Abstract
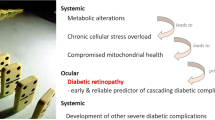
Diabetic retinopathy (DR) is among the leading causes of preventable blindness. Hyperglycemia, hypertension, hyperlipidemia and anemia majorly predispose its pathogenesis. The current treatment modalities of DR include laser photocoagulation therapy, intravitreal corticosteroids, intravitreal anti-vascular endothelial growth factor (VEGF) agents and vitreo-retinal surgery which are costly, highly invasive, unproven for prolonged use and opted in advanced stages of DR. By then retina already encounters a vast damage. Nutrients by their natural physiological, biochemical and molecular action can preserve retinal structure and functions by interfering with the various pathological steps prompting DR incidence, thereby altering the risk of developing this ocular morbidity. Nutrients can also play a central role in DR patients resistant towards the conventional medical treatments. However due to the byzantine interplay existing between nutrients and DR, the worth of nutrition in curbing this vision-threatening ocular morbidity remains silent. This review highlights how nutrients can halt DR development. A nutritional therapy, if adopted in the initial stages, can provide superior-efficacy over the current treatment modalities and can be a complementary, inexpensive, readily available, anodyne option to the clinically unmet requirement for preventing DR. Assessment of nutritional status is presently considered relevant in various clinical conditions except DR. Body Mass Index (BMI) conferred inconclusive results in DR subjects. Subjective Global Assessment (SGA) of nutritional status has recently furnished relevant association with DR status. By integrating nutritional strategies, the risk of developing DR can be reduced substantially. This review summarizes the subsisting knowledge on nutrition, potentially beneficial for preventing DR and sustaining good vision among diabetic subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is among the leading causes of preventable blindness worldwide [1]. It is a microangiopathy, majorly caused by hyperglycemia, hypertension, hyperlipidemia and anemia which through a series of pathological processes, contribute to the pathogenesis of DR [2–4]. The current treatment modalities of DR include laser photocoagulation therapy which is intrinsically harsh, suffering with unavoidable side effects of visual field loss and is not completely successful in reversing or arresting vision loss [5]. Intravitreal corticosteroids involve side effects such as infection, glaucoma and cataract formation [6]. Intravitreal anti-vascular endothelial growth factor (VEGF) agents administration is an invasive procedure, ensuing with the risk of endophthalmitis, retinal detachment along with the probability of imparting deleterious effects on the remaining healthy retina and can cause systemic complications as they can gorge into the systemic circulation which can be hazardous for diabetic patients with expected long-term administration [7]. Vitreo-retinal surgery is an expensive procedure, requiring experienced vitreoretinal specialists and is opted in advanced stages of DR [8]. By that time retina already encounters a vast damage. A wide array of nutrients, by their natural physiological, biochemical and molecular action, can preserve retinal structure and functions by interfering with the various pathological steps prompting DR incidence, thereby altering the risk of developing this ocular morbidity. Nutrients can also play a central role in DR patients resistant towards the conventional medical treatments. Recently nutrition-based approaches have gained momentum in various clinical conditions. As DR is a nutritionally responsive disorder, the defensive role of nutrition in daunting DR deserves spotlight. But probably due to the byzantine interplay existing between nutrients and DR, the worth of nutrition in curbing this vision-threatening disorder remains silent. A nutritional therapy, if adopted in the initial stages, can be an anodyne option proving effective, inexpensive and readily available in halting DR onset or progression. Nutrition-based approaches can provide superior efficacy and propose a complementary solution to the clinically-unmet requirement for preventing DR. This review is an effort to summarize the subsisting information on nutrition which can be applied for arresting the pathogenesis of DR and sustaining good vision among diabetic subjects.
Clinical manifestations of diabetic retinopathy
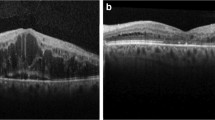
Clinically DR can be classified as Non-Proliferative Diabetic Retinopathy (NPDR) and Proliferative Diabetic Retinopathy (PDR) [9–11]. Non-Proliferative Diabetic Retinopathy is characterized by basement membrane hypertrophy, loss of pericytes and formation of microaneurysms. Pericyte loss causes development of microaneurysms, retinal vascular hyperpermeability and impaired blood-retina barrier resulting in diabetic macular edema and spillage of plasma lipoproteins, leading to formation of retinal hard exudates. Loss of pericyte also leads to capillary acellularity precipitating retinal ischemia. Retinal ischemia is among the primary angiogenic stimulus responsible for the ischemia-driven angiogenic pathology. Non-Proliferative Diabetic Retinopathy is also marked by inflammation which results in increased activation of leukocytes with elevated levels of inflammatory cytokines and adhesion molecules; leading to increased capillary stasis, occlusion and ultimately hypoxia [9, 10]. Hypoxia stimulates development and proliferation of new vessels which in due course, cause pathological vasoproliferation initiating PDR (Fig. 1) which may trigger contraction of the vitreous and fibrovascular proliferation thereby triggering retinal distortion and tractional detachment. Vision loss occurring at this stage is mainly caused by macular ischemia, chronic macular edema (Figs. 2, 3) and macular detachment [11]. Hyperglycemia and diabetes-induced metabolic changes alters the vitreous gel and vitreoretinal interface. Collagen fibril cross-linking and accumulation of advanced glycation end products (AGEs) augment vitreoretinal adhesion, incite retinal glial cell reactivity and alter concentration of various soluble proteins. Vitreous gel is an important regulator of intraocular oxygen tension having important implications in DR which is also precipitated by retinal hypoxia [10, 11].
Processes involved in pathogenesis of diabetic retinopathy
Hyperglycemia, hypertension and hyperlipidemia majorly cause decreased visual acuity or vision loss via certain pathological processes.
Aldose reductase pathway
Elevated intracellular glucose levels cause increased activation of the aldose reductase pathway which is also known as the polyol pathway or the sorbitol pathway. It results in decline of intracellular nicotinamide adenine dinucleotide phosphate (NADP) that reduces the production of nitric oxide (NO) in endothelial cells (ECs). This pathway also leads to chronic galactosemia causing vascular basement membrane changes, pericyte loss, development of microaneurysms and capillary acellularity. Excessive amount of galactose competes with glucose for the glucose transporters (GLUTs), thereby limiting the entry of glucose in retinal cells and diminishing glucose-requiring cellular energy metabolism [9].
Advanced Glycation End products (AGEs)
Formation of AGEs directly damage cells by impairing the function proteins, i.e., extracellular proteins like collagen and the intracellular proteins. The cellular effect of AGEs is mediated by its binding to AGE-receptors which initiates a cascade of signal transduction involving p21ras, p44/p42 mitogen activated protein kinase (MAPK), nuclear factor-κB (NF-κB) and protein kinase C (PKC). Activation of these intracellular kinases subsequently leads to cell dysfunction [9].
Reactive oxygen intermediates
Chronic hyperglycemia also increases oxidative stress. Free radicals such as superoxide anions are produced as byproducts of oxidative phosphorylation of glucose and by glucose autoxidation. High glucose levels increase their production causing oxidative stress. Oxidative stress reduces NO levels, promotes leukocyte adhesion to the endothelium, decreases barrier function of ECs, damages cellular proteins and activate PKC by increasing the formation of diacylglycerol. Free radicals can damage mitochondrial DNA (mtDNA) and cellular proteins [9].
Protein Kinase C (PKC)
Hyperglycemia can result in pathological activation of PKC. Protein Kinase C is a ubiquitous enzyme, capable of promoting vascular damage by increased vascular permeability, disruption of NO regulation, increased leukocyte adhesion to vessel walls and changes in blood flow without the involvement of the aldose reductase pathway. Protein Kinase C activation can influence MAPK or NF-κB pathways [9].
Renin–Angiotensin system(RAS)
Apart from hyperglycemia, RAS is another potential angiogenic mechanism participating in DR incidence. It induces microvascular complications such as vasoconstriction, inflammation, oxidative stress, cell hypertrophy and proliferation, angiogenesis and fibrosis [12]. Renin, an angiotensin-converting enzyme, and angiotensin II receptors are present in retinal and choroidal vessels and angiotensin acts as an angiogenic growth factor stimulating formation of new retinal blood vessels, by upregulating VEGF activity and other growth factors such as platelet-derived growth factor and connective tissue growth factor and also increases exudation from retinal vessels [13, 14].
Hyperlipidemia
Hyperlipidemia increases blood viscosity and alters fibrinolytic system causing hard exudates formation [15]. Hard exudates are lipoprotein deposits that are often associated with macular edema [16]. Apart from retinal thickening, the severity of retinal hard exudates is a significant risk factor for moderate visual loss and decreased visual acuity [17].
Nutrients and diabetic retinopathy
Nutrients can interfere with the discussed pathological processes, impeding the development and progression of DR (Fig. 4). Majority of the beneficial nutrients can be included in the diet of diabetic subjects through various foods rich in these nutrients (Table 1). Hence, the role of these nutrients in terms of this ocular morbidity needs to be highlighted.
Schematic representation showing various nutrients potential in halting the pathogenesis of diabetic retinopathy on earlier steps as compared to the contemporary treatment modalities (laser photocoagulation therapy, intravitreal corticosteroids and intravitreal anti-VEGF agents) which are indicated at the stage of diabetic macular edema. β-car β carotene, BF Bioflavonoids, C Curcuminoids, CA Caffeic acid, Cr Chromium, Cu Copper, DF Dietary fibres, DL Dietary lipids, DR Diabetic retinopathy, Fe Iron, I/R Ischemia Reperfusion, LA α-lipoic acid, L Lycopene, L/Z Lutein/Zeaxanthin, Mg Magnesium, Mn Manganese, Na Sodium, Pr Protein, PUFA Polyunsaturated fatty acids, RA Rosmarinic acid, RAS Renin Angiotensin System, Se Selenium, T Taurine, VEGF Vascular endothelial growth factor, Vit A Vitamin A, Vit B1 Vitamin B1, Vit B6 Vitamin B6 , Vit B9 Vitamin B9, Vit B12 Vitamin B12, Vit C Vitamin C, Vit D Vitamin D, Vit E Vitamin E, Zn Zinc
Carbohydrates
Carbohydrate intake was found associated with the severity of DR and consequent deterioration of visual acuity [18–20]. Carbohydrates when ingested in the form of sucrose, break down into glucose and fructose. Fructose was found responsible for retinal changes. Metabolism of fructose in diabetic retina lead to the formation of lactate which acts as a pathogenic agent in retinopathy development [18]. Fructose-rich diet also increased AGE formation [21].
Dietary fiber
Diabetic patients are advised a low carbohydrate diet recommending at least half of the daily energy intake to be derived from fiber-rich complex carbohydrates [22]. High-glycemic index foods on ingestion induce higher blood glucose concentration and chronically increased insulin demand leading to pancreatic exhaustion thereby resulting in glucose intolerance [23]. Dietary fibers lead to quicker intestinal transit by lessening carbohydrate absorption time in upper jejunum and decrease the insulin demand [24]. Dietary fibers slowed glucose response after ingestion, improved diabetic dyslipidemia, suppressed low-grade systemic inflammation and lowered blood pressure [25, 26]. Dietary fiber intake correlated with the progression and severity of DR [27].
Protein
Although, higher protein intake did not improve DR status [19, 20], it ameliorated glycemic control and hyperglycemia in diabetics and pre-diabetics without any pharmacological intervention [28, 29].
Amino acid ‘taurine’
Taurine is present in very high concentrations in the retina [30]. Dietary taurine supplementation ameliorated DR by normalizing retinal vascular function and attenuating induction of retinal VEGF which is associated with neovascularization [31]. Taurine lowered VEGF levels present in retinal homogenates by reducing oxidative stress [32]. Diffusion of nutrients from the vasculature is prevented in diabetes by AGE deposition along with developing a hypoxic environment for retinal pigment epithelium and photoreceptors. Taurine treatment reduced a high-fructose diet-induced AGE formation [33]. It attenuated glial fibrillary acid protein (GFAP) induction, which is a marker of gliosis and apoptosis in retinal glial cells in diabetes and also decreased retinal carbonyl dienes significantly [31]. Taurine supplementation ameliorated DR by anti-excitotoxicity of glutamate and declined glutamate levels, intermediate filament GFAP, N-methyl-D-aspartate receptor subunit 1 expression and gamma aminobutyric acid levels and it also increased glutamate transporter expression in diabetic retina [34].
Lipids
Hyperlipidemia increases blood viscosity and alters fibrinolytic system causing hard exudates formation [15]. Hard exudates accumulation at the center of the macula deteriorates visual acuity [17]. Elevated lipids caused hypoxia, increased LDL oxidation, release of cytokines and growth factors and endothelial dysfunction (ED). Endothelial dysfunction in diabetic vasculature leads to breakdown of blood–retinal barrier [15]. Lipids exudate through damaged retinal vasculature, causing diabetic macular edema [16]. Retinal hemorrhage and edema also occurs due to triglycerides incorporation into cell membranes causing alteration in membrane fluidity leading to plasma constituent leakage in the retina [15]. Small low-density lipoprotein (LDL) particles easily cross the endothelium and are readily oxidized. Oxidized LDL has prothrombotic effect which is mediated by PKC activation. It is toxic to pericytes and ECs of the retinal capillary contributing to retinal capillary injury. Very low-density lipoprotein (VLDL) increases plasminogen activator inhibitor-1 secretion by ECs. High-density lipoprotein is found to have a protective role against retinopathy due to its paraoxonase activity which detoxifies the lipid peroxidation products [35]. Previous studies reported elevated lipid levels as an important contributing factor in the pathogenesis of DR [15, 16, 35]. Alteration and reduction in dietary lipid intake in order to sequentially reduce serum lipids for halting the pathogenesis of DR has been proposed by previous studies [36, 37].
Fatty acids
α-Lipoic acid
It is an organosulphur compound derived from octanoic acid which is an eight carbon saturated fatty acid [38]. α-Lipoic acid is a biological antioxidant with reactive oxygen species (ROS) scavenging potentials [39]. It inhibited the development of DR by preventing accumulation of oxidatively modified DNA, diabetes-induced increase in nitrotyrosine levels and decrease in retinal mitochondrial and cytosolic ratios of oxidized and reduced nicotinamide adenine dinucleotide (NAD + and NADH) and also prevented activation of NF-κB and decreased VEGF levels and oxidatively modified proteins in retina [40, 41]. It protected retina against ischemia–reperfusion injury which is one of the major cause of vision loss in DR [42].
Poly Unsaturated Fatty Acids (PUFAs)
Poly unsaturated fatty acids can inhibit the development and progression of DR [18, 22, 43–45]. Poly unsaturated fatty acids metabolites i.e., lipoxins, resolvins and protectins exhibited anti-inflammatory action by suppressing interleukin (IL)-6, tumor necrosis factor (TNF)-α, VEGF and ROS and also restored antioxidant homeostasis. Production of brain-derived neurotrophic factor is augmented by PUFA which can protect retinal neuronal cells from degeneration caused by DR [45]. Poly unsaturated fatty acids according to their chemical structure can be classified into two groups which are ω-3 and ω-6 fatty acids [46]. Linoleic acid, an ω-6 fatty acid and α-linoleic acid, an ω-3 fatty acid are essential fatty acids required to be supplemented through diet. ω-3 and ω-6 essential fatty acids are metabolized into longer chain PUFAs by elongase and desaturase enzymes [47]. Linoleic acid is metabolized to arachidonic acid and α-linoleic acid to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Retina is rich in arachidonic acid, EPA and DHA [45]. Neurons need a steady glucose supply and neuronal glucose uptake depends on extracellular glucose concentration. Diabetes-induced persistent hyperglycemia leads to glucose neurotoxicity. ω-3 PUFAs improved glucose tolerance and preserved retinal functions in diabetes [44]. Compromised endothelial progenitor cells lead to inadequate vascular repair in DR. Acid sphingomyelinase participates in dysfunction of endothelial progenitor cells (EPCs) and initial retinal damage. A DHA-rich diet inhibited acid sphingomyelinase in both, EPCs and retina thereby correcting EPCs number and function, preventing diabetes-induced retinal vascular pathology and suppressing retinal inflammation [43]. Poly unsaturated fatty acids, especially EPA and DHA prevented IL-6 and TNF-α production and suppressed intercellular cell adhesion molecule-1 and vascular cell adhesion molecule expression and VEGF secretion. High-glucose-induced retinal vascular endothelial damage is prevented by linoleic acid and arachidonic acid [45].
Vitamins
Vitamin A
Vitamin A or retinol protected retina from hyperglycemia-induced retinal hazards in diabetes as it is required for normal cell growth and its deficiency can initiate retinal changes like cell proliferation in DR [48]. It retarded neovascularization and retinal pigment epithelium cell proliferation [49]. Previous study confirmed relationship between vitamin A deficiency and DR occurrence [48].
Vitamin B
Association of B vitamins and DR have been reported by previous studies [50, 51]. Hyperglycemia in diabetic milieu promotes vascular superoxide production, sequentially inactivating NO expression by ECs leading to vascular degeneration. B vitamins enhance NO production and are specifically essential in maintaining the overall vascular system integrity [50]. Pyridoxamine or vitamin B6 inhibited late stages of glycation reactions that cause AGE formation, thus being capable of protecting against premature pericyte cell death temporally maintaining capillary viability. It also inhibited acellular strand formation in diabetic retina thereby maintaining microvascular cellularity [52]. Biotin or vitamin B7 supplementation improved glucose management by enhancing glucose uptake in skeletal muscle cells and increasing glucose disposal. It was also found potential in improving lipid metabolism [53]. A low concentration folic acid or vitamin B9 and cobalamine or vitamin B12 increased the risk of vascular damage by homocysteine [51]. It hinders retinal arterioles dilation through NO-mediated pathway and induces apoptosis in retinal ganglion cells by increasing expression of Bcl-2-associated X protein, a pro-apoptotic protein expression, found in increased levels in diabetic retinas [50, 54, 55]. Homocysteine, if incorporates with proteins by a disulphide or amide linkage, it causes significant oxidative stress and inflammation [50]. It also disrupts glutamate homeostasis by acting as an agonist at the glutamate site of N-Methyl-D-Aspartate receptors [56, 57]. High homocysteine levels are toxic to pericytes and EC lining of the walls of retinal blood vessels causing vascular thrombosis and microaneurysms [50]. Homocysteine is detoxified by methionine synthetase, which depends on vitamin B9 and B12 as coenzymes for its proper functioning [58]. Dietary deficiency of folic acid and cobalamine causes hyperhomocysteinemia [51]. Supplementation of vitamin B9 and B12 reduced homocysteine levels [59].
Vitamin C
Development of DR may be prevented by vitamin C intake as it is a chain-breaking antioxidant, scavenging ROS directly, preventing breakdown of NO and decreasing LDL oxidation [60]. It reduces platelet aggregation, affects retinal blood flow and acts as an aldose reductase inhibitor of the hyperglycemia-induced polyol pathway [61–63]. It protects against detrimental effects of high oxidative stress occurring due to non-enzymatic glycosylation, auto-oxidative glycosylation and metabolic stress among diabetics [64]. Previous studies reported lower vitamin C levels in DR patients compared to the ones without retinopathy [65, 66].
Vitamin D
Vitamin D is a potent inhibitor of angiogenesis and its anti-angiogenic effect may be mediated by vitamin D receptor present in the retina [67]. Vitamin D suppressed RAS, creating anti-inflammatory and immune-suppressive effect [68, 69]. It decreased VEGF, replication of vascular smooth muscle cells and cytokine production by downregulating NF-κB and increasing IL10 thus reducing inflammation. It reduced negative effect of AGEs on ECs and inhibited production and activity of tissue matrix metalloproteinases that induce thrombosis. Vitamin D is required for efficient insulin secretion and insulin functioning [70]. Insulin-like growth factor-1 was found associated with DR [71]. Active forms of vitamin D regulate several insulin-like growth factor binding protein genes [72]. Prior studies reported association of vitamin D with DR [70].
Vitamin E
Vitamin E is present in retina predominantly in the form of α-tocopherol [73]. The role of vitamin E in preventing DR owes to its free radical scavenger activity outside the cells by non-enzymatic mechanism [74]. It can normalize diabetic retinal hemodynamics and reduce production of VEGF and overproduction of intercellular cell adhesion molecule-1 [75]. It inhibited hyperglycemia-induced diacylglycerol PKC pathway in retinal tissues that caused decrease in retinal blood flow promoting retinopathy [74, 76]. Thus, vitamin E can prevent pathogenesis of DR.
Carotenoids
Carotenoids can be discriminated into pro-vitamin A and non pro-vitamin A carotenoids. β-carotene is the only pro-vitamin A carotenoid present in the ocular tissue. The non-pro-vitamin A carotenoids present in the ocular tissue in high concentrations are lycopene and lutein/zeaxanthin [77].
β-carotene
β-carotene, due to its antioxidant properties, protected retina from the active free radicals rendered degeneration [78]. It can protect against hyperglycemia hazards like initiation of cell proliferation involved in DR [48].
Lycopene
It is the most potent singlet oxygen quencher carotenoid. It modulated lipoxygenase activity and in turn inflammation and immune function. It increased antioxidant activity in ocular capillaries [77]. Significantly lower serum lycopene levels were observed in patients with advanced stages of DR [79].
Lutein/zeaxanthin
They are isomers of each other and the only carotenoids present in the retina. They have antioxidant effect and improved visual acuity. They inhibited free radicals combining with retinal collagen, strengthened retinal collagen structure, reduced vascular permeability and leakage and maintained blood vessel integrity [80]. They can reduce the risk of DR pathogenesis [77, 80].
Bioflavonoids
Bioflavonoids exhibit antioxidant activity by scavenging free radicals directly; inhibiting enzymes responsible for superoxide production and chelating ROS enhancer trace elements. They improved ocular blood flow, decreased angiogenesis and vascular leakage, inhibited aldose reductase activity and also exhibited anti-inflammatory activity [81]. They also prevented neuronal degeneration in inner retina resulting from ischemic injury [82]. The usual source of bioflavonoids are pulp and rinds of fruits and vegetables. They are also present in green tea which can be beneficial in controlling DR when recommended as nutritional supplements [83, 84].
Green tea
Green tea or Camellia sinensis is a rich source of catechins whose antioxidant activity is many times higher than vitamin C and E. Green tea suppressed hyperlipidemia, reduced plasma hydroperoxides, ameliorated retinal superoxide formation and also normalized erythrocyte glutathione [83]. Green tea prevented angiogenesis by inhibiting hypoxia-inducible factor-1 alpha protein expression and sequentially turned down VEGF expression [85]. Green tea also reduced anion production level and restored glutamate transporter, glutamate receptor and glutamine synthetase thereby maintaining retinal functions equivalent to non-diabetics. It inhibited acellular capillaries, pericyte ghost formation and ameliorated structural lesions in DR [84].
Curcuminoids
They are turmeric constituents. Curcumin, which is one of the curcuminoids, prevented diabetes-induced decrease in total antioxidant capacity of retina [86]. It inhibited accumulation of 8-hydroxyl-2′ deoxyguanosine in diabetic retina [87]. The anti-inflammatory action of curcumin targets anti-inflammatory biomarkers such as 5-hydroxy-eicosatetraenoic acid, cyclooxygenase and lipoxygenase [88, 89]. Dietary supplementation of curcumin prevented IL-1β, VEGF, TNF-α and diabetes-induced NF-κB activation [86, 87]. The anti-angiogenic activity of curcumin includes decrease in stromal cell-derived factor-1-induced migration of human retinal ECs, VEGF-induced PKC-β II translocation and inhibition of increased VEGF levels in retina [86, 90, 91]. Oral administration of curcumin inhibited increase of retinal acetylated histones that were noticed in the development of DR [92].
Caffeic acid
It demonstrated antioxidant and potential anti-angiogenic activity on retinal ECs and retinal neovascularization respectively by suppressing ROS-induced VEGF expression. It effectively inhibited VEGF-induced retinal EC proliferation and VEGF-induced migration and tube formation of retinal ECs [93]. Caffeic Acid Phenethyl Esters, a caffeic acid derivative protected retina from ischemia/reperfusion injury by enhancing antioxidation ability and preventing retinal cell apoptosis [94]. It inhibited lipid peroxidation in retina by scavenging peroxy radicals, reduced NO overproduction and nitrosative stress and regulated superoxide dismutase enzyme activity in diabetic retina [95].
Rosmarinic acid
It exhibited anti-inflammatory and antioxidant properties [96]. It decreased intracellular ROS levels, IL-8 release and VEGF expression [97]. It also showed anti-angiogenic activity to retinal neovascularization and inhibited retinal EC proliferation and angiogenesis [98].
Minerals
Zinc
It is present in retina in high concentrations and can protect retina from ROS-induced pericyte apoptosis [99]. Zinc acted as an antioxidant as it halted free radicals formation by inhibiting NADPH oxidase [100]. It is essential in copper–zinc superoxide dismutase formation which is required for maintaining erythrocyte antioxidant defense enzymes, preventing diabetes-induced plasma malondialdehyde increase, protecting retina against diabetes-induced increase in lipid peroxidation by binding and stabilizing cell membranes thus preventing their disintegration by inducing metallothioneins production [101, 102]. Zinc can prevent neovascularization by inhibiting VEGF expression and inflammatory cytokine production by suppressing NF-κB activation. It prevented vascular leakage in DR [99].
Chromium
Chromium deficiency led to elevated blood glucose levels [103]. Since retina is a high energy-demanding tissue, glucose uptake, regulation and utilization is required for maintaining normal retinal functions [104]. Glucose transporter proteins, GLUT-1 and GLUT-3, transport glucose across the blood–retinal barrier in the retina [105, 106]. In DR, GLUT-1 and GLUT-3 expression is reduced. Chromium histidinate, a chromium compound, increased GLUT-1 and GLUT-3 expression for compensating the retinal glucose need [107]. Retina being rich in polyunsaturated lipid membranes is very sensitive to ROS that cause lipid peroxidation. Retinal oxidative stress is induced by hyperglycemia [108]. Trivalent chromium supplementation can reduce cellular oxidative stress and blood levels of pro-inflammatory cytokines and lipids [109]. Chromium supplements can improve retinal functions as well as blurred vision [103, 110, 111]. Dietary chromium supplementation suppressed diabetes-induced retinal tissue damage [108].
Selenium
It is one of the major non-enzymatic antioxidants of the body [112]. Selenium prevented the hazards of DR by downregulating VEGF production, ameliorating diabetes-induced biochemical retinal abnormalities and protecting cells against oxidative damage caused by peroxides generated from lipid metabolism [113–115]. It protected from oxidative stress by modulating cellular response and inhibiting ROS production. Glutathione peroxidase, a very important antioxidant, catalyzing decomposition of ROS, is a selenium-dependent enzyme. Selenium deficiency exacerbates oxidative stress in diabetes. It complements vitamin E functioning against oxidative stress by affecting the absorption and biological activity of vitamin E and preventing its decomposition [116].
Magnesium
Hypomagnesemia is a risk factor in DR development [117]. Magnesium deficiency induced pro-inflammatory and pro-fibrogenic response and also oxidative stress due to reduction of certain protective enzymes [118–120]. Magnesium deficiency can interfere with the normal cell growth and apoptosis regulation as it plays an important role in DNA synthesis and repair [121]. Low magnesium levels caused microvascular complications by inhibiting procyclin receptor function creating imbalance between prostacyclin and thromboxane effect [122]. As magnesium is a physiological calcium antagonist, its low levels can cause vascular calcification and increased platelet aggregation promoting EC dysfunction and thrombogenesis [123, 124]. Increase in peripheral intracellular free calcium concentration also inhibited insulin action on glucose uptake further worsening hyperglycemia [123]. Magnesium also acts as a co-factor of the glucose transport mechanism in the cell membranes and aids carbohydrate oxidation enzymes, insulin secretion, binding and activity [125]. Oral magnesium supplementation improved insulin sensitivity and metabolic control in diabetic subjects with low serum magnesium levels [126].
Manganese
Manganese in the form of manganese superoxide dismutase inhibited oxidative stress and prevented DR development [127]. Diabetes cause dysfunctional retinal mitochondria, decrease in reduced glutathione levels, apoptosis of retinal capillary cells and lesions in retinal ECs. Manganese superoxide dismutase overexpression protects mitochondrial encoded genes and inhibited mtDNA damage preventing the plausible mechanism of DR pathogenesis [128].
Copper
It is an essential dietary micronutrient. Its deficiency caused altered glucose metabolism, hypercholesterolemia, compromised oxidant defense system, increased diabetes-induced glycation, peroxidation and nitration. Copper deficiency led to decreased activity of oxidant defense enzyme copper-zinc superoxide dismutase and selenium-dependent glutathione peroxidase and altered ROS scavengers like glutathione and metallothioneins altering oxidant defense system causing excessive oxidative stress and tissue damage [129]. But hyperglycemia induces fragmentation of copper-containing enzymes like ceruloplasmin and copper–zinc superoxide dismutase releasing copper ions in the blood [130]. Free copper participates in Fenton and Haber–Weiss reactions generating ROS, thus behaving as a potential cytotoxic element by increasing oxidative stress and AGEs formation [131]. Previous studies reported raised serum copper levels with the presence and increasing severity of DR [130, 132].
Iron
Iron deficiency caused by nutritional anemia precipitates DR. Treating nutritional anemia by iron, vitamin B1, B6, B9 and B12 supplements led to spontaneous closure of microaneurysms, reduced superficial hemorrhages and cotton-wool spots and improved visual acuity [133]. Anemia induced retinal hypoxia [134]. Treating anemia increased tissue oxygenation and reduced VEGF production which further halted the stimulus for neovascularization and improved hyperpermeability [135]. One-third of anemia occurs due to nutrient deficiency including iron deficiency, either alone or together with folate or vitamin B12 deficiency [136]. Iron deficiency anemia rapidly led to PDR pathogenesis [3]. Hyperglycemia completely destroys heme molecules of hemoglobin and myoglobin, releasing free iron. Free iron is a highly pro-oxidant molecule capable of generating powerful ROS and stimulates expression of monocyte endothelial adhesion and adhesion molecules which leads to pathogenesis of DR. Free iron catalyzes binding of AGEs to specific receptors and regulates L-glutamate production which is involved in retinal neurodegeneration [137]. As these activities lead to the pathogenesis of DR, maintaining iron homeostasis is essential.
Sodium
Macular edema, a leading cause of visual impairment in DR was found associated with sodium intake. Lowering sodium intake reduced blood pressure. Hypertension is among the risk factors of macular edema. Hence, high sodium intake was reported to be a risk factor in the progression of DR [138].
Nutritional status and diabetic retinopathy
Body Mass Index (BMI)
Many studies found an association between BMI and obesity with DR but the relationship between BMI and the associated risk of DR remained inconclusive [139–143]. This inconsistency could be due to the fact that BMI is deflected by the weight change of diabetic subjects. Diabetic retinopathy increases with uncontrolled diabetes which also causes unintentional weight loss and a low BMI. Hence, a low BMI can be associated with increasing severity of DR. Concurrently, obesity or a high BMI is often correlated with escalating grade of DR. This can be explained by the fact that obesity increases inflammatory markers. Adipose tissue secretes adipokines, such as IL-6, TNF-α, leptin and adiponectin. They regulate lipid levels, inflammation, oxidative stress, insulin resistance and diabetes occurrence [144] Obesity-associated oxidative stress and inflammation caused ED which leads to pathogenesis of DR. [145] Raised plasma leptin levels in obesity caused vascular EC proliferation, angiogenesis and neovascularization whereas; obesity associated low adiponectin levels led to insulin resistance [146, 147] Obesity also initiates hyperlipidemia and hypertension which are amongst the reckoned risk factors of DR.
Subjective Global Assessment (SGA)
Evaluation of nutritional status of DR subjects by BMI conferred ambiguous results. Subjective Global Assessment (SGA) is a consistent, dependable and reproducible clinical assessment method of nutritional status, based on the medical history and physical examination of the subject providing thorough estimation of the nutritional status as demonstrated by the previous studies in different clinical conditions [148, 149]. Subjective Global Assessment scores correlated with the presence and increasing severity of DR in our studies recently [150, 151]. Uncontrolled diabetes leads to increase in the grade of DR and is often accompanied by co-morbidities like diabetic gastropathy and nephropathy. These co-morbidities cause change in dietary intake, gastrointestinal disturbances, edema and weight fluctuations. Subjective Global Assessment scores are calculated after evaluating the overall health status of the subject than merely the anthropometric indices. Hence, SGA might overcome the ambiguities arising from BMI and may provide more discreet results for DR subjects.
Conclusion
In summary, nutritional strategies can substantially reduce the risk of developing DR, proving quite beneficial in DR cases resistant towards the conventional medical treatments. Nutritional treatment can preserve the normal physiology, structure and functions of retina. Nutrition-based approaches have a high potential to be developed as adjunct therapy for arresting the occurrence or progression of DR in early stages and can serve as a non-invasive and cost-effective treatment which can be within the means of every socioeconomic status. As the current treatment modalities are highly invasive, expensive and unproven for prolonged use due to their certain side effects, nutrition-based approaches can evolve as dependable, complementary therapies to the existing DR treatment inhibiting development of retinopathy and subsequent loss of vision in diabetic subjects.
References
Song MK, Roufogalis BD, Huang THW (2012) Modulation of diabetic retinopathy pathophysiology by natural medicines through PPAR-γ- related pharmacology. Br J Pharmacol 165:4–19
Rani PK, Raman R, Rachepalli SR, Pal SS, Koluthungan V, Lakshmipathy P, Satagopan U, Kumaramanikavel G, Sharma T (2010) Anemia and diabetic retinopathy in type 2 diabetes mellitus. J Assoc Physicians India 58:91–94
Shorb SR (1985) Anemia and diabetic retinopathy. Am J Ophthalmol 100(3):434–436
Soro-Paavonen A, Forbes JM (2006) Novel therapeutics for diabetic micro and macrovascular complications. Curr Med Chem 13:1777–1788
Mohamed Q, Gillies MC, Wong TY (2007) Management of diabetic retinopathy: a systematic review. JAMA 298:902–916
Simó R, Hernández C (2009) Advances in the medical treatment of diabetic retinopathy. Diabetes Care 32:1556–1562
Simó R, Hernández C (2008) Intravitreous anti-VEGF for diabetic retinopathy: hopes and fears for a new therapeutic strategy. Diabetologia 51:1574–1580
Joussen AM, Joeres S (2007) Benefits and limitations in vitreoretinal surgery for proliferative diabetic retinopathy and macular edema. Dev Ophthalmol 39:69–87
Zhang K, Ferreyra HA, Grob S, Bedell M, Zhang JJ (2013) Diabetic retinopathy: genetics and etiologic mechanisms. In: Ryan SJ (ed) Retina, 5th edn. Elsevier Saunders, China, pp 925–939
Wiley HE, Ferris FL III (2013) Non proliferative diabetic retinopathy and diabetic macular edema. In: Ryan SJ (ed) Retina, 5th edn. Elsevier Saunders, China, pp 940–968
Silva PAS, Cavallerano JD, Sun JK et al (2013) Proliferative diabetic retinopathy. In: Ryan SJ (ed) Retina, 5th edn. Elsevier Saunders, China, pp 969–1000
Wilkinson- Berka JL (2006) Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol 38(5–6):752–765
Wright AD, Dodson PM (2010) Diabetic retinopathy and blockade of the renin- angiotensin system: new data from the DIRECT study programme. Eye 24(1):1–6
Sjoli AK, Dodson P, Hobbs FRR (2011) Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int J Clin Pract 65(2):148–153
Rema M, Srivastava BK, Anitha B, Deepa R, Mohan V (2006) Association of serum lipids with diabetic retinopathy in urban South Indians- the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-2. Diabet Med 23:1029–1036
Alex AA, Adinortey MB, Lartey S, Amponsah I, Boadi- Kusi SB, Kyei S, Ocansey S, Abu EK, Banahene FO, Okoh C (2014) Is there any association between serum lipids and diabetic retinopathy in type 2 diabetic patients in Ghana? Int J Trop Dis Health 4(4):457–468
Ferris FL III, Patz A (1984) Macular edema: a complication of diabetic retinopathy. Surv Ophthalmol 28:452–461
Solomon YC (1979) Diabetic retinopathy and carbohydrate metabolism. Proc Nutr Soc 38:351–358
Elshazly LH, Samy N, Sebaay FE (2010) Risk factors association with diabetic retinopathy and maculopathy in Egyptian type 2 diabetics. Aust J Basic Appl Sci 4(12):6233–6238
Roy MS, Stables G, Collier B, Roy A, Bou E (1989) Nutritional factors in diabetics with and without retinopathy. Am J Clin Nutr 50:728–730
Krajcovicova-Kudlackova M, Sebekova K, Schinzel R, Klvanova J (2002) Advanced glycation end products and nutrition. Physiol Res 51:313–316
Williams JH, Patel P, Jelfs R, Carter RD, Awdry P, Bron A, Mann JI, Hockaday TDR (1985) Polyunsaturated fatty acids and diabetic retinopathy. Br J Ophthalmol 69:15–18
Willet W, Manson J, Liu S (2002) Glycemic index, glycemic load and risk of type 2 diabetes. Am J Clin Nutr 76(suppl):274S–280S
Cummings JH, Englyst HN (1987) Fermentation in the human large intestine and the available substrates. Am J Clin Nutr 45:1243–1255
Tanaka S, Yoshimura Y, Kawasaki R, Kamada C, Tanaka S, Horikawa C, Ohashi Y, Araki A, Ito H, Akanuma Y et al (2013) Fruit intake and incident diabetic retinopathy with type 2 diabetes. Epidemiology 24(2):204–211
Fujii H, Iwase M, Okhuma T, Ogata-Kaizu S, Ide H, Kikuchi Y, Idewaki Y, Joudai T, Hirakawa Y, Uchida K et al (2013) Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J 12:159
Ganesan S, Raman R, Kulothungan V, Sharma T (2012) Influence of dietary-fibre intake on diabetes and diabetic retinopathy: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetic Study (report 26). Clin Experiment Ophthalmol 40(3):288–294
Campbell AP, Rains TM (2015) Dietary protein is important in the practical management of prediabetes and type 2 diabetes. J Nutr 145(1):164S–169S
Gannon MC, Nuttall FQ (2004) Effect of a high protein, low carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 53:2375–2382
Pasantes-Morales H, Klethi J, Ledig M, Mandel P (1972) Free amino acids of chicken and rat retina. Brain Res 41(2):494–497
Ito T, Schaffer SW, Azuma J (2012) The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids 42:1529–1539
Obrosova IG, Minchenko AG, Marinescu V, Fathallah L, Kennedy A, Stockert CM, Frank RN, Stevens MJ (2001) Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia 44(9):1102–1110
Nandhini AT, Thirunavukkarasu V, Anuradha CV (2004) Stimulation of glucose utilization and inhibition of protein glycation and AGE products by taurine. Acta Physiol Scand 181(3):297–303
Yu X, Xu Z, Mi M, Xu H, Zhu J, Wei N, Chen K, Zhang Q, Zeng K, Wang J et al (2008) Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem Res 33(3):500–507
Lyon TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, Klein RL, The DCCT/EDIC research group (2004) Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci 45(3):910–918
Eck WFV (1959) The effect of a fat diet on the serum lipids in diabetes and its significance in diabetic retinopathy. Am J Med 27(2):196–211
King RC, Dobree JH, Kok DA, Foulds WS, Dangerfield WG (1963) Exudatives diabetic retinopathy. Spontaneous changes and effects of a corn oil diet. Brit J Ophthal 47:666–672
Packer L, Kraemer K, Rimbach G (2001) Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 17:888–895
Packer L, Witt E, Tritchler H (1995) Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250
Kowluru RA, Odenbach S (2004) Effect of long term administration of alpha lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes 53:3233–3238
Lin J, Bierhaus A, Bugert P, Dietrich N, Feng Y, Vom Hagen F, Nawroth P, Brownlee M, Hammes HP (2006) Effect of R-(+)- alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia 49:1089–1096
Chidlow G, Schmidt KG, Wood J, Melena J, Osborne N (2002) Alpha lipoic acid protects the retina against ischaemia-reperfusion. Neuropharmacology 43:1015–1025
Tikhonenko M, Lydic TA, Opreanu M, Li Calzi S, Bozack S, McSorley KM, Sochacki AL, Faber MS, Hazra S, Duclos S et al (2013) N-3 polyunsaturated fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS One 8(1):e55177
Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ et al (2012) Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr. Diabetes 2:e36
Shen J, Bi YL, Das UN (2014) Potential role of polyunsaturated fatty acids in diabetic retinopathy. Arch. Med Sci 10(6):1167–1174
Koto T, Nagai N, Mochimaru H, Kurihara T, Izumi-Nagai K, Satofuka S, Shinoda H, Noda K, Ozawa Y, Inoue M et al (2007) EPA is anti-inflammatory in preventing choroidal neovascularization in mice. Invest Ophthalmol Vis Sci 48(9):4328–4334
Querques G, Forte R, Souied EH (2011) Retina and omega-3. J Nutr Metab 2011:748361
Osman ZM, Gomaa AM, Hussein HM Soliman, El-Shobaki AF (2004) Association between retinol metabolism and diabetic retinopathy. Pol J Food Nutr Sci 13/54(4):391–396
Schonfeld CL (2000) All-trans-retinol (atR) inhibits expression of the metalloproteinase stromelysin in retinal pigment epithelium (RPE). Ophthalmologe 97:532–536
Smolek MK, Notaroberto NF, Jaramillo AG, Pradillo LR (2013) Intervention with vitamins in patients with nonproliferative diabetic retinopathy: a pilot study. Clin Ophthalmol 7:1451–1458
Satyanarayana A, Balakrishna N, Pitla S, Reddy PY, Mudili S, Lopamudra P, Suryanarayana P, Viswanath K, Ayyagari R, Reddy GB (2011) Status of B vitamins and homocysteine in diabetic retinopathy: association with vitamin B12 deficiency and hyperhomocysteinemia. PLoS One 6(11):e26747
Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich M, Baynes JW et al (2002) The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes 51:2826–2832
Singer GM, Geohas J (2006) The effect of chromium picolinate and biotin supplementation on glycemic controlin poorly controlled patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomized trial. Diabetes Technol Ther 8(6):636–643
Kern TS, Du Y, Miller CM, Hatala DA, Levin LA (2010) Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol 176:2550–2558
Martin PM, Roon P, van Ells TK, Ganapathy V, Smith SB (2004) Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 45:3330–3336
Ganapathy PS, White RE, Ha Y, Bozard BR, McNeil PL, Caldwell RW, Kumar S, Black SM, Smith SB (2011) The role of N-methyl-D-aspartate receptor activation in homocysteine-induced death of retinal ganglion cells. Invest Ophthalmol Vis Sci 52:5515–5524
Diederen RM, La Heij EC, Deutz NE, Kijlstra A, Kessels AG, van Eijk HM, Liem AT, Dieudonné S, Hendrikse F (2006) Increased glutamate levels in the vitreous of patients with retinal detachment. Exp Eye Res 83:45–50
Wijekoon EP, Brosnan ME, Brosnan JT (2007) Homocysteine metabolism in diabetes. Biochem Soc Trans 35
Stanger O, Herrmann W, Pietrzik K, Fowler B, Geisel J, Dierkes J, Weger M, DACH-LIGA Homocystein e.V (2003) DACH-LIGA homocystein (german, austrian and swiss homocysteine society): consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: guidelines and recommendations. Clin Chem Lab Med 41:1392–1403
Young I, Tate S, Lightbody J, Mcmaster D, Trimble E (1995) The effect of desferrioxamine and ascorbate on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med 18:833–840
Wilkinson I, Megson I, Maccallum H, Sogo N, Cockcroft JR, Webb DJ (1999) Oral vitamin C reduces arterial stiffness and platelet aggregation in humans. J Cardiovasc Pharmacol 34:690–693
Wang H, Zhang Z, Wen R, Chen J (1995) Experimental and clinical studies on the reduction of erythrocyte sorbitol-glucose ratios by ascorbic acid in diabetes mellitus. Diabetes Res Clin Pract 28:1–8
Millen A, Klein R, Folsom AR, Stevens J, Patla M, Mares JA (2004) Relation between intake of vitamin C and E and risk of diabetic retinopathy in the atherosclerosis risk in Communities Study. Am J Clin Nutr 79:865–873
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9
Sinclair AJ, Girling AJ, Gray L, Lunec J, Barnett AH (1992) An investigation of the relationship between free radical activity and vitamin C metabolism in elderly diabetic subjects with retinopathy. Gerontology 38:268–274
Gupta MM, Chari S (2005) Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Indian J Physiol Pharmacol 49:187–192
Taverna MJ, Selam JL, Slama G (2005) Association between a protein polymorphism in the start codon of the vitamin D receptor gene and severe diabetic retinopathy in C-peptide-negative type 1 diabetes. J Clin Endocrinol Metab 90:4803–4808
Mathieu C, Adorini L (2002) The coming age of 1,25-dihyroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med 8(4):174–179
Judd SE, Tangpricha V (2011) Vitamin D therapy and cardiovascular health. Curr Hypertens Rep 13(3):187–191
Bonakdaran S, Shoeibi N (2015) Is there any correlation between vitamin D insufficiency and diabetic retinopathy? Int J Ophthalmol 8(2):326–331
Wilkinson-Berka JL, Wraight C, Werther G (2006) The role of growth hormone, insulin-like growth factor and somatostatin in diabetic retinopathy. Curr Med Chem 13(27):3307–3317
Matilainen M, Malinen M, Saavalainen K, Carlberg C (2005) Regulation of multiple insulin-like growth factor binding protein genes by 1alpha,25-dihydroxyvitamin D3. Nucleic Acids Res 33(17):5521–5532
Alvarez R, Liou G, Fong S (1987) Levels of alpha- and gamma-tocopherol in human eyes: evaluation of the possible role of IRBP in intraocular alpha-tocopherol transport. Am J Clin Nutr 46:481–487
Bursell S, Clermont A, Aiello LP, Aiello LM, Schlossman DK, Feener EP, Laffel L, King GL (1999) High dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care 22:1245–1251
Mamputu JC, Renier G (2004) Advanced glycation end-products increase monocyte adhesion to retinal ECs through vascular endothelial growth factor-induced ICAM-1 expression: inhibitory effect of antioxidants. J Leukoc Biol 75:1062–1069
Kunisaki M, Bursell SE, Clermont AC, Ishii H, Ballas LM, Jirousek MR, Umeda F, Nawata H, King GL (1995) Vitamin E prevents diabetes-induced abnormal retinal blood flow via the diacylglycerol protein kinase C pathway. Am J Physiol 269:E239–E246
Brazionis L, Rowley K, Itsiopoulos C, O’Dea K (2009) Plasma carotenoids and diabetic retinopathy. Br J Nutr 101:270–277
Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME (1999) Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 18:4633–4644
Li ZZ, Lu XZ, Ma CC, Chen L (2010) Serum lycopene levels in patients with diabetic retinopathy. Eur J Ophthalmol 20(4):719–723
Hu BJ, Hu YN, Lin S, Ma WJ, Li XR (2011) Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int. J Ophthalmol 4(3):303–306
Majumdar S, Srirangam R (2010) Potential of the bioflavonoids in the prevention/ treatment of ocular disorders. J Pharm Pharmacol 62:951–965
Hayashi A, Weinberger A, Kim H, De Juan EJ (1997) Genistein, a protein tyrosine kinase inhibitor, ameliorates retinal degeneration after ischemia-reperfusion injury in rat. Invest Ophthalmol Vis Sci 38:1193–1202
Mustata GT, Rosca M, Biemel KM, Reihl O, Smith MA, Viswanathan A, Strauch C, Du Y, Tang J, Kern TS et al (2005) Paradoxical effects of green tea (Camellia sinensis) and antioxidant vitamins in diabetic rats: improved retinopathy and renal mitochondrial defects but deterioration of collagen matrix glycoxidation and cross-linking. Diabetes 54:517–526
Silva KC, Rosales MAB, Hamassaki DE, Saito KC, Faria AM, Ribeiro PAO, Lopes di Faria JB, Lopes di Faria JM (2013) Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci 54(2):1325–1336
Zhang Q, Tang X, Lu Q, Zhang Z, Rao J, Le AD (2006) Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1 alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther 5:1227–1238
Kowluru RA, Kanwar M (2007) Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab 4:8
Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S (2011) Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, anti oxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther 27:123–130
Flynn DL, Rafferty MF, Boctor AM (1986) Inhibition of 5-hydroxy-eicosatetraenoic acid (5-HETE) formation in intact human neutrophils by naturally-occurring diarylheptanoids: inhibitory activities of curcuminoids and yakuchinones. Prostaglandins Leukot Med 22:357–360
Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH (1991) Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res 51:813–819
Sameermahmood Z, Balasubramanyam M, Saravanan T, Rema M (2008) Curcumin modulates SDF-1alpha/CXCR4- induced migration of human retinal ECs (HRECs). Invest Ophthalmol Vis Sci 49:3305–3311
Williams B, Gallacher B, Patel H, Orme C (1997) Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes 46:1497–1503
Wang L, Sun Y, Huang K, Zheng L (2013) Curcumin, a potential therapeutic candidate for retinal diseases. Mol Nutr Food Res 57:1557–1568
Kim JH, Lee BJ, Kim JH, Yu YS, Kim KW (2009) Anti-angiogenic effect of caffeic acid on retinal neovascularization. Vascul Pharmacol 51(4):262–267
Shi Y, Wu X, Gong Y, Qiu Y, Zhang H, Huang Z, Su K (2010) Protective effect of caffeic acid phenethyl ester on retinal ischemia/reperfusion injury in rats. Curr Eye Res 35(10):930–937
Durmus M, Yilmaz HR, Uz E, Ozcelik N (2008) The effect of caffeic acid phenethyl ester (CAPE) treatment on levels of MDA, NO and antioxidant enzyme activities in retinas of streptozotocin-induced diabetic rats. Turk J Med Sci 38(6):525–530
Peterson M, Simmonds MSJ (2003) Rosmarinic acid. Phytochemistry 62(2):121–125
Kim JH, Lee BJ, Kim JH, Young SY, Kim MY, Kim KW (2009) Rosmarinic acid suppresses retinal neovascularization via cell cycle arrest with increase of p21WAF1 expression. Eur J Pharmacol 615(1–3):150–154
Huang SS, Zheng RL (2006) Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett 239(2):271–280
Miao X, Sun W, Miao L, Fu Y, Wang Y, Su G, Liu Q (2013) Zinc and diabetic retinopathy. J Diabetes Res 2013:425854
Prasad AS (2009) Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 12(6):646–652
Duzguner V, Kaya S (2007) Effect of zinc on the lipid peroxidation and the antioxidant defense systems of the alloxan-induced diabetic rabbits. Free Radic Biol Med 42(10):1481–1486
Moustafa SA (2004) Zinc might protect oxidative changes in the retina and pancreas at the early stage of diabetic rats. Toxicol Appl Pharmacol 201(2):149–155
Sahin K, Onderci M, Tuzcu M, Ustundag B, Cikim G, Ozercan IH, Sriramoju V, Juturu V, Komorowski JR (2007) Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: the fat-fed, streptozotocin-treated rat. Metab Clin Exp 56:1233–1240
Rajah TT, Olson AL, Grammas P (2001) Differential glucose uptake in retina and brain-derived ECs. Microvasc Res 62:236–242
Puchowicz MA, Xu K, Magness D, Miller C, Lust WD, Kern TS, LaManna JC (2004) Comparison of glucose influx and blood flow in retina and brain of diabetic rats. J Cereb Blood Flow Metab 24(4):449–457
Watanabe T, Matsushima S, Okazaki M, Nagamatsu S, Hirosawa K, Uchimura H, Nakahara K (1996) Localization and ontogeny of GLUT3 expression in the rat retina. Brain Res Dev Brain Res 94(1):60–66
Klip A, Paquet MR (1990) Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care 13:228–243
Ulas M, Orhan C, Tuzcu M, Ozercan IH, Sahin N, Gencoglu H, Komorowski JR, Sahin K (2015) Anti-diabetic potential of chromium histidinate in diabetic retinopathy rats. BMC Complement Altern Med 15:16
Jain SK, Rains JL, Croad JL (2007) Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-α, IL-6, CRP, glycated hemoglobin, triglycerides and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radic Biol Med 43(8):1124–1131
Mutuma S, Amuna P, Shukla H, Sumar S (1999) Chromium in food, nutrition and health—an introduction. Nutri Food Sci 2:81–88
Ueda Y, Kanazawa S, Gong H, Miyamura N, Kitaoka T, Amemiya T (2002) The retinal pigment epithelium of Cr-deficient rats. Life Sci 71:1569–1577
Tabar MB (2012) Determination of serum selenium in patients with type II diabetes mellitus. Middle East J Sci Res 12(4):433–435
McCarty MF (2005) The putative therapeutic value of high-dose selenium in proliferative retinopathies may reflect down-regulation of VEGF production by the hypoxic retina. Med Hypotheses 64(1):159–161
Di Leo MA, Ghirlanda G, Silveri NG, Giardina B, Franconi F, Santini SA (2003) Potential therapeutic effect of antioxidants in experimental diabetic retina: a comparison between chronic taurine and vitamin E plus selenium supplementation. Free Radic Res 37(3):323–330
Tuvemo T, Gebre-Medhin M (1983–1985) The role of trace elements in juvenile diabetes mellitus. Pediatrician 12(4):213–219
Joshi U, Raut PD, Agrawal SK, Patra PK, Maheshwari BK, Apurb M, Dhirhe TC (2011) Evaluation of serum selenium levels in patients with uncomplicated diabetes mellitus, Raipur, India. J Clin Diagn Res 5:70–73
McNair P, Christiansen C, Madsbad S, Lauritzen E, Faber O, Binder C, Transbol I (1978) Hypomagnesemia, a risk factor in diabetic retinopathy. Diabetes 27:1075–1077
Shivkumar K (2002) Pro-fibrogenic effect of magnesium deficiency in the cardiovascular system. Magnes Res 15:307–315
Maier JAM, Malpuech-Brugere C, Zimowska W, Rays-siguier Y, Mazur A (2004) Low magnesium promotes EC dysfunction:implications for atheroscelosis, inflammation and thrombosis. Biochem Biophys Acta 1689:3–21
Zhou Q, Olinescu RM, Kummerow FA (1999) Influence of low magnesium concentrations in the medium on the antioxidant system in cultured human arterial ECs. Magnes Res 12:19–29
Hartwig A (2001) Role of magnesium in genomic stability. Mutat Res 475:113–121
Baig MSA, Ali MS, Tejovathi B (2012) Study of serum magnesium in diabetic retinopathy. Int J Biol Res 3(4):2480–2482
Lee CTC, Gayton EL, Beulens JWJ, Flanagan DW, Adler AI (2010) Micronutrients and diabetic retinopathy. Ophthalmology 117:71–78
Rayssignier Y (1984) Role of magnesium and potassium in the pathogenesis of arteriosclerosis. Magnesium 3:226–238
Garfinkel D, Garfinkel L (1988) Magnesium and regulation of carbohydrate metabolism at the molecular level. Magnesium 7(5–6):249–261
Rodriguez MM, Romero FG (2003) Oral magnesium supplementation improves insulin sensitivity and metabolic control in type II diabetic subjects. Diabetes Care 26(4):1147–1152
Kanwar M, Chan PS, Kern TS, Kowluru RA (2007) Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci 48:3805–3811
Madsen-Bouterse SA, Zhong Q, Mohammad G, Ho YS, Kowluru YS (2010) Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Rad Res 44(3):313–321
Uriu-Adams JY, Keen CL (2005) Copper, oxidative stress and human health. Mol Aspects Med 26:268–298
Sindhu P, Shetty B, Sudha K, Rao G (2012) Role of redox metals, oxidative protein products and antioxidant potentials of thiols in diabetic retinopathy. J Med Biochem 31(2):126–130
Mosad A, Abou S, Yousef AA (2004) Evaluation of some biochemical changes in diabetic patients. Clinica Chemica Acta 346:161–170
Walter RM, Uriu-Hare JY, Olin KL, Oster MH, Anawalt BD, Critchfield JW, Keen CL (1991) Copper, zinc, manganese and magnesium status and complications of diabetes mellitus. Diabetes Care 14(11):1050–1056
Singh R, Gupta V, Gupta A, Bhansali A (2005) Spontaneous closure of microaneurysms in diabetic retinopathy with treatment of co-existing anaemia. Br J Ophthalmol 89:248–249
He BB, Wei L, Gu YJ, Han JF, Li M, Liu YX, Bao YQ, Jia WP (2012) Factors associated with diabetic retinopathy in Chinese patients with type 2 diabetes mellitus. Int J Endocrinol 2012:157940
Friedman E, Brown C, Berman D (1995) Erythropoietin in diabetic macular edema and renal insufficiency. Am J Kidney Dis 26:202–208
Guralnik JM, Eisenstaedt, Ferrucci L, Klein HG, Woodman RC (2004) Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 104:2263–2268
Ciudin A, Hernandez C, Simo R (2010) Iron overload in diabetic retinopathy: a cause or a consequence of impaired mechanisms? Exp Diabetes Res 2010:714108
Roy MS, Janal MN (2010) High caloric and sodium intakes as risk factors for progression of retinopathy in type 1 diabetes mellitus. Arch Ophthalmol 128(1):33–39
Tomic M, Ljubic S, Kastelan S, Antunica AG, Jazbec A, Poljicanin T (2013) Inflammation, homeostatic disturbance and obesity: possible link to pathogenesis of diabetic retinopathy in type 2 diabetes. Mediators Inflamm 2013:818671
Katusic D, Tomic M, Jukic T, Kordic R, Sikic J, Vukojevic N, Saric B (2005) Obesity-a risk factor for diabetic retinopathy in type 2 diabetes? Coll Antropol 29(1):47–50
Dirani M, Xie J, Fenwick E, Benarous R, Rees G, Wong TY, Lamoureux EL (2011) Are obesity and anthropometry risk factors for diabetic retinopathy? The diabetes management project. Invest Ophthalmol Vis Sci 52(7):4416–4421
Raman R, Rani PK, Gnanamoorthy P, Sudhir RR, Kumaramanikavel G, Sharma T (2010) Association of obesity with diabetic retinopathy: Sankara nethralaya diabetic retinopathy epidemiology and molecular genetics study (SN-DREAMS Report no. 8). Acta Diabetol 47(3):209–215
Dowse G, Humphrey ARG, Collins VR, Plehwe W, Gareeboo H, Fareed D, Hemraj F, Taylor HR, Tuomilehto J, Alberti KG et al (1998) Prevalence and risk factors for diabetic retinopathy in the multiethnic population of Mauritius. Am J Epidemiol 147(5):448–457
Kastelan S, Tomic M, Antunica AG, Ljubic S, Rabatic JS, Karabatic M (2013) Body mass index: a risk factor for retinopathy in type 2 diabetic patients. Mediators Inflamm 2013:436329
Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M (2009) Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem 16(1):94–112
Klein R, Klein BEK, Moss SE (1997) Is obesity related to microvascular and macrovascular complications in diabetes? The Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med 157(6):650–656
Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ et al (1998) Biological action of leptin as an angiogenic factor. Science 281(5383):P1683–P1686
Oguz M, Kurukahvecioglu O, Salman B, Onuk E, Tatlicioglu E (2005) A subjective global assessment of nutritional status: a study of 1400 surgical patients. Gazi Tip Derg 16(2):66–69
Yang FL, Lee RP, Wang CH, Fang TC, Hsu BG (2007) A cohort study of subjective global assessment and mortality in Taiwanese hemodialysis patients. Ren Fail 29:1–5
SharmaY, Saxena S, Saxena A, Mishra A, Natu SM (2015) Interrelationship of elevated serum Advanced Glycation End- product levels and malnutrition (Subjective Global Assessment) scores with the severity of retinopathy in type II diabetes. Clin Nutr ESPEN 10:e42–e48
SharmaY, Saxena S, Mishra A, Saxena A, Natu SM (2017) Apolipoprotein A-I and B and Subjective Global Assessment relationship can reflect lipid defects in diabetic retinopathy. Nutrition 33:70–75
Acknowledgements
University Grants Commission, India (Funding Agency).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Junior Research Fellowship [No. F. 17-7/2011 (SA-I)] by the University Grants Commission, India.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, Y., Saxena, S., Mishra, A. et al. Nutrition for diabetic retinopathy: plummeting the inevitable threat of diabetic vision loss. Eur J Nutr 56, 2013–2027 (2017). https://doi.org/10.1007/s00394-017-1406-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1406-2