Abstract
Purpose
Beneficial effects of green tea (GT) polyphenols against obesity have been reported. However, until this moment the molecular mechanisms of how green tea can modulate obesity and regulates fat metabolism, particularly in adipose tissue, remain poorly understood. The aim of this study was to evaluate the role of GT extract in the adipose tissue of obese animals and its effect on weight gain, metabolism and function (de novo lipogenesis and lipolysis), and the involvement of AMP-activated protein kinase (AMPK).
Methods and results
Male Wistar rats were treated with GT by gavage (12 weeks/5 days/week; 500 mg/kg of body weight), and obesity was induced by cafeteria diet (8 weeks). Here, we show that obese rats treated with GT showed a significant reduction in indicators of obesity such as hyperlipidemia, fat synthesis, body weight, and fat depots as compared to those treated with standard control diet. AMPK was induced in adipose tissue in rats that were treated with GT and likely restored insulin sensitivity, increased mRNA expression of GLUT4, reducing the concentrations of plasma and liver lipid content, also stimulating fatty acid oxidation in the same tissue. Importantly, repression of de novo lipogenesis in the adipose tissue, reduced lipid droplets in the liver, and the development of insulin resistance in diet-induced obese rats were accompanied by AMPK activation.
Conclusion
Our study identified that metabolic changes caused by GT intake induced AMPK activation and modulate the expression of genes involved in metabolism, particularly in adipose tissue, thus offering a therapeutic strategy to combat insulin resistance, dyslipidemia, and obesity in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the primary fuel reserve in mammals, white adipose tissue (WAT) has the function of storing triacylglycerol for hydrolysis (lipolysis) to provide fatty acids for other organs during times of energy shortage [1]. The dysregulated expression of bioactive factors from adipose tissue, caused by excess adiposity and adipocyte dysfunction, has been linked to the pathogenesis of several disease processes through altered immune responses. However, adipocytes possess functional pathways that might be targeted to complement therapies to restore energy balance.
The active enzymes of lipid metabolism integrate signals from multiple pathways and coordinate the activity of the machinery needed for metabolism and lipid metabolism in adipocytes for the supply of fatty acids and energy for the body. Pharmacological or genetic manipulation of enzymes in the lipogenic and lipolytic pathway may have profound metabolic consequences [2].
Besides the well-established role of nutrients, drugs, hormones, and hypothalamic microcircuits, recent evidence has demonstrated that basic cellular metabolic pathways have a major role in the regulation of whole-body energy homeostasis. Among them, much interest and research have been focused on the AMP-activated protein kinase (AMPK) pathway, a master sensor and regulator of energy homeostasis at the cellular level [3, 4]. Research has shown that understanding pathways that activate AMPK might provide new targets for the treatment of obesity, insulin resistance, endocrine and cardiovascular diseases.
Scientific community is constantly looking for new molecules or plant-derived compounds that could be used to prevent or to treat overweight and obesity. In recent years, a great deal of interest has focused on green tea, brewed from the unfermented dried leaves of Camellia sinensis plant. Green tea has been shown to possess many potential health benefits, including antioxidant, anticarcinogenic, and cardioprotective activities, and may affect multiple aspects of energy balance that, in aggregate, result in body weight and fat loss [5, 6]. Notably, green tea polyphenols are widely reported to have beneficial effects on obesity and metabolic syndrome in humans and in animal models [6–8] mainly by its anti-inflammatory effects. Epigallocatechin-3-gallate (EGCG), the main catechin in green tea, may disturb many inflammatory pathways such as MAPKs, AP-1, and NFκB [8]. EGCG inhibits monocyte chemotactic protein-1 (MCP-1) expression in endothelial cells by blocking NFκB signaling. Also, EGCG blocks the induction of nitric oxide synthase (iNOS) by down-regulating lipopolysaccharide-induced activity of NFκB [9, 10].
Recent studies searching for a signaling pathway for green tea polyphenols action in obesity have reported that several polyphenols stimulate AMPKα1/α2 activity in liver [11, 12]. Then, the purpose of this study was to evaluate the role of green tea in the activation of AMPK in adipose tissue of obese animals and its effect on weight gain, metabolism, and function of adipose tissue mainly focusing on de novo lipogenesis and lipolysis.
Methods and procedures
Chemicals
All chemicals including catechin, epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC), epigallocatechin-3-gallate (EGCG), quercetin, and caffeine were of analytical grade and purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA), except those used in the preparation of common buffers (Labsynth, Diadema, SP, Brazil). The powdered green tea extract was commercially acquired from Tovani-Benzaquen, São Paulo, SP, Brazil. Commercial biochemical test kits were obtained from Bioclin–Quibasa Química Básica (Belo Horizonte, MG, Brazil) for measurements of glucose (prod. code K082), triacylglycerol (prod. code K055), and cholesterol (prod. code k083) concentrations in plasma. Rabbit antibodies recognizing AMPK, p-AMPK, p-ACC, ACC, p-HSL, and HSL were purchased from Cell Signaling Technologies (Danvers, MA, USA), whereas antibodies recognizing b-actin, and HRP-conjugated secondary antibodies recognizing mouse and rabbit IgG were purchased from Santa Cruz Biotechnologies (Dallas, TX, USA).
Animals, supplementation, and feeding protocols
Eight-week-old Wistar male rats, weighing 180 ± 20 g at the beginning of the study, were provided by the Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil. All animals were housed in Plexiglas cage (5 rats/cage) under standard laboratory conditions: 12-h light/dark cycle; lights on at 7:00 a.m.; 22 ± 1 °C; water and a standard rodent chow (NuviLab®). The animals used in this study were handled in accordance with the guidelines of the committee on care and use of laboratory animal resources of the Ethics Committee on Care and Use of Laboratory Animal Resources from Universidade Cruzeiro do Sul, which approved the study protocol (CE/UCS no. 153/2011).
Following a week of adaptation, a standard rodent chow was provided for all experimental animals (n = 40) ad libitum during the first month of experimentation. At this period, half of the animals (n = 20) were daily treated (Monday to Friday) with 500 mg/kg of body weight of green tea (GT) extract by gavage, whereas control animals were treated with water by gavage. GT extract was stored at 4 °C during all period of experimentation.
An increasing volume of green tea up to a maximum of 500 µL was established for the gavage treatment in order to prevent regurgitation or stomach discomfort of the animals. Powdered GT extract was weighed daily and then solubilized in water at 70 °C to be subsequently administered by gavage to the animals prior to the feeding period (between 5:30 and 6:30 p.m.). After 1 month of preventive GT treatment, the animals were divided to receive standard control diet or a high-fat cafeteria diet (cafeteria diet) (n = 10 per group). The cafeteria and standard diets were offered permanently to the animals during 2 months, and the quantity of food offered to the animals was according to a pair-feeding regimen among all four groups. The amount of food ingested by OB + GT group was used as reference. The food items in the cafeteria diet were chosen to reflect the enormous variety, palatability, and energy density of the modern Western diet [13]. They included standard rodent chow (37.5 %), peanut (12.5 %), corn starch biscuit (12.5 %), condensed milk (12.5 %), and chocolate (25 %). This diet provided an average of 4.2 kcal/g, 38 % energy as fat, 14 % protein, and 48 % carbohydrate. Standard commercial rodent chow (Nuvilab®) provided 2.8 kcal/g, 13 % energy as fat, 32 % protein, and 55 % carbohydrate. Diets contained equivalent amounts of macro- and micronutrients as presented in supplemental Table 1S.
Thus, four experimental groups of ten animals each were formed during the experimental period: (1) control (Cont), fed with standard chow and gavage with water; (2) green tea (GT), fed with standard chow and gavage with GT extract; (3) Obese (OB), fed with cafeteria diet and gavage with water; and (4) Obese plus GT (OB + GT), fed with cafeteria diet and gavage with GT extract. The total period of gavage with GT was 12 weeks (Monday to Friday). Body weight of the animals was measured twice per week.
Total polyphenols content and HPLC analysis of flavonoids and caffeine concentration in GT extract
For the determination of the total phenolic content (TPC), Folin–Ciocalteu’s phenol reagent, gallic acid (99 % purity, Sigma), and anhydrous sodium carbonate were used. TPC was determined by spectrophotometry according to the method described by the International Organization for Standardization (ISO) 14502-1 [14]. The concentration of polyphenols in samples was derived from a standard curve of gallic acid ranging from 10 to 50 µg/mL (Pearson’s correlation coefficient: r 2 = 0.9896).
Flavonoids (catechins and quercetin) and caffeine were detected in the methanolic extract (50 %) of GT powder at concentrations around 1000 µg/mL. The GT extract and standards (EC, EGCG, EGC, ECG, catechin, quercetin, and caffeine) were analyzed in an analytical LC (Varian 210) system with a ternary solvent delivery system equipped with an auto-sampler, a photodiode array detector (PDA) monitored at λ = 200–800 nm. The LC column was a C-18 (25 cm × 4.6 mm; particle size, 5 μm; Luna, Phenomenex, Torrance, CA, USA), with a small pre-column (2.5 cm × 3 mm) containing the same packing, used to protect the analytical column. In each analysis, the flow rate and the injected volume were set as 1.0 mL/min and 20 μL, respectively. All chromatographic analyses were performed at 22 °C. Elution was carried out using formic acid 0.06 % in water (solvent A) and methanol (solvent B) following solvent-gradient program: 90 % A by 8 min, 90–70 % A by 15 min, and 70–55 % A by 20 min in a linear gradient. Catechins were quantified at 280 nm, quercetin at 250 nm, and caffeine at 270 nm by using external standard calibration curves (0.5–100 µg/mL for catechins and 0.5–20 µg/mL for quercetin and caffeine). The catechins, quercetin, and caffeine were identified in green tea extract by comparing their retention time with those of standard solutions.
Fasting glucose, GTT, ITT, and Kitt analyses
The animals fasting glucose was measured after 11 weeks of trial. Animals were overnight fasted (8 h), and in the morning the blood was obtained via a small section in the distal end of the animal’s tail. Blood glucose was measured using Infinity glucose monitors and strips (US Diagnostics, Indianapolis, IN, USA).
A week prior euthanasia, the glucose tolerance test (GTT) was performed in all 6-h-fasted rats by assaying blood glucose at various times after i.p. injection of glucose (1.5 g/kg of body weight). Insulin tolerance test (ITT) was also performed in all 6-h-fasted rats by assaying blood glucose at various times after i.p. injection of insulin (Humulin R, Lilly, 1.5 IU/kg of body weight). The tests were performed in different days, one at Monday (GTT) and another (ITT) at Thursday in the same week. Glucose level was measured in blood collected from the tail of the animal before (time 0), and at 5, 15, 30, 60, and 90 min after glucose or insulin i.p. injection by using Infinity glucose monitors and strips (US Diagnostics). Total area under the curve (AUC) was calculated of plasma glucose concentration among the groups. The rate of glucose clearance per minute (Kitt) value was obtained by linear regression from the fall of log glucose value rate between 5 and 15 min after i.p. insulin injection.
Experimental design and histology
After 12 weeks of the experimental period, animals were killed by decapitation under starvation condition, during the first 4 h of the beginning of the light cycle. The liver, brown adipose tissue (BAT), and different WAT depots—retroperitoneal, subcutaneous, and epididymal—were rapidly removed. To calculate the adiposity index, all fat pad depots were weighed immediately after removal and before freezing. Adiposity index was calculated as the sum of all the fat pad depots per animal and expressed per 100 g/body weight (BW). Afterward, samples were immediately frozen and stored at −80 °C for analysis. Total blood was also collected to carry out biochemical and metabolic parameters.
Liver and epididymal adipose tissues were freshly fixed in 4 % paraformaldehyde–phosphate-buffered saline. Fixed samples were dehydrated by sequentially increased ethanol concentrations, cleared in xylene, and then embedded in paraffin. The embedded samples were sectioned and stained with hematoxylin and eosin (HE). A total of 100 adipocytes were analyzed per slide, and four animals were analyzed per group. ImageJ was used to measured adipocytes size (results are presented as mean of µm ± SEM).
Analyses in plasma
Briefly, total blood volume (~5 mL) was collected in EDTA-Vacutainer tubes (5.4 mg K2EDTA spray-dried, BD Biosciences, Franklin Lakes, New Jersey, USA) and centrifuged for 10 min, 300×g at room temperature. The clean plasma fraction was then removed and stocked in Eppendorf 2-mL microtubes at −80 °C for further analyses.
Plasma triglyceride, total cholesterol, LDL cholesterol, HDL cholesterol, uric acid, creatinine, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were analyzed using a detection kit obtained from Bioclin (Minas Gerais, Brazil), in accordance with the manufacturer’s instructions. Non-esterified (or free) fatty acids and glycerol in plasma were measured by an enzymatic colorimetric method using a commercial kit (Cayman Chemical, Ann Arbor, Michigan, USA).
Cytokines and adipokines release
Cytokines IL-1β, IL-6, and TNF-alpha were assayed in plasma with ELISA kits, in accordance with the manufacturer’s instructions (Quantikine, R&D System, Minneapolis, Minn, USA). Leptin and adiponectin levels were quantified by ELISA (Abcam, Cambridge, UK) following the manufacturer’s instructions.
Tissue triglyceride and cholesterol analysis
A sample of liver (100–200 mg) was homogenized in isopropyl alcohol solution using a Polytron homogenizer. Homogenates were centrifuged at 500×g for 10 min, and the supernatant was used for the quantification of triglyceride and cholesterol total content. Triglyceride and cholesterol contents were determined using Triglyceride Determination Kit (Sigma) and Cholesterol Quantification Kit (Sigma).
Western blotting analysis
For evaluation of protein expression in the epididymal WAT (n = 4 per group), the specimens were excised and immediately homogenized in solubilization buffer [1 % Triton X-100, 100 mM Tris–HCl (pH 7.4), 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM sodium orthovanadate, 2.0 mM phenylmethylsulfonyl fluoride, and 0.1 mg aprotinin/mL] at 4 °C with a Polytron PTA 20S generator (model PT 10/35; Brinkmann Instruments, Westbury, NY). Insoluble material was removed by centrifugation for 20 min at 12.000×g. The protein concentration of the supernatants was determined by the Bradford dye-binding method. In immunoblot experiments, 30–80 µg of protein extracts was separated by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with specific antibodies, as described in the figure legends. Membranes containing immunoreactive bands were visualized by enhanced chemiluminescence (Amersham, Little Chalfont, Buckinghamshire, UK) according to the manufacturer’s protocol. The bands were visualized on X-ray film and analyzed by optical densitometry with the program ImageJ. β-Actin was used to check equal protein loading of gels.
RNA extraction
Total RNA was extracted from two different WAT depots (epididymal and subcutaneous) (n = 4 per group), by using TRIzol Reagent (LifeTechnologies, Rockville, MD, USA) according to the manufacturer’s instructions. Isolated RNA was quantified using the NanoDrop ND-1000 spectrophotometer (NadroDrop Technologies, Wilmington, DE), and its integrity was confirmed using agarose gel electrophoresis.
RT-PCR
Reverse transcriptase (RT)-PCR was used to measure mRNA expression levels of fatty acid synthase (FAS), pyruvate dehydrogenase (PDH), stearoyl-coenzyme A desaturase 1 (SCD1), diacylglycerol acyltransferase (DGAT), hormone-sensitive lipase (HSL), adipose triglyceride lipase (ATGL), perilipin, lipoprotein lipase (LPL), glucose transporter type 4 (GLUT4), leptin, adiponectin, peroxisome proliferator-activated receptor-γ (PPAR-γ), carnitine palmitoyltransferase 1 (CPT1), CD36, and fatty acid binding protein 4 (FABP4) in different WAT depots (epididymal and subcutaneous). Table 1 presents nucleotide sequence used in this study.
Total RNA (2 μg) was treated with 1U DNase for 25 min at 25 °C and inactivated with 2.5 mM EDTA. Afterward, the cDNA was synthesized using oligo (dT) in a 20-μL reaction containing 1 mM of each dNTP and 200 U SuperScript II RNase H reverse transcriptase at 42 °C for 50 min according to the manufacturer’s instructions. Heating at 70 °C for 15 min inactivated the reaction. The PCR was performed in a total volume of 25 μL, containing 2.5 μL of buffer DNA polymerase enzyme (50 mm KCl, 1.5 mM MgCl2, 10 mM Tris–HCl, pH 9·0), 10 pmol of the primer, 200 μM of each nucleotide (dATP, dCTP, dGTP, and dTTP) and 2.5 U of Taq DNA polymerase (Sigma) and 2 μL of cDNA. The RT and PCR were carried out in a Veriti Gradient equipment (Applied Biosystems, Grand Island, USA), using parameters described by Innis and Gelfand (1990). For semi-quantitative PCR analysis, the housekeeping β-actin gene was used as reference. RT-PCR endpoint products were analyzed after agarose gel (1.3 %) electrophoresis using blue green loading dye (LGC Biotecnologia, SP, Brazil) and ImageJ processing program.
Statistical analysis
Results are given as mean ± SEM. Levene test was used to verify the variance of the data. When the Levene test was p > 0.05, interaction was evaluated through a factorial two-way ANOVA (diet, D × green tea, GT) using Tukey as post-test (p < 0.05). When interaction was not statistically significant, the main effect (diet, D, and/or green tea treatment, GT) was accessed. The statistical analysis was performed using the SPSS/Windows version 22 statistical package (SPSS Inc., Chicago, IL) and GraphPad Prism statistics software package version 5.0 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Polyphenol content of green tea extract
Total phenolic content analysis revealed that in our GT extract TPC was about 39 % (392 µg/mg of GT extract). HPLC analysis showed that among polyphenols catechins and quercetin contents were about 30 % of total polyphenols (117 µg/mg). HPLC analysis also showed that the GT extract was a mixture of several catechins, namely catechin, EGC, EC, EGCG, and ECG. The sum of EGC and EGCG contributed to more than 85 % of the catechin mixture in the extract. The content of caffeine in GT powdered extract was 0.4 %, whereas quercetin was 1.5 % by dry weight.
Green tea inhibits weight gain and adipose tissue mass induced by the cafeteria diet
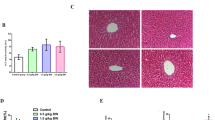
During the experimental period, the body weight (BW) was twice weekly measured. Body weight (initial, final, and BW gain) and fat pad size data are shown in Fig. 1a–h. For the body weight gain (Fig. 1a), there was an interactive effect between diet and GT. Rats fed with standard rodent chow diet supplemented with GT for 60 days decreased the body weight gain by 24 % (vs. cont group), whereas in animals treated with cafeteria diet and GT (OB + GT), the body weight gain was reduced by 39 % (versus OB group). As rats were fed in a pair-feeding regimen, the amount of food intake (in grams) among the groups was similar during all experimental period. However, a 7-day average energy intake conducted in rats during the eight week of treatment revealed an increase in the values of energy intake in Ob/Ob + GT (~714 ± 13 kcal/rat/week) versus control/GT group energy intake (~473 ± 14 kcal/rat/week). Epididymal, subcutaneous, and retroperitoneal fat pad weights/size were decreased in animals treated with GT (GT effect), while cafeteria diet increased all fat pad weights/size (diet effect) as can be seen in Fig. 1d–f. Accordingly, adiposity index were significantly lowest in both groups of animals supplemented with green tea regardless of diet (GT effect) (Fig. 1h). For BAT adipose tissue size (Fig. 1g), there was an interactive effect between diet and GT.
Green tea inhibits weight gain and adipose tissue mass induced by the cafeteria diet. a Body weight gain (g). b Initial body weight (g). c Final body weight (g). d eWAT weight (g/100 g). e scWAT weight (g/100 g). f rpWAT weight (g/100 g). g BAT weight (g/100 g). h Body adiposity index (g/100 g). i Histological images of epididymal adipocytes (×10 magnification). j Morphometric analyses of isolated adipocytes (μm) measured in ImageJ processing program. Data are presented as mean ± SEM of 10 rats per group. An ANOVA 2 × 2 factorial design was used to study the effects of diet (D), green tea (GT), and potential interaction between D × GT. When interaction was statistically significant, superscript letters designate: a statistical difference compared to the control group, b compared to the green tea group, c compared to the obese group
In order to evaluate the modulatory effect of green tea on hypertrophy of adipose tissue, histological analysis (Fig. 1i) revealed larger adipocytes in epididymal fat pads from rats fed with cafeteria diet, indicating increased adipocyte size. Consistent with the body weight and fat depots results, GT inhibits the effects of cafeteria diet-induced hypertrophy of adipose tissue (Fig. 1j).
Green tea protects against glucose intolerance
To evaluate whether cafeteria diet could induce glucose intolerance in rats, we measured the glycemia after 8 h fasting and also after have injecting glucose or insulin. As can be seen in Fig. 2a–c (fasting glucose, GTT, and ITT, respectively), there was a diet effect in fasting glucose and an interactive effect between diet and GT in both GTT and ITT assay (D × GT). Animals treated with cafeteria diet showed an induction of glucose intolerance as evidenced by an increased glucose levels after an overnight fasting, GTT, and ITT AUC values. Green tea was effective in restoring glucose intolerance in obese animals (OB + GT) as well as increased the Kitt (Fig. 2d). Consistent with these results, we found that green tea (GT effect) was able to increase mRNA levels of GLUT4 in subcutaneous and epididymal adipose tissue (Fig. 2e) as well as increase mRNA levels of adiponectin (Fig. 2f) (GT effect), while a significant reduction in the mRNA levels of leptin was seen in subcutaneous adipose tissue due to an interactive effect between diet and GT (Fig. 2g).
Green tea protects against glucose intolerance. a Fasting glucose test. b Glucose tolerance test (GTT). c Insulin tolerance test (ITT). d Rate of glucose clearance per minute (Kitt). e mRNA expression of glucose transporter 4, f adiponectin, and g leptin in epididymal and subcutaneous adipose tissue, assessed by RT-PCR. Data are presented as mean ± SEM of 10 rats per group. An ANOVA 2 × 2 factorial design was used to study the effects of diet (D), green tea (GT), and potential interaction between D × GT. When interaction was statistically significant, superscript letters designate: a statistical difference compared to the control group, b compared to the green tea group, c compared to the obese group
The effect of green tea on plasma lipid parameters
To assess whether treatment with green tea changes the adipokines secretion, we measured the levels of adiponectin and leptin in plasma as well as the inflammatory cytokines IL-6, TNF-α, and IL-1β. Plasma of the animals induced to obesity by the cafeteria diet showed a significant increase in the concentration of the hormone leptin (effect of diet), whereas in adiponectin levels, there was an interactive effect between D × GT. Regarding OB + GT group, there was a significant increase in the secretion of the adiponectin as compared to the OB group (Table 2).
Concerning the IL-6 levels, the group which was supplemented with green tea extract showed a significant decrease regardless of the diet (effect of GT). Cafeteria diet decreased IL-6 levels compared to standard chow diet (diet effect). TNF-α and IL-1β levels were increased by the cafeteria diet (diet effect) (Table 2).
We also evaluate the effect of green tea on the content of lipids and cholesterol in the plasma. We observed a significant increase in FFA concentration in plasma of obese rats, whereas the obese group supplemented with green tea extracts (OB + GT) showed a significant decrease in FFA (interactive effect between D × GT). Glycerol, total cholesterol, and LDL cholesterol were reduced by GT (GT effect), whereas an increase in HDL cholesterol due to GT (GT main effect) was observed as compared to the obese group (Table 2). Biochemical parameters such as uric acid, AST, and ALT were not different among the groups, indicating that the green tea did not induce any kind of kidney or liver damage. However, creatinine level was modified after GT treatment regardless of the diet (interactive effect between D × GT).
To confirm the effectiveness of green tea in reducing the lipid content, we measured the content of triglycerides and total cholesterol in the liver of obese rats supplemented with green tea extract. Cafeteria diet induced a significant increase in the content of cholesterol and triglycerides in the liver of the animals as confirmed by histological images of the liver (Fig. 3a). There was an interactive effect between diet and GT for TG and cholesterol levels in the liver. GT treatment was effective in reducing the content of both lipids in about 50 % as compared to the OB group (Fig. 3b, c).
Green tea extract protects against cafeteria diet-induced obesity. a Histological images stained with hematoxylin and eosin, ×40 magnification, in the liver of rats. b, c Liver triglyceride and cholesterol content in obese rats treated with green tea. Data are presented as mean ± SEM of 6 rats per group. An ANOVA 2 × 2 factorial design was used to study the effects of diet (D), green tea (GT), and potential interaction between D × GT. When interaction was statistically significant, superscript letters designate: a statistical difference compared to the control group, b compared to the green tea group, c compared to the obese group
Green tea modulates the metabolism of adipose tissue, attenuating the effects of the cafeteria diet and increasing the breakdown of lipids
In order to evaluate whether the green tea extract modulates gene expression of key proteins involved in the lipid metabolism of adipose tissue, we measured the mRNA levels of the main lipogenic and lipolytic enzymes by RT-PCR. mRNA expression of lipogenic enzymes FAS, SCD1, and DGAT presented an interactive effect between diet and GT (Fig. 4a). Supplementation of the obese rats with green tea (OB + GT group) caused a significant decrease in mRNA expression of DGAT when compared to the obese group.
Green tea modulates the metabolism of adipose tissue, attenuating the effects of the cafeteria diet and increasing the breakdown of lipids. a mRNA expression of lipogenic enzymes: fatty acid synthase (FAS), pyruvate dehydrogenase (PDH), stearoyl CoA desaturase 1 (SCD1), diacylglycerol acyltransferase (DGAT). b mRNA expression of lipolytic enzymes: hormone-sensitive lipase (HSL), adipocyte triglyceride lipase (ATGL), protein perilipin, lipoprotein lipase (LPL). c mRNA expression of adipogenic transcription factor PPAR-γ. d mRNA expression of intracellular lipid chaperones (FABP4 and CD36). e mRNA expression of carnitine palmitoyltransferase 1 beta (CPT1β) assessed by RT-PCR. Data are presented as mean ± SEM of 4 per group. An ANOVA 2 × 2 factorial design was used to study the effects of diet (D), green tea (GT), and potential interaction between D × GT. When interaction was statistically significant, superscript letters designate: a statistical difference compared to the control group, b compared to the green tea group, c compared to the obese group
The effects of supplementation with green tea extract on lipolytic enzymes in adipose tissue were assessed by measuring the mRNA levels of HSL, ATGL, perilipin, and LPL enzymes. mRNA levels of HSL and ATGL (Fig. 4b) in animals that received the cafeteria diet decreased significantly compared to the control group, whereas in obese animals supplemented with GT, mRNA expression levels of enzymes HSL and ATGL were significantly increased (vs obese group) (interactive effect between diet and GT). The treatment with GT increased the mRNA of perilipin in both conditions (standard and cafeteria diet—GT effect). LPL mRNA level was decreased in obese rats (diet effect) and was increased in the groups that were treated with GT extract (GT effect) (Fig. 4d).
To find out whether treatment with the green tea extract could be modulating the gene expression of lipogenic and lipolytic enzymes by modulating adipogenic transcription factor, we measured the mRNA expression of PPAR-γ. There was an interactive effect between diet and GT treatment for PPAR-γ mRNA levels. Both cafeteria diet and treatment with green tea caused a significant decrease in mRNA expression of PPAR-γ as compared to the control group, although OB + GT increased PPAR-γ mRNA level as compared to obese rats (Fig. 4c).
To investigate whether treatment with GT extract alters intracellular lipid chaperones (CD36 and FABP4), which are involved in lipid metabolism of adipocytes, we carried out an evaluation of mRNA levels of these proteins. The CD36 expression increased significantly in the groups treated with GT extract as compared to the control group (GT effect). However, the fatty acid binding protein 4 (FABP4), a transporter protein of fatty acids and other lipophilic substances through the membrane, was significantly decreased due to GT treatment (effect of GT) (Fig. 4d). mRNA levels of CPT-1β, a key protein involved in fatty acid oxidation, was also increased in animals treated with green tea extract (effect of GT) but not in obese rats supplemented with GT (Fig. 4e).
Proposed mechanism of green tea action on adipose tissue
To find out whether green tea would be generating its actions in epididymal adipose tissue via activation of AMPK, we investigate the phosphorylation state of this metabolic kinase in the experimental groups as well as total AMPK content. Overall GT extract increased AMPK phosphorylation regardless of diet (effect of GT), whereas obesity reduced both total and phosphorylated AMPK (effect of diet) (Fig. 5a–c). As we showed that green tea activates AMPK phosphorylation, we investigate whether lipogenic and lipolytic metabolism of adipose tissue was also been changed. Concerning protein expression and phosphorylation state of lipolytic HSL and lipogenic ACC enzymes, it was notorious that GT extract (GT effect) was able to increase phosphorylation state of ACC altering significantly total ACC protein expression (Fig. 5d, e). Similar profile was observed to both p-HSL and total HSL lipolytic enzyme (Fig. 5f, g).
Proposed mechanism of green tea action on adipose tissue. Protein levels measured by western blotting in epididymal adipocytes. a Phosphorylation state of AMPK on Thr172. b Total AMPK content. c Ratio of phosphorylated AMPK/total AMPK content. d ACC phosphorylation on Ser79. e Total ACC. f HSL phosphorylation on Ser853. g Total HSL. h A model for green tea extract action in fat cells. Data are presented as mean ± SEM of 4 per group. An ANOVA 2 × 2 factorial design was used to study the effects of diet (D), green tea (GT), and potential interaction between D × GT. When interaction was statistically significant, superscript letters designate: a statistical difference compared to the control group, b compared to the green tea group, c compared to the obese group
Discussion
The ability of green tea to prevent deleterious effects of obesity and modulate fat synthesis and fat oxidation pathways suggests that green tea and molecules with similar properties might be valuable tools in the search for key regulators of energy balance and health. The mechanisms whereby green tea can influence body weight loss remain an interesting area of investigation. Overall, the results of this study provide evidence that the supplementation with green tea extract restores obesity-related dysfunction in rats fed with a cafeteria diet, by remodeling adipose tissue metabolism and improving health, as showed by the marked reduction in a variety of metabolic parameters including a lower body weight gain, a prevention in adiposity increase by inhibiting adipocyte hypertrophy, an increase in insulin sensitivity, as well as a considerable increase in levels of adiponectin in plasma associated with a modulation in mRNA of lipolytic and lipogenic enzymes in both subcutaneous and epididymal adipose tissue.
Fat accumulation is determined by the balance between fat synthesis (lipogenesis) and fat breakdown (lipolysis or fatty acid oxidation). The result of fat depot is a chronic positive energy balance that leads to the progressive increase in WAT mass. Such energy balance is controlled by a complex biochemical mechanism that modulates hunger and energy expenditure through hormonal changes, induction/repression of gene expression, and inhibition/activation of signaling proteins. The control of fat storage and fat mobilization is partly mediated by hormones, especially insulin, leptin, and catecholamines, but other factors such as nutritional status, diet composition, bioactive compounds, and exercise can also lead to metabolism for lipogenesis [15]. The integrity of these processes in the liver and adipocytes is essential for maintenance of energy homeostasis.
All changes caused by green tea extract in this study as reducing body weight gain, adipocyte size, and fat pads depots (Fig. 1) and increasing fat cell energy expenditure contributed to improve the blood and liver lipid profile. Notably, we can suppose that some of these effects can be consequence of AMPK activation (Fig. 5). Surprisingly, GT treatment increased cholesterol content in the liver (Fig. 3). As GT has reduced all fat depots in rats likely by reducing gut absorption of lipids as previously described [16], a compensatory mechanism in the liver to increase the cholesterol synthesis would be expected since cholesterol is a precursor in the synthesis of essential steroids hormones and bile salts as well as it is an essential component of eukaryotic membranes. Drugs such as metformin, used in patients with type 2 diabetes, reduce hepatic glucose production and thus decrease blood glucose levels by stimulating AMPK activity [17]. In addition to metformin, Zang et al. reported that several polyphenols stimulate AMPKα1 and AMPKα2 activity and ACC phosphorylation in the liver. The effect of polyphenols was 50–200 times more potent than that of AICAR (analog of AMP) or metformin in AMPK activation. Accumulating evidence has demonstrated that AMPK is also involved in the regulation of energy balance at the whole-body level by responding to hormones and nutrient signals, which leads to changes in energy homeostasis. As shown in in vitro and in vivo studies performed in hepatocytes, inhibition of AMPK activity and ACC phosphorylation increases the accumulation of hepatocellular lipids triggered by elevated glucose [11]. The physiological relevance of this new role of AMPK is demonstrated by the fact that impairment of AMPK function is associated with metabolic alterations, insulin resistance, obesity, hormonal disorders, and cardiovascular disease [3, 4].
In this study, we are describing a mechanism by which green tea can stimulate fatty acid oxidation in epididymal adipose tissue as was previously showed in the liver and skeletal muscle, by possibly activating AMPK [11, 12]. These effects may be partly mediated by an increase in mRNA expression of adiponectin in WAT which was confirmed with its increased plasma levels. Accumulating results have unveiled adiponectin as a promising candidate for the treatment of obesity-associated metabolic syndrome. In animal models and human subjects with obesity and type 2 diabetes, adiponectin stimulates fatty acid oxidation and decreases lipid accumulation in muscles, reduces plasma FFA levels, and enhances insulin sensitivity [18–20]. Adiponectin has been shown to increase fatty acid oxidation via AMPK activation with downstream ACC phosphorylation [21]. It is likely that supplementation with green tea extract can increase the expression and secretion of adiponectin by adipocytes and that this adipokine could be responsible for AMPK protein activity and reduction in lipid accumulation in adipocytes and liver. It seems also that green tea extract can improve glucose sensitivity as showed by improved GTT and ITT by increasing mRNA levels of GLUT 4 in obese animals treated with green tea extract (Fig. 2). Unexpectedly, we observed an increase in plasma leptin levels that was accompanied by a reduction in leptin mRNA expression in subcutaneous WAT. We can speculate that there was an increase in leptin mRNA half-life, which in turn provided an increase in translation of leptin, increasing plasma leptin levels.
It is well recognized that sympathetic nervous system is involved in the regulation of lipolysis, and which sympathetic innervation of WAT may play an important role in the regulation of total body fat [22]. Polyphenolic catechins as well as caffeine can inhibit two enzymes that metabolize norepinephrine (NE), in this way activating thermogenesis [23–25]. Green tea extract has potential activity to inhibit catechol-O-methyl transferase (COMT), an enzyme that degrades NE [25]. The reduction in NE breakdown can prolong and enhance adrenergic-induced lipolysis. Thus, in our study COMT inhibition can be suggested as an additional mechanism by which catechins increase energy expenditure and fat oxidation, either separately or synergistically in the case of green tea which contains both catechin and caffeine.
Here, we showed that the treatment with green tea extract down-regulated the expression of the major genes involved in adipogenic pathway as well as the peroxisome proliferator-activated receptor (PPAR)-γ (Fig. 4c). This effect could be mediated in part via up-regulation of the Wnt/β-catenin as a range of evidence showed that β-catenin knockdown animals attenuated the inhibitory effects of green tea polyphenols on intracellular lipid accumulation. Restoration of β-catenin expression restored the anti-adipogenic effects of polyphenols [26]. PPAR-γ is a pleiotropic transcription factor, and its activity can be linked to countless molecular effects such as control of adipogenesis, lipid metabolism, glucose homeostasis, and inhibition of anti-adipogenic genes [27]. In our study, a decrease in PPAR-γ observed in obese rats could be related with the decrease in adipogenesis which in turn favors adipocyte hypertrophy. Similar results were described by Tsuchida et al. [27] who suggest that hypertrophy of the adipocytes is inhibited with the increase in PPAR-γ. Thus, we can suppose that decreased PPAR-γ mRNA expression observed in our obese rats can be involved in adipocyte hypertrophy. Whether in our model of obesity, polyphenols of the green tea are also acting through this signaling pathway remains to be elucidated.
In summary, our results showed that treatment of obese rats with green tea extract affects fat pad depot size, insulin signaling, and modulates glucose, FFA synthesis, fat acid breakdown (lipolysis/fatty acid oxidation), and adiponectin expression in adipocytes. The effects of green tea in adipocytes contribute to systemic metabolic homeostasis, since 12 weeks with green tea extract supplementation increased glucose transporter (GLUT4) gene expression in fat cells controlling insulin sensitivity of the whole body and the predisposition to inflammation in obese rats induced by cafeteria diet. An unexpected increase in mRNA level of FAS was observed in rats treated with green tea. The increase in FAS mRNA level probably occurred as a compensatory mechanism since all fat pad depots were markedly decreased after GT treatment. Based on these data, a model for green tea action in fat cells is proposed in Fig. 5h. However, our results showed that green tea polyphenols can be acting on AMPK activation in epididymal adipose tissue and then promoting the beneficial effects observed in obese animals supplemented with green tea. As we are supposing GT could be acting through AMPK activation, we are considering the inability to find cause–consequence relationships the main limitation of our study. Considering the results, we can suggest that green tea extract restores obesity-induced metabolic changes and improves health since this compound is able to activate AMPK, a key metabolic enzyme. Further studies using AMPK-knockout mice could be a good strategy to confirm our results.
References
Ahmadian M, Duncan RE, Sul HS (2009) The skinny on fat: lipolysis and fatty acid utilization in adipocytes. Trends Endocrinol Metab: TEM 20(9):424–428. doi:10.1016/j.tem.2009.06.002
Postic C, Girard J (2008) Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Investig 118(3):829–838. doi:10.1172/JCI34275
Zhang BB, Zhou G, Li C (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9(5):407–416. doi:10.1016/j.cmet.2009.03.012
Lage R, Dieguez C, Vidal-Puig A, Lopez M (2008) AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med 14(12):539–549. doi:10.1016/j.molmed.2008.09.007
Basu A, Betts NM, Mulugeta A, Tong C, Newman E, Lyons TJ (2013) Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res 33(3):180–187. doi:10.1016/j.nutres.2012.12.010
Sae-tan S, Grove KA, Lambert JD (2011) Weight control and prevention of metabolic syndrome by green tea. Pharmacol Res 64(2):146–154. doi:10.1016/j.phrs.2010.12.013
Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, O’Connor M, Manautou JE, Bruno RS (2011) Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J Nutr Biochem 22(4):393–400. doi:10.1016/j.jnutbio.2010.03.009
Singh BN, Shankar S, Srivastava RK (2011) Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol 82(12):1807–1821. doi:10.1016/j.bcp.2011.07.093
Hong MH, Kim MH, Chang HJ, Kim NH, Shin BA, Ahn BW, Jung YD (2007) (-)-Epigallocatechin-3-gallate inhibits monocyte chemotactic protein-1 expression in endothelial cells via blocking NF-kappaB signaling. Life Sci 80(21):1957–1965. doi:10.1016/j.lfs.2007.02.024
Lin YL, Lin JK (1997) (-)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol Pharmacol 52(3):465–472
Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA (2006) Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55(8):2180–2191. doi:10.2337/db05-1188
Huang HC, Lin JK (2012) Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct 3(2):170–177. doi:10.1039/c1fo10157a
Hansen MJ, Jovanovska V, Morris MJ (2004) Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J Neurochem 88(4):909–916
Anesini C, Ferraro GE, Filip R (2008) Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agric Food Chem 56(19):9225–9229. doi:10.1021/jf8022782
Kersten S (2001) Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep 2(4):282–286. doi:10.1093/embo-reports/kve071
Raederstorff DG, Schlachter MF, Elste V, Weber P (2003) Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem 14(6):326–332
Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB (2006) Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab 8(3):264–280. doi:10.1111/j.1463-1326.2005.00510.x
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7(8):947–953. doi:10.1038/90992
Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, Liu F, Dong LQ (2009) Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem 284(33):22426–22435. doi:10.1074/jbc.M109.028357
Kadowaki T, Yamauchi T (2011) Adiponectin receptor signaling: a new layer to the current model. Cell Metab 13(2):123–124. doi:10.1016/j.cmet.2011.01.012
Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99(25):16309–16313. doi:10.1073/pnas.222657499
Rg IJ, Stehouwer CD, de Geus EJ, van Weissenbruch MM, Delemarre-van de Waal HA, Boomsma DI (2003) Low birth weight is associated with increased sympathetic activity: dependence on genetic factors. Circulation 108(5):566–571. doi:10.1161/01.CIR.0000081778.35370.1B
Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J (2000) Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disorders 24(2):252–258
Shixian Q, VanCrey B, Shi J, Kakuda Y, Jiang Y (2006) Green tea extract thermogenesis-induced weight loss by epigallocatechin gallate inhibition of catechol-O-methyltransferase. J Med Food 9(4):451–458. doi:10.1089/jmf.2006.9.451
Westerterp-Plantenga MS (2010) Green tea catechins, caffeine and body-weight regulation. Physiol Behav 100(1):42–46. doi:10.1016/j.physbeh.2010.02.005
Lee H, Bae S, Yoon Y (2013) The anti-adipogenic effects of (-)epigallocatechin gallate are dependent on the WNT/beta-catenin pathway. J Nutr Biochem 24(7):1232–1240. doi:10.1016/j.jnutbio.2012.09.007
Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T (2005) Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes 54(12):3358–3370
Acknowledgments
The authors are grateful to the technical assistance of R. C. Macedo, B. A. Guerra, A. C. Morandi, R. G. Cruz, Marinovic M. P., Maia, O. F. Mori M, and R. Holanda. This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2011/19216-8), Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq (139307/2012-5), and Universidade Cruzeiro do Sul.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors of the present manuscript declare that there is no actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations that could inappropriately influence, or be perceived to influence our work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rocha, A., Bolin, A.P., Cardoso, C.A.L. et al. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur J Nutr 55, 2231–2244 (2016). https://doi.org/10.1007/s00394-015-1033-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1033-8