Abstract
Purpose
Virgin coconut oil (VCO) is a medium-chain fatty acid source with popularly attributed benefits on obesity management. However, its role on obesity requires elucidation due to its saturated nature. In the study herein, we investigated acute effects of VCO consumption on energy metabolism, cardiometabolic risk markers, and appetitive responses in women with excess body fat.
Methods
Fifteen adult women with excess body fat (37.43 ± 0.83%) participated in this randomized, crossover, controlled study. Two isocaloric mixed breakfasts containing 25 mL of VCO or control (extra-virgin olive oil-C) were evaluated. Resting energy expenditure (REE), fat oxidation rate (FOR), diet induced thermogenesis (DIT) and appetitive subjective responses were assessed at fasting and postprandial periods (up to 240 min). Cardiometabolic risk markers were assessed at fasting and up to 180 min postprandially.
Results
VCO did not affect REE, FOR, and DIT compared to C. In addition, VCO did not cause deleterious change in triglycerides, total cholesterol, HDL-c, LDL-c, triglycerides/HDL-c ratio, uric acid, glucose and Homeostasis Model Assessment of Insulin Resistance Index (HOMA-IR) (P time×treatment > 0.05). However, VCO suppressed less hunger (P time×treatment = 0.003), total satiety (P iAUC = 0.021) and total fullness (P iAUC = 0.035) responses than C.
Conclusions
VCO consumption did not acutely change energy metabolism and cardiometabolic risk markers when added to a mixed breakfast but promoted less appetitive responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and overweight remain to be a serious public health problem despite of international efforts to combat them. Excess body fat is related to non-communicable diseases (NCDs) such as cardiovascular diseases, hypertension, diabetes, and some types of cancer [1]. Data from the latest World Health Organization report [2] showed that NCDs have been responsible for around 38 million deaths per year since 2012, accounting for 68% of all deaths worldwide. Cardiovascular diseases were the leader in mortality, which claimed 17.5 million lives in 2012 (46% of all NCDs deaths), 6 million of which were premature.
Virgin coconut oil (Cocos nucifera L.) (VCO) is a high quality source of medium-chain fatty acids (MCFA) with commercial weight loss claims. It has been attributed to coconut oil thermogenic effects, increased fullness responses and HDL-c improvement [3, 4]. These are based on the fact that MCFA could be absorbed and metabolized faster than others fatty acids [5]. The theory is that faster metabolization could boost energy and fat metabolism without promoting fat storage and dyslipidemia and could enhance satiety [6, 7] thus favoring weight loss. These claims attract consumers’ attention [8] but it lacks scientific confirmation.
While some studies reported beneficial effects of coconut oil or isolated MCFA on energy metabolism [9], fat oxidation [10, 11], food intake [12, 13], and no detrimental effect on serum cardiometabolic risk markers [14–17], others demonstrated negative results on lipid profile [18], and conflicting results on metabolic rates [11, 19], and satiety [20, 21]. Contradictory results could be partly explained by the distinct fatty acid composition of VCO compared to the synthetic source of MCFA adopted in the majority of studies [9, 11–13, 16, 18, 19], enforcing the need for studies conducted with VCO. Furthermore, randomized controlled clinical trials about VCO are scarce, present methodological limitations such as unusual doses of oil consumption, and/or exhibit inconclusive results [22].
Because women are the main coconut oil consumers due to its weight loss claims and previous studies have lead us to question the applicability of MCFA-rich oil claims in women [23], the aim of this study was to evaluate acute effects of reasonable amounts of VCO intake on energy metabolism, serum cardiometabolic risk markers, and subjective appetitive responses in women with excess body fat.
Methods
Study population
Seventeen women aged 19–42 years with BMI between 25 and 31 kg/m2 and total body fat >32% were recruited through local advertisement (Supplementary Figure 1). Exclusion criteria were: known chronic illnesses except obesity, fasting glucose >5.5 mmol/L, hypertension (>140/90 mmHg), smoking, alcohol consumption (>2 doses/d), pregnancy or lactation, recent changes (previous three months) in diet or physical activities habits, elite athletes (>10 h/week), use of dietary supplements or drugs except oral contraceptive, food allergies or intolerances, and aversion to the tested ingredients. Based on published values of fasting fat oxidation and estimated change of 3.6 g during the postprandial state [24], a sample size of 12 subjects was estimated for this crossover study design using the formula by Mera et al. [25]. Accounting for dropouts (30%), seventeen subjects have been enrolled. Participants gave written consent after receiving verbal and written information. The study was performed at Laboratory of Energy Metabolism and Body Composition of Nutrition and Health Department, Universidade Federal de Viçosa, Brazil. The study protocol was approved by the Ethics Committee of Universidade Federal de Viçosa (protocol number: 541.836/2014), conducted in accordance with Helsinki declaration and its later amendments and registered at http://www.ensaiosclinicos.gov.br/ (RBR-8NGPQ9).
Study design
This was a randomized single-blinded crossover design study in which two different isocaloric breakfasts were tested in non-consecutive days. Subjects were randomly assigned by simple draw to either control (extra-virgin olive oil—EVOO) or VCO breakfast, with washout period of one week.
Prior to test days, participants were instructed to maintain their usual dietary intake and to abstain from alcohol and strenuous physical activity. On the night before each test day, all participants consumed standardized dinner (2.5 MJ; 62E% carbohydrate; 8.5E% protein; 29.4E% fat) and water intake was allowed until 4 h before starting the test. Immediately before dinner consumption, participants emptied their bladder and thenceforth all urine was collected and carried to laboratory.
In each test day, participants attended the laboratory from 7 a.m. to 1 p.m. after 11 h overnight fasting with minimal physical effort possible. The remaining 1 h fasting was completed at laboratory, totaling 12 h of fasting before test drink consumption. Anthropometric measurements were recorded by trained appraiser. Body weight was measured using digital platform scale with resolution of 0.5 kg (Toledo®, Model 2096PP/2, São Paulo, Brazil), while participants were barefoot and wearing lightweight clothing. Height was measured with wall-mounted stadiometer (Wiso, Chapecó, SC, Brazil) to the closest value to 0.1 cm. Total body fat was assessed by bioelectrical impedance (model Y230, InBody Co. Lta., Gyeonggi, Korea). Then, subjects remained in lay supine for mandatory 15 min rest period. After that, fasting resting energy expenditure (REE) and substrate oxidation rates (SOR) were assessed by indirect calorimetry (Carefusion Vmax® Series, Califórnia, EUA) for 30 min and participants remained awake and motionless as much as possible. This protocol comprises fasting REE not very different from a basal energy expenditure measurement obtained immediately on awaking, after an overnight stay in the laboratory [26]. Urine was collected to complete 12 h of urine collection. Fasting (12 h) antecubital blood sample was drawn and subjects consumed one of two mixed breakfasts containing 25 mL of control (EVOO) or test (VCO) oils within 15 min. Meal palatability questionnaire was completed in this period.
Indirect calorimetry followed intermittent protocol previously developed [27], in which measurements were made every half for each hour during 4 h following breakfast. During protocol intervals, subjects remained awake but inactive and they could not leave the laboratory. Water at room temperature (200 mL) was given in each interval and urine collections were made during all postprandial period. Antecubital blood samples were collected at 60, 120, and 180 min and subjective appetitive responses were taken immediately before/after breakfast consumption and immediately before/after each indirect calorimetry until 240 min.
Test breakfasts
Participants were assigned to consume 30 to 40% of their daily energy requirements as test breakfasts. It consisted of standard breakfasts containing white bread with 25 mL of control (EVOO) or test (VCO) oils and 300 mL of milk-derived fat-free drink (Table 1). Virgin coconut oil (FidBō, São Paulo, Brazil) and extra-virgin olive oil (Bunge Alimentos, Santa Catarina, Brazil) were maintained protected from light and heat until consumption and fat-free drink was offered at ~10 °C.
Fatty acid compositions of EVOO and VCO were assessed after esterification [29] by gas chromatography (GC). Chromatographic analysis was carried out using Shimadzu GC Solution instrument (Shimadzu Seisakusho Co., Kyoto, Japan) equipped with flame ionization detector (FID) and Carbowax capillary column (30 m × 0.25 mm). Briefly, 1µL of esterified sample was injected in GC with split ratio of 10. Nitrogen was supplied as carrier gas at flow rate of 43.2 cm/s. The initial oven temperature was 100 °C, maintained for 5 min, then increased to 220 °C at 4 °C/minutes and held for 20 min. Flow rate over column was 1.0 mL/minute. FID and injection port temperature was 200 and 220 °C, respectively. Data handling was carried out using the software GC Solution package (Shimadzu Seisakusho Co., Kyoto, Japan). Fatty acid methyl esters (FAME) were identified by direct comparison of retention time with FAME standard mix (Supelco 37 Component FAME Mix; Sigma-Aldrich®, EUA).
Energy metabolism measurement and calculation
Flow meter and flow sensor were calibrated daily by a 3.0-L syringe. Analyzers were calibrated with gases of known concentration before each testing as recommended by manufacturer’s instructions. The first gas was 26% O2 with N2 balance, the second gas was 4% CO2 16% O2, with N2, and the last gas was ambient air.
During measurements, subjects had their head covered with ventilated canopy to quantify oxygen consumed and carbon dioxide produced. They stayed in quiet room with stable temperature (22 °C) and humidity (55%) during measurements. The first 10 min (adaptation phase), and individual outlier values of oxygen and carbon dioxide were excluded from the analyses [25, 30]. Means of oxygen and carbon dioxide volumes (L/min) from the remaining data were used in the calculations.
REE and SOR (carbohydrate, protein and fat oxidation) from fasting and postprandial states were calculated using oxygen and carbon dioxide volumes and urinary nitrogen excretion of each period of time, as described previously [31]. Values of non-protein respiratory quotient (NPRQ) were also calculated [31]. Diet induced thermogenesis (DIT) was assessed [27] and expressed as percentage of breakfasts energy. Total urinary nitrogen excretion was estimated by Kjeldahl technique. Changes between fasting and posprandial carbohydrate and fat oxidation were calculated by subtracting the total postprandial values over 4 h from fasting value multiplied by duration of measurement (4 h) [24].
Cardiometabolic risk markers
Serum samples were separated from whole blood by centrifugation (3500 rpm, 4 °C, 15 min) and immediately frozen at −80 °C until analyses. Triglycerides (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), glucose, and uric acid were measured by standard colorimetric kits (K082, K117, K083, K071, K088, and K139; Bioclin®, Minas Gerais, Brazil) by automatic biochemical analyzer BS-200 (Mindray Medical International Ldt., Shenzen, China). Insulin was assessed by chemiluminescence method. Very low-density lipoprotein (VLDL) was estimated by Friedewald et al. [32] formula. Insulin resistance was estimated by Homeostasis Model Assessment Index of Insulin Resistance (HOMA-IR) using Matthews et al. [33] equation. Atherogenic Index (TG/HDL ratio) [34] and total area under the curves (tAUC) of each cardiometabolic risk marker were also calculated [35].
The normal ranges used for fasting parameters were: TG (<1.7 mmol/L), total cholesterol (<5.17 mmol/L), HDL-c (>1.3 mmol/L), LDL-c (<2.6 mmol/L), glucose (<5.55 mmol/L), uric acid (89.22–475.84 µmol/L), insulin (<174 pmol/L), and HOMA-IR (<2.7) [36, 37].
Appetitive responses
Hunger (“How hungry do you feel?”), fullness (“How full do you feel?”), satisfaction (“How satisfied do you feel?”), and desire to eat specific food types (“Would you like to eat something sweet?”; “Would you like to eat something salty?”; “Would you like to eat something savoury?”; “Would you like to eat something fatty?”) were assessed using 10 unit visual analog scales (VAS) with words anchored at their ends, expressing the most positive and the most negative rating [38]. Incremental area under the curve (iAUC) was determined for fullness and satiety, and incremental area above the curve (iAAC) was determined for hunger and desire to eat specific food types. Incremental areas were assessed by trapezoidal method [35]. VAS were also used to rate palatability by the following questions: visual appeal, smell, taste, aftertaste, and palatability [38].
Statistical analysis
Statistical analyses were carried out with SPSS 17 for Windows (SPSS, Inc., Chicago, IL, USA). Data are expressed as means and standard errors of the mean (SEM). Individual outlier values of each variable were excluded before analyses. Data normality and homoscedasticity were assessed by Shapiro–Wilk and Levene tests, respectively. Non-parametric data were log transformed prior to the analysis. Paired t test was used to assess significant differences between dietary treatments for areas under (AUC) or above (AAC) the curve and palatability responses. Repeated-measures ANOVA in mixed model setting, considering time as within-subject factor and treatment (breakfast) as between-subject factor were conducted to verify treatment effects on all variables. Paired t test with Bonferroni’s correction was performed to assess differences in individual time-points in which differences were expected to arise. The α level of 5% was considered statistically significant.
Results
From the seventeen recruited participants two of them failed to complete the study protocol due to personal reasons. Both breakfasts were well tolerated by the 15 participants. There were no differences between breakfasts related to palatability questions (P > 0.05). For subjects’ characteristics see Table 2.
Metabolic rates
There were no differences in fasting metabolic rates between the test days. As expected, there was significant time effect in REE, NPRQ, and all SOR (carbohydrate, protein, and fat oxidation) (P time < 0.001). However, there were no treatment effect or time × treatment interaction for any metabolic rate analyzed (Table 3). Similarly, comparisons of fasting and total postprandial states for all metabolic parameters showed only effect of time (P time ≤ 0.001). Absence of significant results was maintained when DIT and total postprandial carbohydrate and fat oxidation were assessed (Table 3).
Cardiometabolic risk markers
All participants had normal uric acid fasting range (minimal/maximal: 130.86/285.50 µmol/L). For other cardiometabolic markers, 13 participants showed normal fasting range for glucose (minimal/maximal, 4.05/6.49 mmol/L), 13 for insulin (25.7/260.44 pmol/L), 8 for HOMA index (0.75/10.82), 10 for triglycerides (0.46/2.09 mmol/L), 13 for total cholesterol (3.13/5.72 mmol/L), 8 for HDL-c (0.59/1.99 mmol/L), and 12 for LDL-c (1.06/3.41 mmol/L). Blood assays indicated typical middle-aged obesity profile with some individuals exhibiting disruption in cardiometabolic risk markers.
There was significant time × treatment interaction for uric acid concentrations between breakfasts (P time×treatment = 0.006); however, such interaction did not persist after post hoc analysis (P > 0.050). Furthermore, there was trend to time × treatment interaction in HDL-c levels at time 60 min (P time×treatment = 0.055). There were no significant changes in total postprandial responses between treatments (Table 4).
Appetitive responses
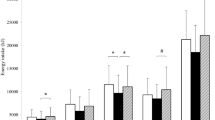
Analyses of subjective hunger responses showed significant time × treatment interaction (P time×treatment = 0.003) with VCO breakfast presenting lesser hunger suppression at 240 min when compared to control breakfast (P = 0.019). Further, VCO had significantly lesser total satiety (P iAUC = 0.021) and total fullness (P iAUC = 0.035) responses when compared to control (Fig. 1). Additional questions regarding desire to eat specific food types showed no significant differences between dietary treatments (Supplementary Fig. 2).
Mean ± SEM changes from baseline of self-reported hunger (a), fullness (b), and satiety (c) responses obtained from visual analog scales (VAS) in response to extra-virgin olive oil (control) or virgin coconut oil (test) intake (n = 15). iAUC incremental area under the curve, iAAC incremental area above the curve. For the sake of clarity, error bars are only given for the maximum and minimum values at each time point. P time×treatment values were obtained from RM-ANOVA in mixed model setting with time as within-subject factor and meal as between-subject factor. *Significantly different from each other (Paired t test, P < 0.05)
Discussion
During decades, coconut oil consumption was discouraged due to its high saturated fat content and consequent potential to raise blood cholesterol and promote dyslipidemia [39]. Analyses of VCO fatty acid composition used in our study support this affirmation since it provided 94% of saturated fat. Nevertheless, we expected that high proportion of easily oxidized medium-chain fatty acid (~62% of total saturated fat, 51.6% from lauric acid) could acutely increase postprandial fat oxidation, resulting in increased thermogenesis, less detrimental changes in blood cardiometabolic risk markers and increased satiety. These effects could contribute to reduce cardiometabolic risks and promote long-term weight loss. In fact, it has long been accepted that MCFA are absorbed and metabolized as rapidly as glucose [5, 40] usually accessed for 120 min postprandially [41]. For this reason, 240 min would be enough to evaluate MCFA metabolism.
The hypothesis that an increase in fat oxidation and thermogenesis would occur after VCO consumption was based on studies showing that acute and long-term consumption of MCFA, but not whole VCO, resulted in increased fat oxidation [11, 23, 42, 43] and energy expenditure [23, 43, 44]. However, few prior studies compared acute effects of VCO on thermogenesis and substrate oxidation rates.
Differences in fatty acid structures, such as chain length, number and position of unsaturation, and stereoisomeric configuration affect fatty acid oxidation rate. Hierarchy seems to exist between saturated and unsaturated fatty acids when consumed individually. Saturated fatty acids oxidation rates decrease with increasing chain length [45, 46] while for unsaturated ones, oxidation decreases with an increase in the number of double bonds [46]. Comparing unsaturated to saturated fatty acids, the former seems to be oxidized more rapidly [47] apart from MCFA, which are oxidized faster than others [48].
In our study, there was no difference between VCO and EVOO fat oxidation rates when incorporated in usual mixed breakfasts. Lauric acid (C12:0) is the predominant MCFA in VCO and it is considered by some authors as a fatty acid with intermediary properties between MCFA and LCFA [49]. We believe that high content of lauric acid in this oil could give it metabolic characteristics more similar to long-chain fatty acid than MCFA present in synthetic oils (rich in C8:0 and C10:0) adopted in the majority of studies [11–13, 17–19, 21, 43, 50]. On the other hand, it was observed increase in carbohydrate oxidation and decrease in fat oxidation in the first hours after both breakfasts consumption. However, after 80 min of breakfast consumption, carbohydrate oxidation started to reduce, and increase in fat oxidation after VCO breakfast seemed to occur (Supplementary Fig. 3). Inclusion of carbohydrate source into breakfasts containing tested oils could change substrate oxidation profile, once carbohydrates are preferably oxidized in order to maintain carbohydrate balance. As consequence, fat oxidation is impaired [51]. Thus, despite the fact that both groups received equal amounts of carbohydrates, we believe that carbohydrate inclusion could have delayed fat oxidation response, masking treatment effects on fat metabolism. We are now testing the role of VCO added to low carbohydrate meal in energy metabolism.
Our study demonstrated no acutely negative effects of MCFA-source VCO in triglycerides, total cholesterol, LDL-c, and triglycerides/HDLc ratio when compared to EVOO rich in cholesterol-neutral oleic acid. There were great differences in the methodology and results from published randomized clinical trials assessing coconut oil effects, and not synthetic MCFA, in lipid-related cardiometabolic risk factors. The majority of these published studies presented the results of chronic coconut oil consumption and there were divergent results even when coconut oil doses and control treatment were similar in different studies [14, 17]. In turn, there was only one published study comparing the effects of coconut oil consumption to cholesterol-neutral MUFA [52], but the lack of methodological criteria prevent us to discuss its results. Some studies published with synthetic MCFA used control fats with cholesterol increasing [53] or cholesterol decreasing [54], making their results difficult to interpret. MCFA cholesterol-increasing effects were observed only when high-doses of MCFA were consumed (e.g., 70 g/d [18] or 43E% [55]). Thus, MCFA cholesterol-increasing effect could be due to its excessive consumption and does not reflect the impact of reasonable doses of MCFA. In the study herein, we showed that acute consumption of 25 mL of VCO, a reasonable amount to be consumed without producing undesirable collateral effects of high-fat meals [14], was not able to cause detrimental changes on lipid profile. Long-term studies are now needed to confirm our results.
As well as postprandial dyslipidemia, increased level of glucose at postprandial state (also referred as postprandial dysmetabolism) is considered an independent predictor of future cardiovascular events, even in nondiabetic subjects [56]. Different fats addition to mixed meals could distinctly affect carbohydrate digestion and insulin secretion, both impacting postprandial glycaemia [57–59]. Long-chain saturated fat appears to be the worst type of fat to promote glycemic control [60]. Because MCFA are hydrolyzed and absorbed faster than other fats, promote more rapidly gastric emptying, and stimulate less secretion of gut hormones with insulinotropic properties than long-chain fatty acids [61, 62], their potential to reduce postprandial glycaemia have long been neglected. However, Clegg et al. [57] demonstrated that while addition of 22.4 g of long-chain saturated fat (butter) had quite similar glycemic response as oil-free control, addition of the same amount of MCFA significantly reduced almost 40% of total glycemic response, and this reduction was similar to the addition of MUFA (olive oil). Our results are in line with these findings since VCO, high in MCFA, presented the same impact as EVOO on postprandial glycaemia.
MCFA influence on satiety was assessed in few studies [12, 13, 20, 21, 63, 64] and the majority enrolled normal weight subjects [12, 13, 20]. Only one was conducted in normal weight to overweight subjects [21]. Reports from published studies failed to demonstrate MCFA effect on appetite suppression [12, 13, 20, 21, 63, 64], despite some showed decreased food intake in subsequent meals [12, 13]. In the current study, we investigated VCO effects on subjective appetitive responses in overweight/obese women. Our study consistently showed that VCO addition to mixed breakfast promoted lesser satiating responses than control breakfast containing similar dose of EVOO rich in long-chain fatty acids. Results were characterized by reduced suppression of total hunger, fullness, and satiating responses, and were independent of palatability differences. These findings refute our previous hypothesis that VCO could increase satiety and, for this reason, reduce food intake contributing for weight loss.
Our study presented several strengths. First we properly used EVOO as control which is a natural source of the cholesterol-neutral oleic acid. Furthermore, we assessed the effects of a dietary source of MCFA, and not synthetic MCFA source, on energy metabolism, cardiometabolic risk markers, and satiety. This could improve the clinical implications of our findings, once VCO is the main commercial MCFA source which is largely available for population consumption worldwide. Also, the only difference between test and control breakfasts was the added oil. Finally, our study differed from most previous studies because it enrolled overweight/obese women and used reasonable dose of VCO.
Our study also has limitations. Despite of assessing metabolic rates for 240 min postprandially was considered adequate in several published studies, it is possible that this period of time was not enough to detect differences in fat oxidation between fat meals in presence of carbohydrate. Furthermore, it could not be neglected that 240 min may be not enough to detect changes in some serum lipid fractions in control group.
Conclusion
Inclusion of reasonable amount (25 mL) of virgin coconut oil into mixed breakfast did not affect energy metabolism, fat oxidation rates, and cardiometabolic risk markers compared to similar control breakfast in generally healthy excess body fat women. Furthermore, this oil suppressed less the hunger, satiety, and fullness responses suggesting that VCO consumption is not effective in improving energy balance. Thus, we recommend caution in prescribing coconut oil as adjuvant in weight loss programs. Long-term studies assessing the role of VCO in obesity control are now necessary to confirm our results.
References
Hruby A, Manson JE, Qi L et al (2016) Determinants and consequences of obesity. Am J Public Heal 106:1656–1662
World Health Organization (2016) Obesity and overweight. WHO
Babu AS, Veluswamy SK, Arena R et al (2014) Virgin coconut oil and its potential cardioprotective effects. Postgrad Med 126:76–83. doi:10.3810/pgm.2014.11.2835
Lockyer S, Stanner S (2016) Coconut oil—a nutty idea? Nutr Bull 41:42–54. doi:10.1111/nbu.12188
Marten B, Pfeuffer M, Schrezenmeir J (2006) Medium-chain triglycerides. Int Dairy J 16:1374–1382. doi:10.1016/j.idairyj.2006.06.015
St-Onge M-P, Jones PJH (2002) Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr 132:329–332
Eyres L, Eyres MF, Chisholm A, Brown RC (2016) Coconut oil consumption and cardiovascular risk factors in humans. Nutr Rev 74:267–280. doi:10.1093/nutrit/nuw002
Poonnakasem N, Pujols KD, Chaiwanichsiri S et al (2016) Different oils and health benefit statements affect physicochemical properties, consumer liking, emotion, and purchase intent: a case of sponge cake. J Food Sci 81:S165–S173. doi:10.1111/1750-3841.13186
Bendixen H, Flint A, Raben A et al (2002) Effect of 3 modified fats and a conventional fat on appetite, energy intake, energy expenditure, and substrate oxidation in healthy men. Am J Clin Nutr 75:47–56
White MD, Papamandjaris AA, Jones PJ (1999) Enhanced postprandial energy expenditure with medium-chain fatty acid feeding is attenuated after 14 d in premenopausal women. Am J Clin Nutr 69:883–889
Alexandrou E, Herzberg GR, White MD (2007) High-level medium-chain triglyceride feeding and energy expenditure in normal-weight women. Can J Physiol Pharmacol 85:507–513. doi:10.1139/y07-034
Van Wymelbeke V, Himaya A, Louis-Sylvestre J, Fantino M (1998) Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr 68:226–234
Van Wymelbeke V, Louis-Sylvestre J, Fantino M (2001) Substrate oxidation and control of food intake in men after a fat-substitute meal compared with meals supplemented with an isoenergetic load of carbohydrate, long-chain triacylglycerols, or medium-chain triacylglycerols. Am J Clin Nutr 74:620–630
Assunção ML, Ferreira HS, dos Santos AF et al (2009) Effects of dietary coconut oil on the biochemical and anthropometric profiles of women presenting abdominal obesity. Lipids 44:593–601. doi:10.1007/s11745-009-3306-6
Liau KM, Lee YY, Chen CK, Rasool AHG (2011) An open-label pilot study to assess the efficacy and safety of virgin coconut oil in reducing visceral adiposity. ISRN Pharmacol 2011:949686. doi:10.5402/2011/949686
Nosaka N, Kasai M, Nakamura M et al (2002) Effects of dietary medium-chain triacylglycerols on serum lipoproteins and biochemical parameters in healthy men. Biosci Biotechnol Biochem 66:1713–1718
Vijayakumar M, Vasudevan DM, Sundaram KR et al (2016) A randomized study of coconut oil versus sunflower oil on cardiovascular risk factors in patients with stable coronary heart disease. Indian Heart J 68:498–506. doi:10.1016/j.ihj.2015.10.384
Tholstrup T, Ehnholm C, Jauhiainen M et al (2004) Effects of medium-chain fatty acids and oleic acid on blood lipids, lipoproteins, glucose, insulin, and lipid transfer protein activities. Am J Clin Nutr 79:564–569
Roynette CE, Rudkowska I, Nakhasi DK, Jones PJH (2008) Structured medium and long chain triglycerides show short-term increases in fat oxidation, but no changes in adiposity in men. Nutr Metab Cardiovasc Dis 18:298–305. doi:10.1016/j.numecd.2006.11.004
Poppitt SD, Strik CM, MacGibbon AKH et al (2010) Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol Behav 101:161–167. doi:10.1016/j.physbeh.2010.04.036
Kovacs EM, Westerterp-Plantenga MS, de Vries M, et al. (2001) Effects of 2-week ingestion of (-)-hydroxycitrate and (-)-hydroxycitrate combined with medium-chain triglycerides on satiety and food intake. Physiol Behav 74:543–549
Rego Costa AC, Rosado EL, Soares-Mota M (2012) Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: a systematic review. Nutr Hosp 27:103–8. doi: 10.1590/S0212-16112012000100011
St-Onge M-P, Ross R, Parsons WD, Jones PJH (2003) Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res 11:395–402. doi:10.1038/oby.2003.53
Soares MJ, Cummings SJ, Mamo JCL et al (2004) The acute effects of olive oil v. cream on postprandial thermogenesis and substrate oxidation in postmenopausal women. Br J Nutr 91:245–252. doi:10.1079/BJN20031047
Mera R, Thompson H, Prasad C (1998) How to calculate sample size for an experiment: a case-based description. Nutr Neurosci 1:87–91. doi:10.1080/1028415X.1998.11747217
Soares MJ, Piers LS, Kraai L, Shetty PS (1989) Day-to-day variations in basal metabolic rates and energy intakes of human subjects. Eur J Clin Nutr 43:465–472
Piers LS, Soares MJ, Makan T, Shetty PS (1992) Thermic effect of a meal: methodology and variation in normal young adults. Br J Nutr 67:165–175
Núcleo de Estudosde e pesquisas em Alimentação – NEPA (2011) Tabela Brasileira de Composicao de Alimentos - TACO, 4th edn. NEPA- UNICAMP, Campinas
Hartman L, Lago RC (1973) Rapid preparation of fatty acid methyl esters from lipids. Lab Pract 22:475–476 (passim)
Cummings NK, James AP, Soares MJ (2006) The acute effects of different sources of dietary calcium on postprandial energy metabolism. Br J Nutr 96:138–144
Ferrannini E (1988) The theoretical bases of indirect calorimetry: a review. Metabolism 37:287–301
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Gaziano JM, Hennekens CH, O’Donnell CJ et al (1997) Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation 96:2520–2525. doi:10.1161/01.cir.96.8.2520
Food and Agriculture Organization of the United Nations., Joint FAO/WHO Expert Consultation on Carbohydrates in Human Nutrition (1997 : Rome I (1998) Carbohydrates in human nutrition : report of a joint FAO/WHO expert consultation, Rome, 14-18 April 1997. World Health Organization
Alberti KGMM, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 120:1640–1645
Geloneze B, Vasques ACJ, Stabe CFC et al (2009) HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq Bras Endocrinol Metabol 53:281–287. doi:10.1590/S0004-27302009000200020
Flint A, Raben A, Blundell JE, Astrup A (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24:38–48
Nevin KG, Rajamohan T (2004) Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clin Biochem 37:830–835. doi:10.1016/j.clinbiochem.2004.04.010
Babayan VK (1987) Medium chain triglycerides and structured lipids. Lipids 22:417–420
Brouns F, Bjorck I, Frayn KN et al (2005) Glycaemic index methodology. Nutr Res Rev 18:145. doi:10.1079/NRR2005100
White MD, Papamandjaris AA, Jones PJ (1999) Enhanced postprandial energy expenditure with medium-chain fatty acid feeding is attenuated after 14 d in premenopausal women. Am J Clin Nutr 69:883–889
St-Onge M-P, Bourque C, Jones PJH et al (2003) Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord 27:95–102. doi:10.1038/sj.ijo.0802169
Hill JO, Peters JC, Yang D et al (1989) Thermogenesis in humans during overfeeding with medium-chain triglycerides. Metabolism 38:641–648
Leyton J, Drury PJ, Crawford MA (1987) Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br J Nutr 57:383–393
DeLany JP, Windhauser MM, Champagne CM, Bray GA (2000) Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr 72:905–911
Jones PJ, Pencharz PB, Clandinin MT (1985) Whole body oxidation of dietary fatty acids: implications for energy utilization. Am J Clin Nutr 42:769–777
Papamandjaris AA, MacDougall DE, Jones PJ (1998) Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci 62:1203–1215
Sáyago-Ayerdi SG, Vaquero MP, Schultz-Moreira A et al (2008) Utilidad y controversias del consumo de ácidos grasos de cadena media sobre el metabolismo lipoproteico y obesidad. Nutr, Hosp, p 23
Liu Y, Wang J, Zhang R et al (2009) A good response to oil with medium- and long-chain fatty acids in body fat and blood lipid profiles of male hypertriglyceridemic subjects. Asia Pac J Clin Nutr 18:351–358
Labayen I, Forga L, Martínez JA (1999) Nutrient oxidation and metabolic rate as affected by meals containing different proportions of carbohydrate and fat, in healthy young women. Eur J Nutr 38:158–166
Ng TK, Hayes KC, DeWitt GF et al (1992) Dietary palmitic and oleic acids exert similar effects on serum cholesterol and lipoprotein profiles in normocholesterolemic men and women. J Am Coll Nutr 11:383–390
Beveridge JMR, Connell WF, Haust HL, Mayer GA (1959) Dietary cholesterol and plasma cholesterol levels in man. Can J Biochem Physiol 37:575–82
Roels OA, Hashim SA (1962) Influence of fatty acids on serum cholesterol. Fed Proc 21(4)Pt 2:71–76
Cater NB, Heller HJ, Denke MA (1997) Comparison of the effects of medium-chain triacylglycerols, palm oil, and high oleic acid sunflower oil on plasma triacylglycerol fatty acids and lipid and lipoprotein concentrations in humans. Am J Clin Nutr 65:41–45
O’Keefe JH, Bell DSH (2007) Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 100:899–904. doi:10.1016/j.amjcard.2007.03.107
Clegg ME, Pratt M, Markey O et al (2012) Addition of different fats to a carbohydrate food: impact on gastric emptying, glycaemic and satiety responses and comparison with in vitro digestion. Food Res Int 48:91–97. doi:10.1016/j.foodres.2012.02.019
Joannic JL, Auboiron S, Raison J et al (1997) How the degree of unsaturation of dietary fatty acids influences the glucose and insulin responses to different carbohydrates in mixed meals. Am J Clin Nutr 65:1427–1433
Thomsen C, Rasmussen O, Lousen T et al (1999) Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr 69:1135–1143
Gatti E, Noè D, Pazzucconi F et al (1992) Differential effect of unsaturated oils and butter on blood glucose and insulin response to carbohydrate in normal volunteers. Eur J Clin Nutr 46:161–166
Fernandes J, van de Kamer JH, Weijers HA (1962) Differences in absorption of the various fatty acids studied in children with steatorrhea. J Clin Invest 41:488–494. doi:10.1172/JCI104502
de Jong AJ, Hopman WP, Jansen JB, Lamers CB (1985) Effect of medium-chain triglycerides and long-chain triglycerides on plasma pancreatic polypeptide secretion in man. Regul Pept 11:77–81
Barbera R, Peracchi M, Brighenti F et al (2000) Sensations induced by medium and long chain triglycerides: role of gastric tone and hormones. Gut 46:32–36
Feinle C, Rades T, Otto B, Fried M (2001) Fat digestion modulates gastrointestinal sensations induced by gastric distention and duodenal lipid in humans. Gastroenterology 120:1100–1107. doi:10.1053/gast.2001.23232
Acknowledgements
We thank Dr. Orgânico, company affiliated to the FidBō group, for kindly donate virgin coconut oil for this research. We also thank Bioclin® for providing biochemical assays kits and Fundação de Amparo à Pesquisa do Estado de Minas Gerais—FAPEMIG, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq for financial support. These companies had no role in manuscript design, analysis, or writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study protocol was approved by the Ethics Committee of Universidade Federal de Viçosa (protocol number: 541,836/2014), conducted in accordance with 1964 Declaration of Helsinki and its later amendments and registered at http://www.ensaiosclinicos.gov.br/(RBR-8NGPQ9). All participants gave written consent after receiving verbal and written information.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
394_2017_1448_MOESM1_ESM.doc
Suppl. Figure 1 CONSORT diagram showing participants flow through each stage of the trial. CONSORT: Consolidated Standards of Reporting Trials (DOC 35 kb)
394_2017_1448_MOESM2_ESM.docx
Suppl. Figure 2 Mean ± SEM values of carbohydrate oxidation (A) and fat oxidation (B) rates in response to extra-virgin olive oil (control) or virgin coconut oil (test) intake (n = 15). For the sake of clarity, error bars are only given for the maximum and minimum values at each time point. P time×treatment values were obtained from RM-ANOVA in mixed model setting with time as within-subject factor and meal as between-subject factor. (DOCX 110 kb)
394_2017_1448_MOESM3_ESM.docx
Suppl. Figure 3 Mean ± SEM changes from baseline of self-reported desire to eat specific food types (a, b, c, and d) responses obtained from visual analog scales (VAS) in response to extra-virgin olive oil (control) or virgin coconut oil (test) intake (n = 15). iAUC: incremental area under the curve. For the sake of clarity, error bars are only given for the maximum and minimum values at each time point. P time×treatment values were obtained from RM-ANOVA in mixed model setting with time as within-subject factor and meal as between-subject factor. There were no significant changes in iAUC values (Paired t test, P > 0.05). (DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Valente, F.X., Cândido, F.G., Lopes, L.L. et al. Effects of coconut oil consumption on energy metabolism, cardiometabolic risk markers, and appetitive responses in women with excess body fat. Eur J Nutr 57, 1627–1637 (2018). https://doi.org/10.1007/s00394-017-1448-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1448-5