg/day vegetable oil capsules without DHA in the control group did not result in improved cognitive and language development in the offspring during early childhood [38]. Other researchers have evaluated the effects of DHA, DHA + AA, or placebo during pregnancy and lactation on neurodevelopment at 18 months. Maternal DHA (220 mg/day, n = 41) or DHA + AA (220 mg/day, n = 39) supplementation did not influence neurodevelopment at that age, although some parameters of brain development are related to perinatal DHA and AA status [42].
The review study of Jensen et al. has shown that DHA supplementation of breastfeeding mothers has resulted in higher scores on the Bayley psychomotor development index at 30 months of age, but caused no other difference either at or before this age [6].
The results of a meta-analysis showed no significant or consistent effects of supplementation with LCPUFA in the different areas of neurodevelopment [4].
Makrides et al., in a review study, have assessed the results of two large-scale clinical trials in which equal dietary doses of DHA were evaluated. They concluded that DHA supplementation after birth appeared to be more effective in improving the neurodevelopmental outcome of preterm children, rather than supplementation with DHA capsules during pregnancy [43].
In another study, Auestad et al. have followed full-term infants for 39 months and did not observe a significant improvement in the results of different mental and motor development tests after prescription of DHA (n = 65), or ARA and DHA (n = 66) to 1 year of age [44].
Bouwstra et al. have also studied healthy full-term infants, reporting improvement effects of formula supplemented with LCPUFA on 2- or 3-month-old infants by measuring the quality of general movements as an indicator of brain function [45].
Fewtrell et al., have indicated significant positive effects in the mental development index component of the Bayley scale of 195 formula-fed preterm infants (birth weight <1750 g, gestation <37 weeks), at 18 months [46].
In the review study, the effect of LCPUFA supplementation on children’s neurodevelopment has been studied in full-term infants who received supplemented formula after birth. Included studies indicated a positive effect of LCPUFA on neurodevelopment in early infancy, but its positive effects at a later stage have not been observed [47]. There is no accessible data on the long-term neurodevelopmental effects. It is possible that, the effects of LCPUFA supplementation re-emerge at school age, much like the effect of breastfeeding on children’s development [42].
It appears that maternal and infant supplementation might be more effective on enhancing the neurodevelopment outcomes in vulnerable individuals such as preterm infants [46]. The various regions of the brain differ in terms of fatty acid composition. Thus, it may fail to influence the domains’ outcomes of the developmental tests [48].
The mothers and infants with suboptimal baseline status of LCPUFA, especially DHA, benefit more than others from supplementation. The beneficial effects of DHA in maternal supplementation studies are mainly found in toddlers and older infants, and their effects do not seem to be dose-dependent [49, 50].
The Cochrane systematic review, based on limited evidence, reports that LCPUFA supplements for breastfeeding mothers do not appear to improve the neurological development of term born children [4].
A high variety of developmental tests was used in the different studies; these tests may not be sensitive or consistent enough.
The results of our study showed no significant differences between the two groups in terms of anthropometric measures (weight, length, or head circumference).
Gonzalez–Casanova, in accordance with our study, observed that prenatal DHA supplementation did not affect height, weight, or BMI during 60 months of age [51].
Rogers et al. found that DHA supplementation does not appear to affect the growth of infants [39], although a few studies have shown benefits for preterm infants [52, 53].
The Cochrane systematic review evaluated the effect of LCPUFA supplementation of breastfeeding mothers on improving child growth and development. They indicated that no significant differences were found for children’s weight at the short- and long-term follow ups. Supplementation with LCPUFA had not shown a significant effect on children at short (up to 12 months) and middle terms. There were significant differences in favour of the control group for long-term data. Supplementation with LCPUFA has not showed a significant effect on the head circumference of children in the short-term. For the long-term data, significant differences were found in one study in favour of the experimental group [4].
According to the results of present study, the maternal serum composition of DHA and EPA was very low in comparison to the European and USA studies [20]. The PUFA status of the developing foetus depends on the maternal PUFA status [54]. Therefore, Fish oil supplementation along with presenting dietary recommendations about proper intake of fish products by health care providers seems beneficial during pregnancy especially in the women with low consumption of these products.
We evaluated the short-term effects of FO supplementation on the neurodevelopment and growth of infants, with the use of ASQ questionnaire as a parent-reported instrument in early ages. The pregnant women referred to the healthcare centres with low-risk pregnancies were recruited in our study. Therefore, the scope of generalization of the results might be limited. It is suggested that further studies be conducted with a larger sample size of people representative of the whole community, with short- and long-term follow-up especially in the settings where there is low consumption of fish. The gold standard research testing along with ASQ should ideally be used for such studies. Furthermore, it is proposed to evaluate the impact of FO supplementation on neurodevelopment and growth measures of preterm compared to term infants.
Another limitation of this study was not measuring the omega-3 fatty acid in the breast milk and the plasma lipid fractions of the offspring.
This study has several strong points: it is well designed with random assignment of participants, triple blinded and nearly complete follow-up. Pregnant women were equally allocated into two groups according to parity using the stratification method. To ensure the safety of the medications, the levels of mercury were re-investigated in the FO capsules.
Conclusion
The main concern of our research was to determine if DHA consumption in women of child-bearing age in Iran, especially in Azerbaijan province, has an impact on optimal neurodevelopment. Based upon the study results, fish oil supplementation can be concluded to be beneficial only for the communication domain of neurodevelopment among 4-month-old infants. In this study, the results related to the supplementation effect on the other domains were inconclusive, but establish a need for further research in this field, with up-to-date lipidomic analysis to find biochemical correlate relative to intervention and developmental findings. Although N-3 LCPUFAs are important for children’s development, multiple studies have not supported this conclusion. There may be several reasons: most trials have been short studies of supplementation after birth, perhaps the endpoints selected have not been sensitive enough to detect changes, and the dosage may have been inadequate.
References
Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B (2007) Developmental potential in the first 5 years for children in developing countries. Lancet 369:60–70. doi:10.1016/S0140-6736(07)60032-4
World Health Organization (2011) e-Library of Evidence for Nutrition Actions (eLENA). Marine oil supplementation to improve pregnancy outcomes. Biological, behavioural and contextual rationale. World Health Organization, Geneva. http://www.who.int/elena/titles/bbc/fish_oil_pregnancy/en/
Simmer K, Patole SK, Rao SC (2008) Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev Issue. doi:10.1002/14651858.CD000376.pub2
Delgado-Noguera MF, Calvache JA, Bonfill Cosp X (2010) Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst Rev 12:CD007901. doi:10.1002/14651858.CD007901.pub2
McCann JC, Ames BN (2005) Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr 82:281–295 PMID:16087970
Jensen CL (2006) Effects of n-3 fatty acids during pregnancy and lactation. Am J Clin Nutr 83:1452S–1457S PMID:16841854
Tinoco M, Sic hieri R, Moura S, Santos Fda S, Carmo MG (2007) The importance of essential fatty acids and the effect of trans fatty acids in human milk on fetal and neonatal development. Cad de Saude Publ 23:525–534. doi:10.1590/S0102-311X2007000300011
Uauy R, Mena P, Wegher B, Nieto S, Salem N Jr (2000) Long chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatr Res 47:127–135
Calder PC, Dangour AD, Diekman C, Eilander A, Koletzko B, Meijer GW et al (2010) Essential fats for future health. Proceedings of the 9th Unilever Nutrition Symposium, 26–27 May. Eur J Clin Nutr 64:S1–S13. doi:10.1038/ejcn.2010.242
Burdge GC, Calder PC (2006) Dietary alpha-linolenic acid and health-related outcomes: a metabolic perspective. Nutr Res Rev 19:26–52. doi:10.1079/NRR2005113
Zivkovic AM, Telis N, German JB, Hammock BD (2011) Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif Agric 65(3):106–111. doi:10.3733/ca.v065n03p106
Uauy R, Dangour AD (2006) Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev 64:S24–S33 (discussion S72–S91. PMID: 16770950)
Koletzko B, Lien E, Agostoni C, Böhles H, Campoy C, Cetin I et al (2008) The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 36:5–14. doi:10.1515/JPM.2008.001
Coletta JM, Bell SJ, Roman A (2010) Omega-3 Fatty Acids and Pregnancy. Rev Obstet Gynecol 3:163–171
Sajedi F, Vameghi R, Kraskian-Mojembari A, Habibollahi A, Lornejad H, Delavar B (2012) Standardization and validation of the ASQ developmental disorders. screening tool in children of Tehran city. TUMJ 70:436–446
Squires J, Bricker D, Potter L (1997) Revision of a parent-completed developmental screening tool: ages and stages questionnaires. J Pediatr Psychol 22:313–328 PMID: 9212550
Squires J, Potter L, Bricker D, Potter L (1999) The ASQ user’s guide, 2nd edn. Paul H. Brookes, Baltimore
Elbers J, Macnab A, McLeod E, Gagnon F (2008) The ages and stages questionnaires: feasibility of use as a screening tool for children in Canada. Can J Rural Med 13:9 (PMID: 18208647)
Vameghi R, Sajedi F, Ak Mojembari, Abbas H, Hr Lornezhad, Delavar B (2013) Cross-cultural adaptation, validation and standardization of ages and stages questionnaire (ASQ) in Iranian children. Iran J Public Health 42:522–528
Farshbaf-Khalili A, Mohamad-Alizadeh S, Darabi M, Hematzadeh S, Mehdizadeh A, Shaaker M, Ostadrahimi A (2016) The effect of fish oil supplementation on serum phospholipid fatty acids profile during pregnancy: a double blind randomized controlled trial. Women Health 24:1–17
Birch EE, Garfield S, Castaneda Y et al (2007) Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev 83:279–284
Birch EE, Castaneda YS, Wheaton DH et al (2005) Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am J Clin Nutr 81:871–879
Wainwright PE (2002) Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc 61(1):61–69
Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW (1980) Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev 4:121–129
Martinez M (1992) Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr 120:S129–S138
Elias SL, Innis SM (2001) Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr 73:807–814
Bell SJ, Bradley D, Forse RA et al (1997) The new dietary fats in health and disease. J Am Diet Assoc 97:280–286
Kris-Etherton PM, Taylor DS, Yu-Poth S et al (2000) Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 71:179S–188S
Burdge G (2004) α-Linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 7:137–144
Bonham MP, Duffy EM, Wallace JMW, Robson PJ, Myers GJ, Davidson PW et al (2008) Habitual fish consumption does not prevent a decrease in LCPUFA status in pregnant women (the Seychelles Child Development Nutrition Study). Prostaglandins Leukot Essent Fat Acid 78(6):343–350
Endres S, Ghorbani R, Kelley VE et al (1989) The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of inter-leukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 320:265–271
Herrera E (2002) Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 19(1):43–55
Herrera E (2000) Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr 54(Suppl 1):S47–S51
Yaghini O, Kelishadi R, Keikha M, Niknam N, Sadeghi S, Najafpour E, Ghazavi MR (2015) Prevalence of developmental delay in apparently normal preschool children in Isfahan, central Iran. Iran J Child Neurol 9:17–23 (PMCID: PMC4577694)
Sajedi F, Vameghi R, Kraskian Mujembari A (2014) Prevalence of undetected developmental delays in Iranian children. Child Care Health Dev 40:379–388. doi:10.1111/cch.12042
Hibbeln JR, Davis JM, Steer C et al (2007) Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet 369:578–585. doi:10.1016/S0140-6736(07)60277-3
Helland IB, Smith L, Saarem K et al (2003) Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 111:e39–e44 PMID:12509593
Makrides M, Gibson RA, McPhee AJ, DOMInO Investigative Team et al (2010) Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA 304:1675–1683. doi:10.1001/jama.2010.1507
Rogers LK, Valentine CJ, Keim SA (2013) DHA supplementation: current Implications in pregnancy and childhood. Pharmacol Res 70:13–19. doi:10.1016/j.phrs.2012.12.003
Hadders-Algra M, Bouwstra H, van Goor SA, Dijck-Brouwer DA, Muskiet FA (2007) Prenatal and early postnatal fatty acid status and neurodevelopmental outcome. J Perinat Med 35:S28–S34. doi:10.1515/JPM.2007.034
Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT (2005) The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med 3:11. doi:10.1186/1741-7015-3-11
Van Goor SA, Dijck-Brouwer DA, Erwich JJ, Schaafsma A, Hadders-Algra M (2011) The influence of supplemental docosahexaenoic and arachidonic acids during pregnancy and lactation on neurodevelopment at 18 months. Prostaglandins Leukot Essent Fat Acid 84:139–146. doi:10.1016/j.plefa.2011.01.002
Makrides M (2013) DHA supplementation during the perinatal period and neurodevelopment: do some babies benefit more than others? Prostaglandins Leukot Essent Fat Acid 88:87–90. doi:10.1016/j.plefa.2012.05.004
Auestad N, Scott DT, Janowsky JS et al (2003) Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics 112:e177–e183 PMID: 12949309
Bouwstra H, Dijck-Brouwer DA, Wildeman JA et al (2003) Long-chain polyunsaturated fatty acids have a positive effect on the quality of general movements of healthy term infants. Am J Clin Nutr 78:313–318 PMID: 12885715
Fewtrell MS, Morley R, Abbott RA et al (2002) Double-blind, randomized trial of long-chain polyunsaturated fatty acid supplementation in formula fed to preterm infants. Pediatrics 110:73–82 PMID:12093949
Hadders-Algra M (2011) Prenatal and early postnatal supplementation with long-chain polyunsaturated fatty acids: neurodevelopmental considerations. Am J Clin Nutr 94:1874S–1879S. doi:10.3945/ajcn.110.001065
Rao PS, Rao KS (1973) Fatty acid composition of phospholipids in different regions of developing human fetal brain. Lipids 8(7):374–377
Judge MP, Harel O, Lammi-Keefe CJ (2007) A docosahexaenoic acid-functional food during pregnancy benefits infant visual acuity at 4 but not 6 months of age. Lipids 42:117–122. doi:10.1007/s11745-006-3007-3
Hadders-Algra M (2008) Prenatal long-chain polyunsaturated fatty acid status: the importance of a balanced intake of docosahexaenoic acid and arachidonic acid. J Perinat Med 36:101–109. doi:10.1515/JPM.2008.029
Gonzalez-Casanova I, Stein AD, Hao W, Garcia-Feregrino R, Barraza-Villarreal A, Romieu I et al (2015) Prenatal supplementation with docosahexaenoic acid has no effect on growth through 60 months of age. J Nutr 145:1330–1334. doi:10.3945/jn.114.203570
Schulzke SM, Patole SK, Simmer K (2011) Long chain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev 16:CD000375. doi:10.1002/14651858.CD000375.pub4
Makrides M, Gibson RA, Udell T, Ried K (2005) Supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. Am J Clin Nutr 81:1094–1101
Hornstra G (2000) Essential fatty acids in mothers and their neonates. Am J Clin Nutr 71:1262S–1269S
Acknowledgements
This study was extracted from the PhD thesis approved by Tabriz Health Services Management Research Centre (Grant No. 5/77/5241) and financially supported by the Research Vice-chancellor, Tabriz University of Medical Sciences. Hereby, we appreciate the Zahravi Pharmacy Company, Health Vice-chancellor, Tabriz University of Medical Sciences, and its authorities, all personnel in the healthcare centres, and all the women who patiently assisted us in completing this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ostadrahimi, A., Salehi-pourmehr, H., Mohammad-Alizadeh-Charandabi, S. et al. The effect of perinatal fish oil supplementation on neurodevelopment and growth of infants: a randomized controlled trial. Eur J Nutr 57, 2387–2397 (2018). https://doi.org/10.1007/s00394-017-1512-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1512-1