Abstract
Purpose
Functional foods can prevent/reduce the risks related to obesity. Lentinula edodes is a highly nutritious mushroom rich in protein, vitamins and minerals. Some studies have demonstrated the hypocholesterolemic effects from L. edodes in high doses, which does not represent the consumption in humans. We evaluated ingestion of a realistic dose of L. edodes associated with a high-fat diet (HFD) on hematologic, biochemical and oxidative stress parameters.
Methods
Eighteen male Wistar rats were divided into three groups: control (normal diet); HFD; and HFD + L. edodes (100 mg/kg/day). After 30 days, blood was collected. Biochemical and hematologic parameters were analyzed, as well as oxidative stress biomarkers.
Results
The HFD increased levels of total cholesterol and triglycerides. Lentinula edodes reduced these parameters significantly to concentrations found in the control group. The HFD increased levels of alanine transaminase and aspartate transaminase (markers of liver damage). Lentinula edodes returned the levels of these enzymes to normal levels and normalized serum levels of urea (which were also increased owing to consumption of the HFD). Lentinula edodes reduced levels of urea and glucose. Lipid peroxidation was increased in rats receiving the HFD, and L. edodes reduced malondialdehyde levels, thereby preventing oxidation of fatty acids.
Conclusions
Lentinula edodes was shown to have hypolipidemic, hypoglycemic, hepatoprotective and renoprotective features in doses that are suitable for humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a major public health problem worldwide. Environmental and genetic factors have important roles in obesity, but inappropriate eating habits and sedentary lifestyles also contribute [1]. Cavalheira et al. [2] stated that excessive ingestion of food and, consequently, storage of carbohydrate and fat result in metabolic dysfunction, which activates innate and adaptive inflammation in adipose tissue. The inflammatory reaction promotes the resistance to insulin and metabolic diseases caused by obesity [3].
Hutcheson and Rocic [4] have hypothesized that the metabolic syndrome is associated with oxidative stress, which is a risk factor for the development of cardiovascular diseases. They have suggested that antioxidants can be useful for the treatment and prevention of cardiovascular diseases in patients with the metabolic syndrome.
Several resources have been used for the prevention and treatment of obesity and related diseases, including “nutraceuticals” [2]. For example, mushrooms have been used as food for thousands of years and have nutritional and medical properties [5, 6]. Lentinula edodes is a mushroom low in calories and lipids yet rich in proteins, vitamins, minerals and fibers [7–9].
β-Glucans are polysaccharides found in the cellular walls of L. edodes. They are bioactive compounds that can activate leukocytes, stimulate the immune system to produce anti-inflammatory mediators and have anticarcinogenic activity [10]. β-Glucans can also participate in lipid metabolism to reduce levels of total cholesterol and reduce body weight [11].
Lentinula edodes also contains glycoproteins as well as compounds with antioxidant [12], antitumor [13] and antibiotic activities [14, 15]. Lentinula edodes has antiviral [16], antihypertensive [17], antihypercholesterolemic [18, 19], hypoglycemic [20, 21] and antithrombotic activities [17, 18]. However, many of the studies mentioned were carried out in vitro or with high concentrations of L. edodes, which does not correspond with human consumption.
We wished to evaluate the effects of ingestion of L. edodes under biochemical, hematologic and oxidative stress parameters in rats receiving a high-fat diet (HFD).
Materials and methods
Reagents and solutions
Ethyl ether, sulfuric acid, copper sulfate, potassium sulfate, boric acid, hydrochloric acid (HCl), acetic acid, nitric acid, trichloroacetic acid (TCA), potassium hydroxide (KOH), sodium acetate buffer (SAB), Triton X-100, potassium phosphate buffer (TFK), 5-5-dithiobis(2-nitrobenzoic acid) (DTNB), ethylenediamine tetra-acetic acid (EDTA), reduced glutathione (GSH), glutathione reductase (GR), nicotinamide adenine dinucleotide phosphate (NADPH), sodium azide, hydrogen peroxide, exo-β-1,3-glucanase, β-glycosidase, glucose oxidase/peroxidase, amiloglycosides and invertase, sodium hydroxide (NaOH), malondialdehyde (MDA), thiobarbituric acid (TBA), phosphoric acid (H3PO4), sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich (St. Louis, MO, USA) or from Yeast and Mushroom, Megazyme, Bray, Eire.
Ethical approval of the study protocol
The study protocol was approved by the Commission of Ethics in the Usage of Animals of the University of Sorocaba (approval number 008/2012; São Paulo, Brazil).
Preparation of Lentinula edodes
Lentinula edodes (Berk.) Pegler-cultivated strain H600 (Hokken, Shimotsuga-gun, Japan) was provided by the commercial company Yuri Cogumelos (São Paulo, Brazil). Fresh samples of L. edodes were sliced and dehydrated in a greenhouse at 38 ± 2 °C. Material was ground in a mill to obtain a fine, homogeneous powder and then diluted in water.
To verify humidity, samples (4 g) were dried at 105 °C until they reached constant weight. To obtain ashes, 3 g of the sample was carbonized and incinerated at 550 °C. For extraction of lipids, ethyl ether was employed for 6 h [22].
Nutritional composition of Lentinula edodes
Proteins were quantified using a nitrogen distiller following the method of Zhou et al. [23] with some alterations. This method comprised three phases: digestion, distillation and titration. Digestion was based on oxidation of 70 mg of sample at 390 °C with sulfuric acid, cooper sulfate and potassium sulfate. In the distiller, the nitrogen in proteins was reduced to ammonium sulfate. Upon contact with boric acid, the ammonium sulfate forms ammonium borate, with release of HCl. Centesimal composition of L. edodes was determined by summating the humidity, ashes, lipids, proteins and carbohydrates [24].
Fibers were quantified following the method of Mccleary et al. [25]. Dry defatted samples were digested with glacial acetic acid, nitric acid and trichloroacetic acid with heating for 40 min. Samples were filtered, desiccated, weighed and washed with boiling water until pH neutralization. Then, samples were washed (alcohol and ether), dried and incinerated at 550 °C until ashes were obtained. The difference between the initial weight of the sample and the weight of the ashes was the quantity of total fibers.
Total glucans were determined using a β-Glucan Assay kit (Yeast and Mushroom; Megazyme, Bray, Eire). Briefly, L. edodes (100 mg) and 1.5 mL of 37 % HCl were incubated for 45 min at 30 °C. Volume was adjusted to 10 mL with deionized water followed by incubation for 2 h. Then, 10 mL of KOH (2 M) was added and sample volume adjusted to 100 mL with SAB (200 mM, pH 5.0). The solution was centrifuged at 1500×g for 10 min at room temperature. The supernatant (0.1 mL) was mixed with 0.1 mL of exo-β-1,3-glucanase and β-glycosidase and incubated for 60 min at 40 °C. Then, 1.5 mL of glucose oxidase/peroxidase was added followed by incubation for 20 min at 40 °C. Absorbance was measured at 510 nm against a blank reagent.
For α-glucans, 0.2 mL of KOH (2 M) was added to 100 mg of sample. After 20 min of agitation, 0.2 mL of aminoglycosides and invertase were added and incubated for 30 min at 40 °C. Tubes were centrifuged at 1500×g 10 min at room temperature. To 0.1 mL of supernatant were added 0.1 mL of SAB and 0.3 mL of enzymatic reagent and incubated for 20 min at 40 °C. Absorbance was measured at 510 nm. α-Glucans were subtracted from total glucans for quantification of β-glucans.
Animal experiments
The dose of L. edodes (100 mg/kg) was chosen based on a study from our research team [26]. A HFD (60 % of lipids) [27] and control diet (10 % lipids) were used (Table 1).
Male Wistar rats (45 ± 3 days) were obtained from Anilab (São Paulo, Brazil). They were maintained individually in cages at the Laboratory of Toxicological Research at a controlled temperature (21 ± 2 °C), humidity (50 ± 5 %) and lighting (12-h light–dark cycle). Standard rat chow and filtered tap water were provided ad libitum. Eighteen rats were allocated randomly (random number table generated by computer) into three groups of six: control (standard diet); HFD (60 % lipids); and HFD + L. edodes (100 mg/kg). Administration of L. edodes was by oral gavage at <3 mL/kg body weight. Mushroom powder was reconstituted in water every day. Rats were treated for 30 days. Rats were weighed every week. At the study end, rats were killed with an overdose of ketamine (100 mg/kg) and xylazine (6 mg/kg). Blood was collected and stored at −80 °C.
Hematologic parameters
Hematologic parameters were measured using a Sysmex XS 1000i™ Hematology Analyzer (Roche, Basel, Switzerland). Parameters were: white blood cells (WBCs) or leukocytes; red blood cells (RBCs) or erythrocytes; hemoglobin (Hb); hematocrit; mean corpuscular volume; mean corpuscular hemoglobin; mean corpuscular hemoglobin concentration (MCHC); platelets.
Biochemical profile
Levels of glucose, triglycerides, cholesterol (total and fractions), fructosamine and gamma-glutamyl transferase (γ-GT) were evaluated using commercial kits according to manufacturers Bioclin (Pretoria, South Africa), in vitro (Placentia, CA, USA), and Wiener Laboratorios (Buenos Aires, Argentina) instructions. Analyses were undertaken on a spectrophotometer (Lambda 35; PerkinElmer, Waltham, MA, USA).
Hepatic enzymes aspartate (aminotransferase (AST), alanine aminotransferase (ALT)) and renal biomarkers (urea, creatinine) were analyzed on automated equipment (Cobas C111; Roche).
Oxidative stress
Determination of reduced glutathione (GSH) levels was based on quantification of total reduced thiols following the method of Ellman [28]. Briefly, 150 μL of blood maintained in an ice bath was vortex-mixed with 100 μL of 10 % Triton X100 (10 %) and 100 μL of 30 % trichloroacetic acid. The solution was centrifuged at 4000×g for 10 min at 4 °C. In the cuvette, 900 μL of trifluoromethyl ketone (TFK; 1 M), 50 μL of the supernatant and 50 μL of 5,5′-dithiobis-(2-nitrobenzoic acid) (10 mM) were pipetted and formed a yellow complex. Absorbance was measured at 412 nm using a spectrophotometer. To calculate the GSH concentration, a calibration curve with predefined concentrations of GSH (0.005, 0.01, 0.025, 0.05 and 0.1 mM) was employed.
Levels of the antioxidant enzyme glutathione peroxidase (GSH-Px) were determined based on the oxidation of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) following the method of Paglia and Valentine [29]. Blood was diluted (1:40) in TFK (pH 7.0). Then, 20 μL of the diluted sample was added to 880 μL of a solution containing GSH, glutathione reductase, NADPH, sodic azide and 100 μL of hydrogen peroxide (H2O2). GSH-Px level was monitored in a spectrophotometer at 340 nm for 2 min.
Measurement of catalase levels followed the method of Aebi [30], which is based on H2O2 decomposition by catalase at 240 nm. Briefly, blood was diluted (1:60) in TFK 50 mM. An aliquot of 20 μL was mixed to 1910 μL of TFK, and 70 μL of H2O2 was added, thereby initiating a reaction that was monitored for 5 min. A constant of variation (k), which is related to Hb, was used to obtain a value for blood activity (k/g Hb).
Thiobarbituric acid reactive substances (TBARS) are an important method to evaluate the lipid peroxidation, including malondialdehyde [31]. Plasma aliquots (150 μL) were mixed with 50 μL of NaOH and 50 μL of Milli-Q Water™ (Millipore, Billerica, MA, USA). The mixture was incubated for 20 min at 60 °C with agitation. Then, 6 % H3PO4 (250 μL), 0.8 % thiobarbituric acid (TBA; 250 μL) and 100 μL of 10 % sodium dodecyl sulfate were added to samples, which were bathed for 1 h at 80 °C. Lipid peroxidation products reacted with TBA in acidic conditions to form a pink substance, the absorbance of which was read at 532 nm in a spectrophotometer. A calibration curve was used (predefined concentrations of 0.28, 0.56, 1.7, 3.4 and 6.6 μM) to calculate the concentration of TBARS in plasma.
Statistical analyses
Data are the mean ± standard deviation. Results were analyzed using one-way analysis of variance followed by Duncan’s test to identify differences among groups. Differences with a p value of <0.05 were considered to be significant. Results were analyzed using Statistica version 8.0 (Dell, Round Rock, TX, USA) and GraphPad Prism version 6.0 (San Diego, CA, USA).
Results
Nutritional composition of Lentinula edodes
Nutritional composition of L. edodes was based on dry weight (with the exception of humidity analyses) and is shown in Table 2 (which also shows the nutritional composition of other varieties of the same mushroom).
Body weight
Rat weights are presented in Fig. 1. Besides lipid content, the HFD administered to the two treatment groups caused a significant increase in body weight only in weeks 3 and 4 compared with control (p = 0.03 and p = 0.04 in HFD; p = 0.02 and p = 0.05 in HFD + L. edodes, respectively). On the other hand, oral administration L. edodes (100 mg/kg) for 30 days was not sufficient to reduce body weight compared with the HFD group (p = 0.68; 0.98; 0.67; 0.81; 0.97 in week 0, 1, 2, 3 and 4, respectively).
Body mass (g) in the control group, high-fat diet (HFD) and HFD + L. edodes (100 mg/kg) group through the weeks of the treatment. Data are the mean ± standard deviation. *Statistically different from control group: week 3 p = 0.03 in HFD; p = 0.023 in HFD+ L. edodes; week 4 p = 0.043 in HFD; p = 0.049 in HFD+ L. edodes. No differences were observed comparing HFD+ L. edodes to HFD
Hematologic parameters
Data for hematologic parameters are shown in Table 3. The group receiving a HFD + L. edodes displayed a significant increase in the number of WBCs compared with the control group (p = 0.03). The MCHC showed a significant (p = 0.04) increase in the HFD group compared with the control group. Values for RBC and platelet counts, as well as those for hemoglobin, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin, did not show significant differences among the three groups. Hence, apart from WBC count, a HFD and co-exposure of L. edodes did not modify hematologic parameters.
Liver function
Rats receiving the HFD had a significant increase in levels of ALT (p = 0.0001; p = 0.0002) and AST (p = 0.003; p = 0.002) compared with the control group and HFD + L. edodes group, respectively (Fig. 2a). In animals who received L. edodes, hepatic enzymes were preserved, without significant differences compared with the control group (p = 0.61 to ALT; p = 0.97 to AST). There were no significant differences in γ-GT levels among groups when compared with the control group (p = 0.53; p = 0.09).
a Enzyme activities [alanine transaminase (ALT); aspartate transaminase (AST); gamma-glutamyl transferase (ɣ-GT)]. b Kidney biomarkers concentrations of rats treated for 30 days with a standard diet (control), high-fat diet (HFD) and HFD + L. edodes (100 mg/kg). Data are the mean ± standard deviation. *Statistically different from control group (p = 0.0001 for ALT; p = 0.003 for AST; p = 0.047 for urea; p = 0.044 for Glucose-U). #Statistically different from HFD+ L. edodes (p = 0.0002 for ALT; p = 0.002 for AST)
Kidney function
Urea levels were increased in the group receiving a HFD in comparison with the control group (p = 0.05) (Fig. 2b). There was no significant difference in creatinine levels among the study groups (p = 0.68; p = 0.72, compared with control). Urinary glucose levels in the group receiving a HFD did not show a significant difference in comparison with the control group (p = 0.73). Animals that consumed a HFD + L. edodes had reduced urinary glucose levels compared with the control group (p = 0.04), suggesting that L. edodes has a hypoglycemic action.
Lipid profile
A significant increase in levels of total cholesterol (p = 0.027) and triglycerides (p = 0.042) was noted in the group receiving the HFD compared with that of the control group. In animals receiving a HFD + L. edodes, levels of cholesterol and triglycerides did not show a significant difference compared with those of the control group (p = 0.59; p = 0.08, respectively), thereby suggesting a hypocholesterolemic action from L. edodes (Fig. 3a). Furthermore, L. edodes administration contributed to a reduction in the level of very-low-density lipoprotein (p = 0.002 when compared with the HFD group) and an increase in those of high-density lipoprotein (HDL) compared with the control group (p = 0.01) and HFD group (p = 0.03). These effects could be attributed to the high concentration of fibers and β-glucans in L. edodes.
a Lipid profile [cholesterol (COL); triglycerides (TRIGL); high-density lipoprotein (HDL); very-low-density lipoprotein (VLDL); low-density lipoprotein (LDL)]. b, c Glycemic profile of rats after 30 days receiving a standard diet (control group), high-fat diet (HFD) and HFD + L. edodes (100 mg/kg). Data are the mean ± standard deviation. *Statistically different from control group (p = 0.027 for CHOL; p = 0.049 for TRIG; p = 0.001 for HDL). #Statistically different from HFD+ L. edodes (p = 0.0018 for TRIG). &Statistically different from HRD (p = 0.03 for HDL; p = 0.002 for VLDL; p = 0.043 for Glucose)
Glycemic profile
The fructosamine concentration (Fig. 3b) was not significantly different among the three groups (p = 0.27; 0.73, compared with the control group). The serum concentration of glucose (Fig. 3c) was reduced significantly in the HFD + L. edodes group compared with the HFD group (0.043). These findings suggested a hypoglycemic action arising from β-glucans.
Oxidative stress
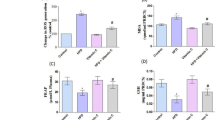
The HFD increased TBARS levels significantly compared with those of the control group (p = 0.02) and L. edodes group (p = 0.006) (Fig. 4a). Nevertheless, the L. edodes group had decreased levels of TBARS similar to those of the control group (p = 0.45). No significant differences were observed among the groups with respect to catalase (Fig. 4b) (p > 0.38), reduced GSH (Fig. 4c) (p > 0.43) or GSH-Px (Fig. 4d) (p > 0.11).
Biomarkers of oxidative stress in rats treated for 30 days with a standard diet (control), high-fat diet (HFD) and HFD + L. edodes (100 mg/kg). Data are the mean ± standard deviation. *Statistically different from control group (p = 0.02 for TBARS). #Statistically different from HFD + L. edodes (p = 0.006 for TBARS)
Discussion
Our findings for the nutritional composition of L. edodes exhibited similarities and differences compared with those from other studies. Differences in the concentrations of macronutrients are dependent upon the location, substrate, weather, crop conditions, phase of growing and harvest of mushrooms [35].
We found high concentrations of β-glucans that were comparable with those observed by Bak et al. [36] (29.7–56.5 %) in ten crops of L. edodes. Brauer and colleagues observed, in extracts and lyophilized samples of L. edodes, 5.0 and 5.6 % of β-glucans, respectively [37]. Manzi et al. [38] found 13.9–66.6 % of β-glucans in samples of Pleurotus ostreatus. Those wide variations of concentrations of bioactive compounds are due to differences in cultivation and species.
Handayani et al. [39] evaluated the ingestion of powdered L. edodes (7, 20 and 60 g/kg) in rats receiving a HFD (50 %) during 6 weeks, along with mushrooms. The group receiving the higher dose of L. edodes gained less corporeal weight in comparison with all other groups. Despite those results, the consumption reported does not match realistic consumption of mushrooms in humans.
Increases in WBC count can be related to: (i) activation of inflammation from excessive consumption of lipids and (ii) stimulation of the immune system by L. edodes [40, 41]. Harada et al. [42] made rats eat extracts of β-glucans from mushrooms to induce leukopenia. They noted improvement in the hematopoietic system, suggesting that this extract could contribute to the increase in production and maturation of thymocytes [42, 43], results that were corroborated by our study. β-Glucans could activate the hematopoietic system.
The MCHC was increased significantly in the HFD group compared with the control. The high concentration of fat could have caused an increase in the level of hemoglobin, causing blood thickening [44]. Tai et al. [45] showed that the lipid source can influence hematologic parameters, since they observed increase in the viscosity of blood, serum and plasma in rats receiving soy oil, palm oil and vegetable fat.
Levels of ALT and AST in blood can be increased if the metabolism of the liver is compromised or if the liver is injured [46, 47]. Increased levels of saturated fatty acids induce liver damage, which results in high levels of AST and ALT in blood [48]. The HFD increased ALT and AST levels by 74 and 20 %, respectively, compared with the control group. L. edodes reduced levels of this enzyme by 63 and 20 %. Chung et al. [49] presented that 250 mg/kg of L. edodes reduced the levels of ALT and AST along with a reduction in inflammation, necrosis and fatty deposition in liver tissue in rats chemically injured.
In the present study, the main reason for increases in urea levels is the kidney damage triggered by excessive amounts of lipids, which hinders blood filtration (Fig. 2b). Urea and creatinine are sensitive markers of early damage to the kidneys [50]. L. edodes acted to reduce urea levels. To verify the renoprotective properties of Inonotus obliquus, researchers induced diabetes in rats using streptozotocin. Results showed reductions in urea levels in groups eating I. obliquus compared with the control group [51], following the same line our findings. In another study, Ganoderma lucidum decreased concentrations of creatinine and urea in the nephrotoxicity induced by cisplatin [52], thereby denoting kidney protection by mushroom ingestion. Moreover, Vaidya et al. [50] showed significant protection of L. edodes against the kidney injuries induced by carbon tetrachloride (CCl4) by normalizing the levels of urea and creatinine.
Fukushima et al. [53] detailed hypocholesterolemic effects in the same line to our findings. Upon feeding of isolated fibers of Grifola frondosa and Flammulina velutipes mushrooms to rats, the concentration of total cholesterol was reduced 11 and 25 %, respectively. Similarly, Yoon et al. [54] administered 5 % L. edodes in powder form to hypercholesterolemic rats and found reductions in levels of triglycerides and cholesterol, findings that are in accordance with our data. However, the concentration of L. edodes used was very high and not consistent with ingestion in humans.
Hyperlipidemia and high blood pressure are the two main risk factors for atherosclerosis. In excess, low-density lipoprotein (LDL) is deposited in the walls of blood vessels and becomes an important component to platelet injuries. HDL facilitates translocation of cholesterol from peripheral tissue to be catabolized in the liver [54]. Yang et al. [55] used 5, 10 and 20 % L. edodes (from total ingestion of food) to feed rats with hypercholesterolemia. The hypocholesterolemic effect was proportional to the increase in percentage of L. edodes administered. Authors have proposed that such action is due to eritadenine and β-glucans in mushrooms. Viscosity and gel-formation properties of β-glucans facilitate fat excretion to reduce the time cholesterol is present in the organism and thus diminish its absorption [56]. Mircea et al. [20] evaluated the effects of three species of fungi—extracts and powders—on levels of glucose and cholesterol. The hypoglycemic effect was more pronounced in groups treated with extracts, whereas groups treated with powder had better results for hypocholesterolemia. This hypoglycemic action could be attributed to the insulin secretion and action after mushroom extracts ingestion [21].
TBARS is a technique that evaluates products of lipid peroxidation, which are involved in the oxidative degradation of polyunsaturated fatty acids [57]. The diet given to rats was rich in lipids, so the increase in levels of total cholesterol and triglyceride was related to the increase in TBARS levels. Conversely, L. edodes could decrease lipid peroxidation levels to those seen in the control group, suggesting an antioxidant action of L. edodes that prevents oxidation of fatty acids. Jayakumar et al. [57] evaluated the CCl4 effects in liver of rats exposed to P. ostreatus. They noticed as increase in liver MDA levels in rats exposed only to CCl4. And the group co-exposed P. ostreatus had a significant reduction in MDA concentrations, suggesting a decreased oxidative damage to tissue liver, similar to our results in plasma.
Kanagasabapathy et al. [58] observed a significant reduction in hepatic and renal lipid peroxidation and an increase in catalase and GPx activities in rats treated with a HFD and three doses of Pleurotus sajor-caju. Acharya et al. [59] noted an increase in lipid peroxidation and decreases in levels of catalase and GSH in liver of rats treated with CCl4 and the restored antioxidant status upon administration of Macrocybe gigantean, which was proposed to be a potent scavenger of free radicals.
Conclusions
This is the first study to report on the different parameters associated with HFD ingestion and L. edodes. The most important finding was the dose of L. edodes that is suitable for human consumption. HFD consumption induced alterations in the lipid profile, hepatic function, renal function and lipid peroxidation. L. edodes was shown to be a good nutraceutical against metabolic disorders through its hypocholesterolemic action as well as its ability to increase HDL levels, restore the concentration of urea to normal levels, decrease levels of transaminases and stimulate the immune system (by increasing the WBC count). Lentinula edodes protected against the oxidative damage induced by excessive ingestion of lipids, thereby suggesting an antioxidant effect.
Bioactive properties of Lentinula edodes can be suggested to the high concentrations of β-glucans and fibers, among other. Thus, even though L. edodes does not aid weight loss, it could be an excellent nutritional source in food supplements (especially in metabolic disorders associated with high consumption of fat).
References
Landsberg L, Aronne LJ, Beilin LJ et al (2013) Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment–a position paper of the The Obesity Society and The American Society of Hypertension. Obesity 21(1):8–24. doi:10.1002/oby.20181
Carvalheira JBC, Qiu Y, Chawla A (2013) Blood spotlight on leukocytes and obesity. Am Soc Hematol 122:3263–3267. doi:10.1182/blood-201304-459446
WHO (2013) World Health Organization. Fact sheet no 311. Disponível em. http://www.who.int/mediacentre/factsheets/fs311/en/. Acesso em 29 de março de 2014
Hutcheson R, Rocic P (2012) The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp J Diabetes Res. doi:10.1155/2012/271028
Roupas P, Keogh J, Noakes M et al (2012) The role of edible mushrooms in health: evaluation of the evidence. J Funct Foods 4:687–709. doi:10.1016/j.jff.2012.05.003
Israilides C, Kletsas D, Arapoglou D et al (2008) In vitro cytostatic and immunomodulatory properties of the medicinal mushroom Lentinula edodes. Phytomedicine 15:512–519. doi:10.1016/j.phymed.2007.11.029
Wani BA, Bodha RH, Wani AH (2010) Nutritional and medicinal importance of mushrooms. JMPR 4:2598–2604. doi:10.5897/JMPR09.565
Regula J, Siwulski M (2007) Dried shiitake (Lentinulla edodes) and oyster (pleurotus ostreatus) mushrooms as a good source of nutrient. Acta Sci Pol Technol Aliment 6:135–142
Nile SH, Park W (2014) Total, soluble, and insoluble dietary fiber contents of wild growing edible mushrooms. Czech J Food Sci 32:302–307
Rop O, Mlcek J, Jurikova T (2009) Beta-glucans in higher fungi and their health effects nure. Nutr Rev 67:624–631. doi:10.1111/j.1753-4887.2009.00230
Giavasis L (2014) Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr Opin Chem Biol 26:162–173. doi:10.1016/j.copbio.2014.01.010
Ferreira ICFR, Barros L, Abreu RMV (2009) Antioxidants in wild mushrooms. Curr Med Chem 16:1543–1560
Hazama S, Watanabe S, Ohashi M et al (2009) Efficacy of orally administered superfine dispersed lentinan (β-1,3-glucan) for the treatment of advanced colorectal cancer. Anticancer Res 29:2611–2618
Attitalla IH (2011) Lentinus sp. RJ-2 mushroom is important source of natural antioxidative polysaccharides. PJBS 14:1070–1071. doi:10.3923/pjbs.2011.1070.1071
Kitzberger CSG, Smânia A Jr, Pedrosa RC et al (2007) Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J Food Eng 80:631–638. doi:10.1016/j.jfoodeng.2006.06.013
Rincão VP, Yamamoto KA, Ricardo NMPS et al (2012) Polysaccharide and extracts from Lentinula edodes: structural features and antiviral activity. Virology 9:37. doi:10.1186/1743-422X-9-37
Guillamón E, García-Lafuente A, Lozano M et al (2010) Edible mushrooms: role in the prevention of cardiovascular diseases. Fitoterapia 81:715–723. doi:10.1016/j.fitote.2010.06.005
Alam N, Yoon KN, Lee TS et al (2011) Hypolipidemic activities of dietary Pleurotus ostreatus in hypercholesterolemic rats. Mycobiology 39:45–51. doi:10.4489/MYCO.2011.39.1.045
Zhu M, Nie P, Liang Y, Wang B (2013) Optimizing conditions of polysaccharide extraction from shiitake mushroom using response surface methodology and its regulating lipid metabolism. Carbohydr Polym 95:644–648. doi:10.1016/j.carbpol.2013.03.035
Mircea C, Bild V, Zavastin D (2013) The protective effect of mushrooms in experimentally induced diabetes in Mice. Farmacia 61:268–275
Fortes RC, Recôva VL, Melo AL et al (2008) Effects of dietary supplementation with medicinal fungus in fasting glycemia levels of patients with colorectal cancer: a randomized, double-blind, placebo-controlled clinical study. Nutr Hosp 23:591–598
Araujo GS, Matos LJBL, Fernandes JO et al (2013) Extraction of lipids from microalgae by ultrasound application: prospection of the optimal extraction method. Ultrason Sonochem 20:95–98. doi:10.1016/j.ultsonch.2012.07.027
Zhou N, Li W, Wu Z et al (2015) Sequential extractions: a new way for protein quantification data from peanut allergens. Anal Biochem 484:31–36. doi:10.1016/j.ab.2015.05.013
Pinela J, Barros L, Carvalho AM et al (2012) Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem Toxicol 50:829–834. doi:10.1016/j.fct.2011.11.045
Mccleary BV, Devries JW, Plymouth AN et al (2010) Determination of total dietary fiber (CODEX Definition) by enzymatic-gravimetric method and liquid chromatography: collaborative study. J AOAC 93:221–233
Grotto D, Gerenutti M, Souza VCO et al (2015) Deficiency of macro-and micronutrients induced by Lentinula edodes. Toxicol Rep 2:401–404. doi:10.1016/j.fct.2011.11.045
Fraulob JC et al (2010) A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr 46:212–223. doi:10.3164/jcbn.09-83
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. doi:10.1016/0003-9861(59)90090-6
Paglia DE, Valentine WN (1967) Study on the quantitative and qualitative caracte- rization of erythrocyte glutathione peroxide. J Lab Clin Med 70:158–169
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by Thiobarbituric Acid reaction. Analyt Biochem 95:351–358
Mattila P, Vaananen PS, Konko K et al (2002) Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agric Food Chem 50:6419–6422
Reis FS, Barros L, Martins A et al (2012) Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food Chem Toxicol 50:191–197. doi:10.1016/j.fct.2011.10.056
Gaitán-Hernández R, Esqueda M, Gutiérrez A et al (2006) Bioconversion of agrowastes by Lentinula edodes: the high potential of viticulture residues. Appl Microbiol Biot 71:432–439. doi:10.1007/s00253-005-0241-1
Brauer D, Kimmons TE, Phillips M et al (2011) Starch concentrations in log-grown shiitake mushrooms (Lentinula edodes (Berk.) Pegler). TOMYCJ 5:1–7. doi:10.2174/1874437001105010001
Bak WC, Park JH, Park YA et al (2014) Determination of glucan contents in the fruiting bodies and mycelia of Lentinula edodes cultivars. Mycrobiology 42:301–304. doi:10.5941/MYCO.2014.42.3.301
Brauer D, Kimmons T, Phillips M (2007) Comparison of two methods for the quantitation of 13-glucans from shiitake mushrooms. J Herbs Spices Med Plants 13:15–26. doi:10.1300/J044vl3n03_02
Manzi P, Aguzzi A, Pizzoferrato L (2001) Nutritional value of mushrooms widely consumed in Italy. Food Chem 73:321–325. doi:10.1016/S0308-8146(00)00304-6
Handayani D, Chen J, Meyer BJ et al (2011) Dietary shiitake mushroom (Lentinus edodes) prevents fat deposition and lowers triglyceride in rats fed a high-fat diet. J Obes 2011:1–8. doi:10.1155/2011/258051
Ellinger VCM, Carlini LT, Moreira RO et al (2006) Relation between insulin resistance and hematological parameters in a brazilian sample. Arq Bras Endocrinol Metabol 50:114–117. doi:10.1590/S0004-27302006000100016
Barazzoni R, Cappellari GG, Semolic A et al (2014) The association between hematological parameters and insulin resistance Is modified by body mass index results from the North-East Italy MoMa population study. PLoS ONE 9:101590. doi:10.1371/journal.pone.0101590
Harada T, Miura N, Adachi Y et al (2002) Effect of SCG, 1,3-b-D-Glucan from sparassis crispa on the hematopoietic response in cyclophosphamide induced leukopenic mice. Biol Pharm Bull 25:931–939. doi:10.1248/bpb.25.931
Pini M, Gove ME, Sennello JA et al (2008) Role and regulation of adipokines during zymosan-induced peritoneal inflammation en mice. Endocrinology 149:4080–4085
Hernandéz GN, Dabin C, del Gayol C et al (2002) Haemorheological variables in a rat model of hypertriglyceridaemic obesity and diabetes. Vet Res Commun 26:625–635
Tai CJ, Chen CH, Chen HH et al (2010) Differential effect of high dietary fat intakes on haemorheological parameters in rat. Br J Nutr 103:977–983. doi:10.1017/S0007114509992704
Schindhelm RK, Diamant M, Dekker JM et al (2006) Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Ver 22:437–443. doi:10.1002/dmrr.666
Akamatsu S, Watanabe A, Tamesada M et al (2004) Biol hepatoprotective effect of extracts from Lentinus edodes mycelia on dimethylnitrosamine-induced liver injury. Pharm Bull 27:1957–1960. doi:10.1248/bpb.27.1957
Wang D, Wei Y, Pagliassotti MJ (2006) Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. doi:10.1210/en.2005-0570
Chung WS, Huawang J, Bose S et al (2015) Hepatoprotective effect of Lentinus edodes mycelia fermented formulation against alcoholic liver injury in rats. J Food Biochem. doi:10.1111/jfbc.12124
Vaidya VS, Ramirez V, Ichimura T et al (2006) Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290:517–529. doi:10.1152/ajprenal.00291.2005
Cha JY, Jun BS, Lee CH et al (2005) Hypoglicemic and antioxidative effects of fermented Chaga Mushroom (inonotus obliquus) on streptozotocin-induced diabetic rats. J Life Sci 15:809–818. doi:10.5352/JLS.2005.15.5.809
Sheena N, Ajith TA, Janardhanan KK (2003) Prevention of nephrotoxicity induced by the anticancer drug cisplatin, using Ganoderma lucidum, a medicinal mushroom occurring in South India. Curr Sci 85:478–482
Fukushima M, Ohashi T, Fujiwara Y et al (2001) Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp Biol Med 226:758–765
Yoon KN, Alam N, Lee JS et al (2011) Antihyperlipidemic effect of dietary Lentinus edodes on plasma, feces and hepatic tissues in hypercholesterolemic rats. Mycobiology 39:96–102. doi:10.4489/MYCO.2011.39.2.096
Yang H, Hwang I, Kim S et al (2013) Lentinus edodes promotes fat removal in hypercholesterolemic mice. Exp Ther Med 6:1409–1413. doi:10.3892/etm.2013.1333
Novak M, Vetvicka V (2008) Beta-Glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol 5:47–57. doi:10.1080/15476910802019045
Jayakumar T, Ramesh E, Geraldine P (2006) Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem Toxicol 44:1989–1996
Kanagasabapathy G, Malek NA, Mahmood AA et al. (2013) Beta-glucan-rich extract from Pleurotus sajor-caju (Fr.) singer prevents obesity and oxidative stress in C57BL/6J mice fed on a high-fat diet. Hindawi 1–10, ID 185259. doi:10.1155/2013/185259
Acharya K, Chatterjee S, Biswas G et al (2012) Hepatoprotective effect of a wild edible mushroom on carbon tetrachloride-induced hepatotoxicity in mice. Int J Pharm Pharm Sci 4:285–288
Acknowledgments
The authors are grateful for financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-2013/05765-5) and Financiadora de Estudos e Projetos (FINEP-01.10.0659.00) and the Programa de Suporte à Pós-Graduação de Instituições de Ensino Particulares (PROSUP/CAPES). We also thank Thaisa Borim Pickler for technical support.
Funding sources
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-2013/05765-5) and Financiadora de Estudos e Projetos (FINEP-01.10.0659.00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Spim, S.R.V., de Oliveira, B.G.C.C., Leite, F.G. et al. Effects of Lentinula edodes consumption on biochemical, hematologic and oxidative stress parameters in rats receiving high-fat diet. Eur J Nutr 56, 2255–2264 (2017). https://doi.org/10.1007/s00394-016-1266-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1266-1