Abstract
Purpose
Vegetarian and vegan diets have gained popularity in Switzerland. The nutritional status of individuals who have adopted such diets, however, has not been investigated. The aim of this study was to assess the intake and status of selected vitamins and minerals among vegetarian and vegan adults living in Switzerland.
Methods
Healthy adults [omnivores (OVs), n OV = 100; vegetarians (VGs), n VG = 53; vegans (VNs), n VN = 53] aged 18–50 years were recruited, and their weight and height were measured. Plasma concentrations of the vitamins A, C, E, B1, B2, B6, B12, folic acid, pantothenic acid, niacin, biotin and β-carotene and of the minerals Fe, Mg and Zn and urinary iodine concentration were determined. Dietary intake was assessed using a three-day weighed food record, and questionnaires were issued in order to assess the physical activity and lifestyle of the subjects.
Results
Omnivores had the lowest intake of Mg, vitamin C, vitamin E, niacin and folic acid. Vegans reported low intakes of Ca and a marginal consumption of the vitamins D and B12. The highest prevalence for vitamin and mineral deficiencies in each group was as follows: in the omnivorous group, for folic acid (58 %); in the vegetarian group, for vitamin B6 and niacin (58 and 34 %, respectively); and in the vegan group, for Zn (47 %). Despite negligible dietary vitamin B12 intake in the vegan group, deficiency of this particular vitamin was low in all groups thanks to widespread use of supplements. Prevalence of Fe deficiency was comparable across all diet groups.
Conclusions
Despite substantial differences in intake and deficiency between groups, our results indicate that by consuming a well-balanced diet including supplements or fortified products, all three types of diet can potentially fulfill requirements for vitamin and mineral consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because they abstain from consuming meat and fish, vegetarians predominantly consume plant products. The so-called ovo-lacto vegetarians avoid all meat and fish, but do consume eggs and dairy products. Vegans follow a stricter form of vegetarianism and thus avoid all animal products [1].
In Switzerland, a growing proportion of the population follows a vegetarian diet. According to the 2012 Swiss Health Survey [2], 2.7 % of the investigated subjects (women 3.9, men 1.3 %) never consume meat or sausages, showing a steady increase from 1.9 % in 1992. At the same time, the percentage of people consuming meat daily decreased from 32.7 % in 1992 to 23.9 % in 2012 (based on personal communication with the Federal Office of Statistics). In the Nutri-Trend-Study in 2000, 9 % of those surveyed stated that they “almost always” followed a vegetarian diet [3]. The Swiss Association of Vegetarians estimates that about 3 % of the population are vegetarian—a conclusion based on a number of surveys between 1997 and 2013. Although no exact data are available, it is estimated that about 10 % of Swiss vegetarians adhere to a vegan diet and the majority of the remaining 90 % follows an ovo-lacto vegetarian eating pattern [4].
Various studies have previously reported differences in nutrient intake in vegetarians and vegans as compared to omnivores [5–8]. In general, total energy intake does not seem to differ significantly between the three groups, but the contribution of proteins to the total energy intake is typically lower in the non-meat-eating diet groups. Total fat intake, expressed as a percentage of total energy, is lowest in vegans and highest in omnivores. Furthermore, meat-eaters consume by far the highest amounts of saturated fatty acids. On the other hand, the intake of polyunsaturated fatty acids is substantially higher in vegans than in the other two groups, particularly in meat-eaters. Vegans have also been found to have the highest fiber intakes, while omnivores have the lowest values. In the EPIC-Oxford study [9], the vegan group was reported to have the highest consumption of vitamin B1, folate, vitamin C and vitamin E and the lowest intakes of retinol, vitamin B12 and vitamin D. Regarding mineral consumption, vegans showed the highest intakes of Mg and Fe and the lowest intakes of Ca and Zn. However, Appleby et al. [9] did not find significant group differences for the intake of vitamin B2, niacin, vitamin B6 and potassium.
Several studies have evaluated the health benefits associated with vegetarian eating patterns as compared to omnivorous diets. Convincing and consistent evidence exists regarding lower rates of coronary heart disease (CHD), colorectal cancer, obesity and diabetes. Total cancer incidence appears to be slightly lower, and life expectancy greater in vegetarians as compared to the general population [10]. The observed positive health effects may not only be due to the avoidance of meat and fish; a diet rich in vegetables, fruits, nuts and whole grain products is known to contribute to better health, independent of the consumption of meat and fish [11]. Moreover, it is widely acknowledged that vegetarians often have healthier lifestyles due to adequate physical activity and lower alcohol and tobacco consumptions [1].
On the other hand, changing from an omnivorous to a vegetarian or even to a vegan diet may lead to deficiencies of some vitamins, minerals and trace elements, as nutrients contained in high amounts in animal products may be difficult to replace. Of particular importance is vitamin B12, which occurs in substantial amounts only in foods derived from animals. The same is true for vitamin D, which can, however, be synthesized in human skin under light exposure. Other nutrients of concern are Zn, Fe, Se, Ca and the long-chain n-3-fatty acids [1].
Therefore, the aim of this study was to investigate micronutrient status and intake in healthy subjects following an omnivorous, a vegetarian or a vegan diet and to investigate associations between health status and intake.
Subjects and methods
Subjects
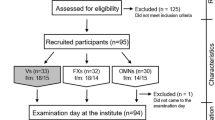
For this study, 206 healthy adult female and male subjects between the age of 18 and 50 years, who have been following either a vegan (VN), vegetarian (VG) or omnivorous (OV) diet for at least 1 year prior to the study, were recruited. We recruited 53 ovo-lacto vegetarians, 53 vegans and 100 omnivores. OV and VG subjects taking antibiotics or vitamin/mineral supplements, as well as pregnant and lactating women, were excluded from the study. Vitamin/mineral supplementation is very common among VNs. As it was impossible to recruit the required number of vegan subjects otherwise, the criteria regarding supplementation were relaxed for this group. VN subjects who were regularly consuming supplements were included in this group, but supplementation had to be interrupted for at least 14 days prior to the start of the study. The usual supplementation was documented. Any subject who was suffering from a chronic disease or had surgery less than 3 months prior to the study was excluded. Signed written informed consent was obtained from each subject. The study has been approved by the Ethical Committees of ETH Zurich and of the Canton of Vaud.
Subjects were recruited in the area of Lausanne by the Swiss Vitamin Institute (Epalinges, Switzerland) as well as in the area of Zurich by ETH Zurich (Zurich, Switzerland) using advertisements in schools, restaurants and shops.
Study design
The subjects had only one meeting with the investigators which took place on the first day of the study. After an overnight fast, the subjects came to the ISV in Epalinges or to ETH in Zurich where their body weight and height were measured and a spot urine sample (for the determination of iodine status) was collected. Subsequently, a venous blood sample of approximately 25 ml was drawn into three heparinized tubes for the determination of micronutrient status including the concentration of the vitamins A, C, E, B1, B2, B6, B12, folic acid, pantothenic acid (B5), niacin (B3), biotin (B7) and β-carotene as well as several minerals and trace elements, namely Fe, Zn, Se and Mg. In the following week, each subject collected three additional spot urine samples. The samples were sent to ETH Zurich and stored at −20 °C until determination of the iodine status was performed.
Assessment of vitamin and mineral status
Plasma concentrations of the vitamins A, C, E, B1, B2, B6, B12, folic acid, pantothenic acid (B5), niacin (B3), biotin (B7) and β-carotene were measured at the ISV in Epalinges. For the determination of vitamin A and E concentrations, HPLC with a UV detector was used [13]. Vitamin C was measured by HPLC with an electrochemical detector [12]. Isocratic liquid chromatography was applied to assess β-carotene concentration [13]. The vitamins B1, B2, B6, B12, pantothenic acid, niacin, biotin and folic acid were measured using microbiologic methods on microplates with different bacterial strains [14].
Urinary iodine concentration was measured in duplicate at the ETH using a modification of the Sandell–Kolthoff reaction [15]. Each of the four samples collected per person was measured individually, and the mean was used for subsequent data analysis. Four samples were collected per person to account for within-subject variations between days. Fe status was assessed using the parameters hemoglobin (Hb), plasma ferritin (PF) and C-reactive protein (CRP). Immediately after blood collection, Hb concentration was analyzed using an electronic counter (HemoCue, Angelholm, Sweden). The measurements were taken at either the ISV or ETH. PF and CRP were analyzed at ETH using an automated chemiluminescent immunoassay system (Immulite 2000, Siemens Healthcare Diagnostics Inc., Tarrytown, New York, USA). If CRP values were >5 mg/l, the samples were excluded from further Fe status analysis. Determination of Mg and Zn concentration in plasma was carried out at ETH Zurich by flame atomic absorption spectrometry (FAAS). Se analysis was performed at the Federal Office of Public Health, Bern (Bundesamt für Gesundheit, BAG) using coupled plasma mass spectrometry (ICP-MS).
Assessment of dietary intake
To assess dietary intake, a three-day weighed food record was done. This dietary protocol was completed over three non-consecutive days during the week after the day of blood collection by the participants at home. The sampling period included 2 week days and one weekend day. Participants were provided with kitchen scales for weighing their consumed food in order to reduce measurement errors. The completed food records were returned to ETH where dietary intake was calculated using the software EBISpro for Windows. Food records were excluded if under- or over-reporting was apparent. The Goldberg cutoffs for over- and underreporting were applied [16].
Assessment of lifestyle factors
In addition to the evaluation of food intake and micronutrient status, subjects were asked to fill in two questionnaires at home assessing lifestyle factors such as their physical activity and their consumption of alcohol and tobacco. Physical activity was evaluated using the International Physical Activity Questionnaire (IPAQ). Alcohol and tobacco consumption were evaluated by means of a questionnaire similar to the one used in the Swiss Health Survey [17].
Data analysis
Statistical analysis was carried out using the statistical package IBM SPSS Statistics 20 for Windows. Non-normally distributed variables were log-transformed prior to analysis. The weighed food records were analyzed using the nutrition software EBISpro for Windows (4.0, Dr. Jürgen Erhardt, Universität Hohenheim, Germany), which allows calculation of energy as well as the macro- and micronutrient intakes of each individual. All protocols were tested for over- and underreporting (REE*2.4 and REE*1.2, respectively) prior to further analysis. One-way ANOVA with post hoc Bonferroni correction was used to assess differences in biochemical as well as dietary intake parameters and physical activity scores between the three groups. The Chi-square test was used to calculate whether the prevalence of micronutrient deficiency, micronutrient status above the reference range, low micronutrient intake and smoking were the same across the three diet groups. To assess continuous associations between dietary intake and biochemical parameters, Pearson correlation was used. Spearman’s rho correlation was used to calculate correlations between nonparametric data (e.g., tobacco and alcohol consumption). The level of significance for all analysis was set at p < 0.05.
Results
Subject characteristics
A total of 206 subjects participated in the study. Table 1 shows the basic characteristics of the study population by diet group. The groups did not differ in terms of gender distribution (p = 0.712) or age (p = 1.000). BMI was significantly lower in the VN group only when compared to OVs (p = 0.016).
The questionnaires regarding lifestyle and physical activity were returned by 196 and 195 subjects, respectively. Ninety-nine percent of all subjects judged their general health status as good or very good. The majority of subjects (64 % of OV, 74 % of VG and 61 % of VN) declared themselves to be health conscious, and this score correlated negatively with alcohol consumption (r = 0.245, p = 0.001) and positively with total physical activity (r = 0.149, p = 0.039). Of the total study population, 24.2 % were current smokers with no difference between the groups. Alcohol consumption was more frequent in the OV and VG groups with 36.6 and 34.7 % reporting to consume alcohol 1–2 times per week, respectively. In comparison, only 15.4 % of VNs reported the same consumption. Similarly, alcohol abstinence was most frequent in the VN group (28.8 % compared to 3.2 % for OV and 6.1 % for VG). Total physical activity was not significantly different between the three groups nor any of the sub-categories of physical activity (work, transport, domestic and leisure time activity).
Micronutrient status and prevalence of micronutrient deficiencies
There were some missing values in the vitamin and mineral analysis due to insufficient sample volume or technical problems during analysis. The following measurements could not be taken: β-carotene status for one OV subject, vitamin C for one VG subject, PF, Mg and Zn status for seven OV subjects and UIC status for seven OV, three VG and one VN subject. A total of 12 female OV, four male OV and one male VN subjects had to be excluded from further analysis of PF data due to elevated CRP values (CRP > 5 mg/l).
Vitamin and mineral status is reported in Table 2 which also indicates significant differences between groups. While OV subjects showed the highest status in PF, Zn, iodine, vitamin A, vitamin E and niacin, VN participants had the highest status in Mg, Vitamins C, B1, B6 and folic acid. Table 3 shows the percentage deficiency for each assessed parameter by diet group including the normal ranges used. As a substantial amount of the VN subjects was consuming vitamin B12 supplements, their vitamin B12 status was calculated separately. Median vitamin B12 status of vegans consuming B12 supplements was 342 pmol/l (27-5166) compared to 274 pmol/l (24-2632) in those reporting no consumption of supplements. In the vegan group consuming no supplements, three subjects had vitamin B12 concentrations below the cutoff for sufficiency (150 pmol/l), whereas only one subject was deficient in the group consuming supplements.
Dietary intake
Out of all 206 three-day weighed food records, 194 (94 %) were returned to ETH Zurich and analyzed (n OV = 93, n VG = 49, n VN = 52). Underreporting occurred in a total of 31 cases (n OV = 16, n VG = 6, n VN = 9) which were excluded from further intake analysis. Over-reporting was not found in any case. Table 4 describes macronutrient and energy intakes reported, and Table 5 shows the intake of micronutrients (n OV = 77, n VG = 43, n VN = 43). Energy and fat intake did not differ significantly between the groups, but protein intake was higher in the OV group compared to the two others (p < 0.001). Carbohydrate intake was significantly higher in the VN group (p < 0.01). The intake of fiber was highest in the VN and lowest in the OV group (p < 0001). Ca intake was highest in the OV group, while the intakes of Mg, Fe and K were highest in the VN group. Similarly, vitamin D and vitamin B12 intakes were highest in the OV group, while the intakes of vitamin E, C, B1, B6, niacin and folic acid were highest in the VN group.
Correlation between dietary intake and biochemical status analysis
To investigate the associations between dietary intake of a nutrient and the respective micronutrient status, Pearson correlations were used. No significant associations between dietary intake and status were determined in any of the three diet groups for retinol, vitamin C, vitamin E, vitamin B1, vitamin B2 and vitamin B12. Mg intake was significantly positively correlated with Mg status in the OVs (r = 0.288, p = 0.011) but not the two other groups (VG: r = 0.056, p = 0.723; VN: r = 0.118, p = 0.451). Iron intake was positively correlated with PF in the OV (r = 0.247, p = 0.030) and the VG (r = 0.331, p = 0.030) groups but not the VN group (r = 0.168, p = 0.281). Zn intake was also significantly positively correlated with Zn status in OV subjects (r = 0.301, p = 0.008) but not in the two other groups (VG: r = 0.040, p = 0.800; VN: r = 0.020, p = 0.901). Significant positive correlations between intake and status in all groups were identified for vitamin B6 (OV: r = 0.460, p < 0.001; VG: r = 0.304, p = 0.048; VN: r = 0.427, p = 0.004) and folic acid (OV: r = 0.298, p = 0.009; VG: r = 0.447, p = 0.003; VN: r = 0.302, p = 0.049).
Discussion
As VG and especially VN lifestyles are currently emerging in Western societies, it is important to understand the impact of those dietary patterns on micronutrient status and thereby health. Health benefits of VG and VN diets have been discussed in association with cardiovascular disease and certain cancers, but their impact on the supply of certain micronutrients is of concern. To our knowledge, this is the first study investigating the status and intake of a broad range of micronutrients, including both vitamins and minerals, in OVs, VGs and VNs in Switzerland.
Nutrients with highest status in omnivores
We found PF to be significantly higher in our OV study population, despite the highest iron intakes in the VNs. The phenomenon of high iron intake in VNs has been reported previously by Davey et al. [5] and Janelle and Barr [18]. A lower status despite high intakes can mainly be explained by the lower iron bioavailability from plant foods compared to animal foods. Generally, non-heme iron is much less well absorbed compared to heme iron found in meat. In addition, most plant foods contain certain iron absorption inhibitors such as polyphenols or phytic acid which reduce the bioavailability of iron even further [19]. The fact that we found PF to be positively correlated with iron intakes in the OV and the VG groups but not the VN group can be explained by the fact that the consumption of iron absorption inhibitors was highest in the VN group.
In contrast to previous studies indicating higher zinc intake in OVs compared to VGs or VNs [5, 18, 20], we found similar zinc intake in all three groups. Nevertheless, zinc status was significantly lower in VGs and VNs as compared to OVs, which is in line with a previous study by Krajčovičová-Kudláčková et al. [21]. Similarly to iron, there was a significant positive association between zinc intake and zinc status in the OV group, but not in the VG or VN groups. Zinc bioavailability also depends on the presence of absorption inhibitors and enhancers [22]. Consequently, zinc-rich plant foods such as legumes, whole grains, nuts and seeds—which are consumed in higher amounts by VGs and VNs—also contain high amounts of phytic acid, a zinc absorption inhibitor [23].
As dietary iodine in Switzerland is largely supplied by iodized salt, assessment of iodine intake using dietary assessment is near to impossible. However, urinary iodine provides a reliable measure of individual iodine intake if collected repeatedly. However, ideally a higher number of samples per subject than used in the current study would have been desirable [24]. We found a significantly lower urinary iodine concentration in VNs as compared to the two other groups. This result confirms findings of Krajčovičová-Kudláčková et al. [25] and Remer et al. [26], both of whom reported a significantly lower urinary iodine concentration in VGs and particularly in VNs as compared to OVs. With a median of 56 µg/l, the urinary iodine concentration of our VN population was far below the current WHO cutoff for iodine sufficiency of 100 µg/l. This may be due to the fact that VNs do not consume milk and dairy products, which are, besides iodized salt, one of the most important dietary iodine sources in Switzerland [27].
Because the most common dietary sources for calcium are milk and dairy products, VNs were expected to have low-calcium intakes. This has also been demonstrated in several previous studies [5, 18, 20, 28]. In agreement with those findings, we found that the VN subjects in our study consumed significantly lower amounts of calcium compared to the other groups. Fifty-four percent of the VN subjects consumed less Ca than recommended by the EAR (800 mg/dl), as compared to 28 % of OVs and 17 % of VGs. Serum calcium is tightly regulated, and only in extreme circumstances concentrations below or above the normal range can be detected [29]; consequently, it is not a good indicator of calcium nutritional status. For this reason, we had to rely solely on intake assessment for this mineral. Low-calcium intake is considered a risk factor for the development of osteoporosis [30], and it is thus important that VNs are informed regarding alternative calcium sources and/or supplements.
Serum retinol is determined by both retinol and β-carotene intake. While preformed retinol is found predominantly in animal foods such as liver, butter, cream, cheese, fish and eggs, all β-carotene is of plant origin. High amounts are found in carrots, herbs, green leafy vegetables, apricots, mangos, pepper, etc. [31]. The total intake of retinol equivalents (RE) is usually calculated as 1 RE = 1 µg retinol = 12 µg β-carotene [32]. We could not demonstrate differences in the intake of RE between the three diet groups which is supported by some [28], but not all, previous studies [18, 33]. Nevertheless, serum retinol status was significantly higher in the OV group as compared to the VN group. As the conversion factor of β-carotene to RE is very low and has further been suggested to depend on the cell’s vitamin A needs [34], it is highly likely that the lower serum retinol status found in VNs is due to the fact that they consume no foods of animal origin and therefore also consume no preformed vitamin A. Our findings seem to indicate that a general conversion factor of 12:1 for β-carotene to RE is too high. Nevertheless, plasma retinol concentrations in our subjects were mainly in the normal range with <10 % showing deficiencies, even in the VN subjects. Thus, β-carotene intake seems to be sufficient in this setting.
Despite higher intakes of vitamin E (composed of α-, β-, γ- and δ-tocopherol) in VGs and VNs, vitamin E status was found to be highest in our OV group. Vitamin E acts as an antioxidant for polyunsaturated fatty acids [35]. As those fatty acids were consumed at higher amounts by our VN subjects, this may explain the higher use and thus lower status of vitamin E in this group. Furthermore, in order to judge vitamin E status correctly, one should also determine cholesterol levels. This was not done in our current study, and we can therefore not determine the α-tocopherol:cholesterol ratio. As α-tocopherol is mainly transported in low-density lipoproteins, which are thus protected from oxidation, the α-tocopherol:cholesterol ratio is an important indicator for oxidative damage [36]. Cholesterol intakes and plasma cholesterol are generally higher in OVs as compared to VGs and VNs, and thus, despite higher absolute plasma vitamin E concentrations in our OV subjects, we expect their α-tocopherol:cholesterol ratio to be lower as compared to the two other groups. This has been demonstrated in a previous study by Krajčovičová‐Kudláčková et al. [21, 35].
Similar to Davey et al. [5], we found the lowest niacin intakes in our VG participants and a significantly higher niacin consumption in OVs and VNs. In other studies [18, 28, 33], significantly lower niacin intakes in VNs as compared to OVs were reported. Despite the high intakes in VNs, their niacin status was still significantly lower compared to the OVs and was similar to the VGs. Consequently, 30 % of all VGs and VNs were found to be niacin deficient. However, in the human body niacin can be converted into not only the metabolically active form of nicotinamide adenine dinucleotide (NAD) but also the amino acid tryptophan, and the classical deficiency symptoms of pellagra only occur in a deficiency of both niacin and tryptophan. Thus, there is no direct need for concern in our study population [34].
As plant foods generally do not contain any vitamin B12, VGs—and particularly VNs—were expected to show lower intakes and plasma concentrations of this nutrient. In accordance with many other studies [5, 18, 20, 28, 33, 37, 38], we found very low dietary vitamin B12 intakes in VNs, higher but still low intakes in VGs and an appropriate vitamin B12 supply from diet only in OVs. Because no supplements were consumed by the subjects throughout the duration of the study, habitual use of supplements was not assessed in the dietary record. However, we have asked all subjects at the beginning of the study whether they consume supplements on a regular basis and if yes, what kind of supplements. Of our vegan subjects, 43 % reported that they normally consume supplements containing vitamin B12, but we did not assess exact frequencies of consumption. This was reflected by the median plasma vitamin B12 concentrations determined in our subjects, which was similar and in all groups. Similarly, the prevalence of vitamin B12 deficiency was low in all groups; nevertheless, with four out of 53 subjects it was highest in the vegan group. However, even when analyzing only the group which abstained from taking regular vitamin B12 supplements, their status was still relatively high (median of 274 pmol/l vs. 342 pmol/l in the group taking supplements) and only three subjects were B12 deficient. This can be explained by the fact that the human body only requires relatively low amounts of vitamin B12; body stores are therefore depleted slowly [39]. Throughout the 1–3 years during which our subjects followed their diets, they may not have completely depleted their vitamin B12 stores despite not having taken supplements. Furthermore, we did only as for supplements taken on a regular basis and some subjects may only take supplements irregularly. In addition, not all fortified products may have been captured by the dietary record. Furthermore, several plant foods such as algae, edible mushrooms and fermented products have been reported to contain significant amounts of vitamin B12, even though their biological activity and consequential effect on B12 status is inconclusive [40]. It may well be that some processed foods specifically made for vegans contain such ingredients which again were not captured by our dietary software. In contrast to our work, several previous studies reported lower vitamin B12 status in both VG and VN subjects [20, 28, 37–39], including a recent comprehensive review [41]. Despite the low prevalence of clinical vitamin B12 deficiency in our study, it has to be considered that the so-called subclinical vitamin B12 deficiency is not well defined and its impact unclear [42, 43]. On the other hand, it was reported that a serum vitamin B12 concentration of < 148 pmol/l has a sensitivity to diagnose 97 % of true, clinical deficiency [43].
We have not been able to assess vitamin D status in this study but have only examined intake. However, as vitamin D—or rather its active metabolite 1,25-dihydroxyvitamin D3—can be synthesized in the skin if exposed to sunlight [44], vitamin D intake is not expected to clearly determine status. As dietary sources of vitamin D are generally animal foods, the finding of lower vitamin D intake in our VG and VN groups is not unexpected and is also in line with previous data [5, 28]. Overall, vitamin D intake was low in all three diet groups which may indicate a potential risk for low vitamin D status especially in winter time. During this season, vitamin D synthesis in the skin is not possible in Switzerland due to the irradiation angle of the sun [45]. The low vitamin D intake together with the low-calcium intake observed in the VN group may potentially put them at risk for the development of osteoporosis. This clearly is a factor which should be closely observed especially in the VN group.
Nutrients with highest status in vegans
Similar to other studies [5, 18], we have found highest magnesium intakes in the VN group. Furthermore, in our study population, the highest magnesium status was seen in the same group, a finding not confirmed by all previous studies [21, 46]. Nevertheless, we have only found a significant positive correlation between magnesium intake and status for the OV subjects. Two main factors can help explain this finding: (1) magnesium is absorbed at higher proportions from low-magnesium diets [47, 48] (in our case the OV diets); (2) dietary factors such as phytic acid and oxalate can inhibit magnesium absorption [49]. These may explain the differences in correlations found between groups as well as the relatively small differences in status compared to intake.
In line with many other studies [5, 18, 20, 28, 33], we found the lowest vitamin C consumption in OVs, intermediate consumption in VGs and the highest intake in VNs with significant differences between all groups. Accordingly, the lowest mean vitamin C status as well as the highest prevalence of vitamin C deficiency (12 %) occurred in the OV group. Similarly, in earlier studies, Krajčovičová‐Kudláčková et al. [21, 35] also measured plasma vitamin C concentration in VG and non-VG subjects and reported significantly higher values for VGs [21]. Despite this, in our data, calculated vitamin C intakes did not correlate with status. However, because storage, processing and preparation can destroy more than 50 % of the vitamin C content of a fresh product [34], and because vitamin C content depends also on variety and ripeness (especially in fruits), it is very difficult to precisely assess vitamin C intake with dietary assessment methods This may explain the lack of correlations found in this study.
In the present study, vitamin B1 intake and status were significantly higher in VNs compared to the other diet groups. Similar results were reported in previous studies [5, 18, 33, 38], but not all agree [28]. Sprouts, seeds and nuts, which are the best sources for vitamin B1, most likely account for the high vitamin B1 consumption in VNs, whereas OVs can benefit from high vitamin B1 concentrations in pork [31]. Despite intake and status showing similar results in our subjects for vitamin B1, no correlations could be shown between the two. This may be due to the fact that the dietary records were completed in the week after blood sampling and that vitamin B1 has a relatively short half-life [34]. Thus, the intake reflected in the measured plasma concentration may not have been the same as the one assessed in the food records.
Vitamin B6 is contained in many different foods. Good sources are wheat germs, sprouts, soy beans, whole oats, pulses, liver, chicken, pork, fish and beef [31]. Sufficient vitamin B6 intake could thus be obtained in all examined diets. Even though the mean vitamin B6 consumption was adequate in all groups, VNs showed significantly higher vitamin B6 intakes in comparison with OVs and VGs. This finding is in agreement with two studies by Haddad et al. and Majchrzak et al. who also report highest vitamin B6 intakes in the VN group [33, 38]; however, other studies do not agree [5, 18, 28, 37]. We found vitamin B6 status to be significantly lower in VGs compared to the two other groups, with a vitamin B6 deficiency of almost 60 % in this group. This finding is in contrast to some [38, 39], but not all, previous studies [37]. It can likely be explained by the fact that VGs do not consume meat—one of the major sources of vitamin B6 in OVs—but also consume germs, sprouts and soy beans—other good vitamin B6 sources—in lower extents than VNs.
Yeast, germs, pulses, bran, sprouts, liver, green vegetables and nuts are good sources of folic acid [31]. It is thus not surprising that we found highest intakes of folic acid in VNs, intermediate intakes in VGs and the lowest intakes in OVs; this is in agreement with the results of several other studies [5, 18, 28, 33, 38]. Accordingly, folic acid status was also found to be significantly higher in the VN group compared to the two others—a finding in line with most earlier studies [20, 37, 38], but not all [50]. Based on the low intake and status of folic acid, 58 % of the OVs in our study were diagnosed with folic acid deficiency compared with only 13 % of our VN population.
Nutrients with no differences among groups
We found no significant differences between groups in selenium, vitamin B2, pantothenic acid and biotin status. As all those micronutrients can be found in a variety of food groups not specific to any of the three diet groups, this finding was not unexpected.
Lifestyle
The common assumption that VGs and particularly VNs follow a healthier lifestyle could not be confirmed in the present study. With a mean of 24 % of all subjects, the smoking rate in our study was relatively high compared to that in previous studies (3–18 %) [5, 6, 51–53]. However, this high smoking rate is not uncommon for Switzerland: in a study from 2010, 27 % of 14- to 65-year-olds and 39 % of 20- to 24-year-olds reported to smoke cigarettes [54]. Alcohol abstinence on the other hand was found to be more prevalent in our VN group (28.8 % compared to 6.1 % in VG and 3.2 % in OV). This shows a similar tendency as previous reports showing that up to 73 % of VNs never consume alcohol [51–53]. However, this is the only factor where VNs seemed to be more health conscious compared to the subjects in the other diet groups. Physical activity in any domain was not found to be significantly different among groups. However, it can of course be argued that volunteers of the OV group who participate in nutrition and lifestyle studies are usually more health conscious than the average population [5, 7]. The so-called healthy volunteer effect may explain why no significant differences between OV, VG and VN participants could be detected in the present study. Besides, we need to consider that our study sample, especially the OV group, was clearly not a representative sample of the total population, and therefore, the general applicability of these results is limited. A further limitation in the VN group may be that we cannot rule out the possibility that there was a certain difference between those who consume dietary supplements and those who don’t. Even though we did not find significant differences between the two groups in their general characteristics, some subtle differences (e.g., regarding health consciousness) which we could not detect may exist.
In conclusion, it can be said that all diet groups show low intakes and deficiencies for some micronutrients. Therefore, based on our results (and focusing only on micronutrients), it is impossible to define which of the three types of diet would be most beneficial to human health. Irrespective of the type of diet a person chooses, it is important for them to be well aware of its potential limitations and to take the corresponding precautions (for example by taking vitamin B12 supplements). Fortified products may also be a good alternative to supplements in all diet groups as they may help cover the needs for the different nutrients.
References
Walter P et al (2005) Gesundheitliche Vor- und Nachteile einer vegetarischen Ernährung. Bericht der Arbeitsgruppe ‘Vegetarische Ernährung’ der Eidgenössischen Ernährungskommission. Bern, Bundesamt für Gesundheit
BfS. Schweizerische Gesundheitsbefragung (SGB). 2012 (cited 26 November 2014). http://www.bfs.admin.ch/bfs/portal/de/index/infothek/erhebungen__quellen/blank/blank/ess/04.html
Exl-Preysch BM, Mühlemann P (2001) Nutri-Trend-Studie 2000 der Nestlé Suisse SA mit Unterstützung durch das Bundesamt für Gesundheit. Vevey, Bern
Walter P, Baerlocher K (2005) Vegetarismus in der Schweiz. In: Eichholzer M et al (eds) Fünfter Schweizerischer Ernährungsbericht. Bundesamt für Gesundheit, Bern, pp 321–340
Davey G et al (2003) EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33883 meat-eaters and 31546 non meat-eaters in the UK. Public Health Nutr 6(3):259–268
Bedford J, Barr S (2005) Diets and selected lifestyle practices of self-defined adult vegetarians from a population-based sample suggest they are more ‘health conscious’. Int J Behav Nutr Phys Act 2:4
Appleby P et al (1999) The Oxford vegetarian study: an overview. Am J Clin Nutr 70(3 Suppl):525S–531S
Spencer E et al (2003) Diet and body mass index in 38,000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes 27(6):728–734. doi:10.1038/sj.ijo.0802300
Appleby P, Davey G, Key T (2002) Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public Health Nutr 5(5):645–654. doi:10.1079/PHN2002332
Fraser G (2009) Vegetarian diets: What do we know of their effects on common chronic diseases? Am J Clin Nutr 89(5):1607S–1612S
Esmaillzadeh A et al (2006) Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr 84:1489–1497
Bui MH et al (1992) Dietary vitamin C intake and concentrations in the body fluids and cells of male smokers and nonsmokers. J Nutr 122(2):312–316
Bui MH (1993) Simple determination of retinol, a-tocopherol and carotinoids (lutein, all-trans-lycopene, a- and b-carotenes) in human plasma by isocrtic liquid chromatography. J Chromatogr B Biomed Sci Appl 654(1):129–133
Bui MH (1999) A microbiological assay on microtitre plates of thiamine in biological fluids and foods. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition 69(5):362–366
Pino S, Fang S, Braverman L (1996) Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin Chem 42(2):239–243
Goldberg G et al (1991) Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 45(12):569
Bundesamt für Statistik (not dated) Erhebungen, Quellen – Schweizerische Gesundheitsbefragung (SGB). http://www.bfs.admin.ch/bfs/portal/de/index/infothek/erhebungen__quellen/blank/blank/ess/04.html. Accessed 6 July 2011
Janelle K, Barr S (1995) Nutrient intakes and eating behavior see of vegetarian and nonvegetarian women. J Am Diet Assoc 95(2):180–189
Aggett PJ (2012) Iron. In: Erdman JW, Macdonald IA, Zeisel SH (eds) Present knowledge in nutrition. ILSI International Life Sciences Institute, Washington
Li D et al (2000) Selected micronutrient intake and status in men with differing meat intakes, vegetarians and vegans. Asia Pac J Clin Nutr 9(1):18–23
Krajčovičová-Kudláčková M et al (1995) Selected vitamins and trace elements in blood of vegetarians. Ann Nutr Metab 39(6):334–339
Holt RR, Uriu-Adams JY, Keen CL (2012) Zinc. In: Erdman JW, Macdonald IA, Zeisel SH (eds) Present knowledge in nutrition. ILSI International Life Sciences Institute, Washington
Hunt J (2003) Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr 78(3):633–639
König F et al (2011) Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 141(11):2049–2054
Krajčovičová-Kudláčková M et al (2003) Iodine deficiency in vegetarians and vegans. Ann Nutr Metab 47(5):183–185
Remer T, Neubert A, Manz F (1999) Increased risk of iodine deficiency with vegetarian nutrition. Br J Nutr 81:45–50
Haldimann M et al (2005) Iodine content of food groups. J Food Compos Anal 18(6):461–471. doi:10.1016/j.jfca.2004.06.003
Larsson C, Johansson G (2002) Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr 76(1):100–106
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (2011) In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB (eds) Dietary reference intakes for Calcium and Vitamin D. National Academies Press, Washington DC, P 1132
Weaver CM (2012) Calcium. In: Erdman JW, Macdonald IA, Zeisel SH (eds) Present knowledge in nutrition. ILSI International Life Sciences Institute, Washington
Federal Food Safety and Veterinary Office (not dated) Schweizerische Nährwertdatenbank. http://www.naehrwertdaten.ch Accessed 14 May 2012
Trumbo P et al (2001) Dietary reference intakes Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. J Am Diet Assoc 101(3):294–301
Haddad E et al (1999) Dietary intake and biochemical, hematologic, and immune status of vegans compared with nonvegetarians. Am J Clin Nutr 70(3):586S–593S
Biesalski H, Grimm P (2005) Pocket atlas of nutrition, 3rd edn. Georg Thieme Verlag, New York
Krajčovičová-Kudláčková M et al (1996) Lipid and antioxidant blood levels in vegetarians. Food/Nahrung 40(1):17–20
Rauma A, Mykkänen H (2000) Antioxidant status in vegetarians versus omnivores. Nutrition 16(2):111–119
Huang Y et al (2003) The status of plasma homocysteine and related B-vitamins in healthy young vegetarians and nonvegetarians. Eur J Nutr 42(2):84–90
Majchrzak D et al (2006) B-vitamin status and concentrations of homocysteine in Austrian omnivores, vegetarians and vegans. Ann Nutr Metab 50(6):485–491
Herrmann W et al (2003) Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am J Clin Nutr 78(1):131–136
Watanabe F et al (2013) Biologically active Vitamin B-12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J Agric Food Chem 61(28):6769–6775. doi:10.1021/Jf401545z
Pawlak R, Lester SE, Babatunde T (2014) The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr 68(5):541–548. doi:10.1038/Ejcn.2014.46
Devalia V et al (2014) Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol 166(4):496–513. doi:10.1111/bjh.12959
Carmel R (2013) Diagnosis and management of clinical and subclinical cobalamin deficiencies: Why controversies persist in the age of sensitive metabolic testing. Biochimie 95(5):1047–1055. doi:10.1016/j.biochi.2013.02.008
Norman AW, Henry HL (2012) Vitamin D. In: Erdman JW, Macdonald IA, Zeisel SH (eds) Present knowledge in nutrition. ILSI International Life Sciences Institute, Washington
FSVO. Vitamin D—the key facts. 2012 (cited 2014 26 November 2014). http://www.blv.admin.ch/themen/04679/05065/05104/index.html?lang=en&download=NHzLpZeg7t,lnp6I0NTU042l2Z6ln1ad1IZn4Z2qZpnO2Yuq2Z6gpJCFfYB9fmym162epYbg2c_JjKbNoKSn6A
Srikumar T, Öckerman P, Åkesson B (1992) Trace element status in vegetarians from Southern India. Nutr Res 12(2):187–198
Rude R (1998) Magnesium deficiency: a cause of heterogenous disease in humans. J Bone Miner Res 13(4):749–758
Fine K et al (1991) Intestinal absorption of magnesium from food and supplements. J Clin Invest 88(2):396
Bohn T (2003) Magnesium absorption in humans. Swiss Federal Institute of Technology, Zurich
Herrmann W et al (2001) Total homocysteine, vitamin B12, and total antioxidant status in vegetarians. Clin Chem 47(6):1094–1101
Herrmann W, Geisel J (2002) Vegetarian lifestyle and monitoring of vitamin B-12 status. Clin Chim Acta 326(1–2):47–59
Key T et al (2009) Cancer incidence in British vegetarians. Br J Cancer 101(1):192–197
Waldmann A et al (2003) Dietary intakes and lifestyle factors of a vegan population in Germany: results from the German vegan study. Eur J Clin Nutr 57(8):947–955
Keller R (2010) Der Tabakkonsum der Schweizer Wohnbevölkerung in den Jahren 2001 bis 2009. Universität Zürich-Psychologisches Institut, Sozial-und Gesundheitspsychologie
IOM (2006) Dietary reference intakes—DRI. Institute of Medicine, Washington
Acknowledgments
We thank all the study participants for their cooperation during the study. Furthermore, we would like to thank Doris Schutz from the Swiss Vitamin Institute for her excellent support during the study and for the vitamin measurements. A big thank you also goes to Stefanie B. Murer, Jasmin Tajeri Foman, Sara Stinca, Valeria Galetti and Christophe Zeder at the Human Nutrition Laboratory at ETH Zurich for their support during the study, for help with translations, sample organization and laboratory analyses. For financial support, we would like to thank the Swiss Foundation for Nutrition Research (SFEFS) and the Swiss Vitamin Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest regarding this work.
Rights and permissions
About this article
Cite this article
Schüpbach, R., Wegmüller, R., Berguerand, C. et al. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur J Nutr 56, 283–293 (2017). https://doi.org/10.1007/s00394-015-1079-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1079-7