Abstract
Purpose
Tremendous differences in iodine status and daily iodine intake persist across provinces of China. The objective of the present study was to evaluate the iodine status and dietary iodine intake of Shandong adults before the implementation of the salt reduction program and a new salt iodization standard.
Methods
Data from a baseline survey of the Shandong and Ministry of Health Action on Salt Reduction and Hypertension project (2011) were analyzed. The iodine contents of 1949 24-h urine samples and 136 drinking water samples were assayed. Daily urinary iodine excretion and daily iodine intake were calculated, analyzed, stratified by different analytical variables and compared with Chinese Dietary Reference Values.
Results
The median urinary iodine concentration and median daily iodine intake of Shandong adults were 248.5 μg/L and 368.2 μg/day, respectively. The median iodine intake of different groups was between the estimated average requirements and the upper limit, except group in water iodine >300 μg/L with median iodine intake of 1200.7 μg/L. Salt intake and iodine levels in drinking water related to iodine intake significantly.
Conclusions
Shandong adults had more than adequate iodine nutrition, and the dietary iodine intake of the population was generally appropriate and safe except residents in high water iodine areas. In the context of the implementation of a salt reduction program and a new salt iodization standard, the iodine status of high water iodine areas may remain in the recommended level, but in low water iodine areas, the risk of inadequate iodine intake may increase, needing monitoring of urine iodine excretion, dietary iodine intake and iodized salt consumption regularly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iodine is an essential micronutrient that is required for thyroid hormones synthesis and release. The recommended daily iodine intake for adults to maintain normal thyroid hormones production is 100–150 μg. The relation between iodine intake and the occurrence of thyroid diseases can be represented by a U-shaped curve, i.e., both deficient and excessive iodine intake will lead to thyroid disorders [1].
Iodine deficiency occurs when iodine intake is below a certain limit: It affects all stages of the life cycle and causes a broad spectrum of disorders termed iodine deficiency disorders (IDDs) [2]. IDDs include goiter, cretinism, isolated deaf mutism, stillbirths, abortions, congenital abnormalities and varying degrees of intellectual disability [3, 4]. Although substantial progress in eliminating iodine deficiency has been achieved worldwide through universal salt iodization (USI), iodine deficiency remains a severe public health problem and is devastating to population health and development, particularly in high-risk populations such as infants and pregnant women [5, 6]. Recent global estimates showed that 246 million school-aged children (SAC) and 1.92 billion individuals had insufficient iodine intake [5]. In 2013, there were still 30 countries classified as iodine-deficient [7].
Recently, attention has also been drawn to iodine excess due to over-iodization of salt and/or poor surveillance of salt iodization [7, 8]. Excessive iodine intake may also have detrimental effects on thyroid function despite the compensatory mechanism. The upper limit (UL) of daily iodine intake for healthy adults is 1100 μg/day, which is considered to be safe and well tolerated [9, 10]. If iodine consumption exceeds the UL, the risks of iodine-induced hyperthyroidism, hypothyroidism, goiter and autoimmune thyroid disease progressively increase [11–13].
Iodine deficiency was previously endemic in China, which had the world’s largest population affected by iodine deficiency [14]. In 1995, the USI program with all edible salt iodized mandatorily was initiated to correct iodine deficiency. This prophylactic strategy has had remarkable success, and IDDs have been sustainably eliminated at the national level [15, 16]. Meanwhile, there are some iodine excess areas attributed to high iodine levels in drinking water in China. When USI first launched, iodized salt was also supplied in these areas because of adjacent to iodine deficiency areas. In the 1970s, water source iodine excess goiter cases were first discovered among fisherman in Hebei Province in China. Since then, high water iodine areas were found in 12 provinces (municipality, autonomous region). The Chinese national investigation about geographical distribution of drinking water with high iodine level, conducted in 2005, demonstrated that seven provinces including Shandong Province had counties with extremely high iodine levels in drinking water [17]. In these high water iodine areas, iodine in drinking water was the key contributor to excessive iodine intake [18].
Shandong Province, the second most populous province in China, was formerly one of the provinces with the most severe iodine deficiency. Seventy million people live in iodine-deficient areas of Shandong. To date, no provincial epidemiological surveys are available to evaluate the iodine status of the adult population in China or in Shandong in particular. By analyzing the baseline survey data of Shandong and Ministry of Health Action on Salt Reduction and Hypertension (SMASH) project, our objectives were to characterize the iodine nutritional status of Shandong adults and to assess dietary iodine intake compared with various nutrient reference values.
Materials and methods
Study population
Shandong Province, located on the eastern coast of China and in the downstream area of the Yellow River, covers a total of 156,700 km2; it has 17 cities, including 140 counties and districts, with a total population of 95.79 million. The province has a varied geography with hills in the east, mountains in the center and south, and the Yellow River alluvial plain in the north and west.
The target population for the present analysis was a subsample of the baseline survey of the SMASH project. This baseline survey was an epidemiological cross-sectional study conducted in Shandong Province between June and July 2011. A provincially representative sample of the adult population (aged 18–69) was selected by multistage cluster sampling. A total of 15,350 subjects from 20 counties and districts (8 urban areas and 12 rural areas) participated in this survey. The subsample comprised 2184 respondents who were selected for a 24-h urine sample collection. Details about the baseline survey design and implementation have been published previously [19]. The Ethics Committee of the Shandong Center for Disease Control and Prevention approved the survey. Written informed consent was obtained from all respondents.
Study subjects
For the present analysis, we only considered the subsample with 2184 respondents from whom a 24-h urine sample had been collected. The variables collected were sex, age, educational level, annual household income level, residence (urban vs. rural; coastal vs. inland) and geographical region.
The study subjects were divided into five age groups: 18–30, 30–39, 40–49, 50–59 and 60–69 years old. Educational levels were classified by the highest level of education completed by subjects. The following five groups were considered: group I (illiterate and functional illiterate), group II (primary school graduates), group III (junior high school graduates), group IV (senior high school graduates) and group V (college and above graduates). Annual household income levels were collapsed into three groups (low, middle and high levels), which were defined as follows: low, households earning less than 10,000 yuan per year; middle, households earning 10,000–20,000 yuan per year; high, households earning more than 20,000 yuan per year. The province was divided into three geographical districts (i.e., Eastern, Central Southern and Northwestern) in accordance with the geography of the province.
Anthropometry
Physical characteristics of subjects, including height and weight, were measured. The body mass index (BMI) was defined as weight/height2 (kg/m2). BMI categories were defined as follows: underweight (BMI < 18.5), healthy weight (18.5 ≤ BMI < 24.0), overweight (24.0 ≤ BMI < 28.0) and obese (BMI ≥ 28.0).
Urine sample collection and iodine concentration determination
The 24-h urine samples were collected for the measurement of urinary iodine concentration (UIC). The study subjects were instructed to void their bladders in the morning, discarding this urine completely, and to note the time of this morning micturition as the start time of urine collection. For the following 24 h, all urine, including the first urine of the next morning, was collected. All urination time points were recorded during the collection. If any urine was lost, the time of this loss was also noted. The time period over which urine was lost was calculated and subtracted from the total 24-h period. Urine volume was measured and recorded. Urine collections were discarded if the urine volume was less than 0.5 L or if urine samples were reported to contain more than two omissions. Five milliliters of each urine sample was extracted and stored at −20 °C until further analysis.
UIC was measured by arsenic cerium catalytic spectrophotometry after ammonium persulfate digestion of the samples. Creatinine was analyzed using the modified Jaffe method. Urinary sodium was measured by the ion-selective electrode method. The completeness of 24-h urine collection was assessed using creatine excretion. Urine samples were regarded as complete when its creatine content was in the mean ± 2SD from the population mean (males 1.91–18.27 mmol/day; females 1.36–14.28 mmol/day).
Calculation of daily iodine intake and comparison with reference values
Daily iodine excretion and daily iodine intake were calculated using the following formulas: daily iodine excretion (μg/day) = UIC (μg/L) × 24-h urine volume (L), and daily iodine intake (μg/day) = UIC (μg/day) × 24-h urine volume (L)/0.92 [20].
The calculated daily iodine intake was compared with three standard indicators, including the estimated average requirement (EAR), the recommended nutrient intake (RNI) and the upper limit (UL). Reference values of iodine intake for adults (18 years and older) were the following: EAR 120 μg/day, RNI 150 μg/day and UL 1000 μg/day [21]. These values, which are used in China, differ from the international nutrient reference values [22]. The international standard EAR for iodine intake (95 μg/day) is lower and UL (1100 μg/day) is higher, than the corresponding reference values used in China.
Estimation of daily salt intake
Daily salt intake was estimated by determining 24-h urinary sodium excretion based on the assumption that all sodium was ingested in the form of sodium chloride. Daily salt intake was calculated by the formula below: daily salt intake (g/day) = daily salt excretion (g/day) = urinary sodium concentration (mmol/L) × 24-h urine volume (L) × 58.5 × 10−3.
Determination of the iodine content of drinking water
The method of water samples collection was as follows. In areas with a centralized water supply, two drinking water samples were collected randomly from two households in the survey village. In areas served instead by wells, five wells were selected from five locations (i.e., east, west, south, north and center) in the survey village, to provide a total of five samples. In total, we collected 136 drinking water samples. Water samples were stored at 4 °C before analysis. The cerium sulfate catalytic spectrophotometric method was used to determine the iodine content in the drinking water.
According to the national standard “Delimitation for the Endemic Areas of Iodine Deficiency Disorders (IDD)” [23] and “Determination and Classification of the Areas of High Water Iodine and the Endemic Areas of Iodine Excess Goiter” [24], the iodine concentrations of the drinking water were divided into four categories for analysis: <10, 10–150, 150–300 and >300 μg/L.
Statistical analysis
The SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used to analyze data and prepare tables and figures. The iodine results were expressed as mean, median and interquartile range (IQR). The nonparametric Mann–Whitney test and Kruskal–Wallis test were used to compare variables. We used Spearman correlation analysis to quantify associations between pairs of variables. The EAR and UL cutoffs were used to characterize the prevalence of inadequate and excessive iodine intake. P < 0.05 was considered to be statistically significant.
Results
There were 2184 respondents selected for a 24-h urine sample collection. However, only 2112 urine samples were collected and determined due to no respondence or site rejection (on the basis of urine volume less than 0.5 L). Ultimately, 163 samples were excluded due to incomplete collections (according to creatinine excretion), leaving 1949 samples (1022 from males and 927 from females) in the final analysis. The characteristics of the respondents are shown in Table 1.
Urinary iodine concentration and daily iodine intake
The median UIC of the adult population in Shandong province was 248.5 μg/L, which is considered to be more than adequate. As shown in Fig. 1, only 8.5 % of the samples had insufficient iodine intake, and no participant had a UIC < 20 μg/L; 91.5 % of samples had UIC > 100 μg/L, and 40.1 % of the study participants had UIC > 300 μg/L.
The median daily iodine intake of the Shandong adults studied was 368.2 μg/day, and 80.6 % of participants had iodine intakes between the RNI and UL. The percentage of individuals with intakes below the EAR, RNI and above the UL was 3.2, 6.7 and 12.6 %, respectively (Table 2).
Daily iodine intake and analytical variables
Daily iodine intake, by sex and age
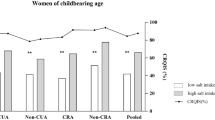
The median daily iodine intake was 402.7 μg/day for males and 336.7 μg/day for females. Iodine intake was significantly different between the sexes (p < 0.001). Women of childbearing age (18–45 years) in Shandong Province had a median iodine intake of 337.8 μg/L, which was not significantly different from the iodine intake of older females (46–69 year).
As shown in Table 2, the age-specific daily iodine intake demonstrated an inverse U-shaped distribution, with the highest iodine intake in the 40- to 49-year-old age group (403.3 μg/day). There were significant differences between the age groups (p < 0.05); however, these differences were significant for women (p < 0.001), but not for men (p = 0.073).
Daily iodine intake by geographical region and area of residence
Shandong Province was divided into three regions: the Eastern, Central Southern and Northwestern geographical regions. The highest median daily iodine intake was found in the Northwestern region, compared with the other two areas. The median iodine intake in rural areas was higher than that in urban areas and was also higher in inland areas than in coastal areas (Table 2). The highest values for daily iodine intake were found in rural inland areas (432.6 μg/day).
Daily iodine intake by educational level
The daily iodine intake decreased significantly with educational levels (p < 0.001). As shown in Table 2, the higher the level of education, the lower the iodine intake. Compared to group I (median daily iodine intake 410.1 μg/day), the median daily iodine intake of the educational group who had completed college or more education was the lowest (312.2 μg/day).
Daily iodine intake and iodine in drinking water supplies
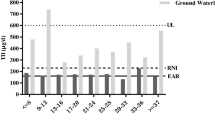
Iodine concentrations in drinking water were also assayed in this study. The means and distributions of the water iodine concentrations are shown in Fig. 2. The iodine concentration in drinking water was less than 150 μg/L in Eastern and Central Southern areas, but it was more than 150 μg/L in most areas of the Northwestern region. In areas where the drinking water iodine content was below 10, 10–150, 150–300 and >300 μg/L, the median daily iodine intake was 342.9, 313.2, 849.7 and 1200.7 μg/day, respectively.
A significant positive correlation was found between daily iodine intake and water iodine concentration (r = 0.351, p < 0.001). The correlation coefficients between iodine excretion and drinking water iodine status stratified by residence and geographical region were shown in Table 3. Specifically, there were significantly positive correlations between daily iodine intake and iodine levels of drinking water in the Northwestern, rural and inland areas (r = 0.513, 0.446, 0.436, respectively), where the iodine concentration in drinking water (>100 μg/L) was higher than the corresponding comparable areas.
Daily iodine intake and salt intake
Considering all participants, the mean daily salt intake evaluated by 24-h urinary sodium excretion was 12.53 g (95 % CI 12.32–12.72 g). The mean salt intake of residents in Northwestern areas (13.31 g) was significantly higher than those in Eastern (12.31 g) and Central Southern areas (12.03 g) (p < 0.001). No significant differences in salt intake were seen between residence groups (urban vs. rural 12.32 vs. 12.63 g; coastal vs. inland 12.36 vs. 12.58 g).
We also found a positive correlation between iodine intake and salt intake (r = 0.350, p < 0.001). As shown in Table 3, the correlation coefficients between iodine excretion and salt intake in different residence and geographical region categories were considerably significant, even though in Northwestern areas (r = 0.211, p < 0.001).
Daily iodine intake and other variables
Iodine intake decreased significantly as annual household income level increased (p < 0.001) and increased significantly with BMI (p < 0.001).
Discussion
The iodine status of the Chinese people has improved remarkably, since the USI policy was introduced for correcting iodine deficiency. Six national surveys targeted at school-aged children (SAC) were conducted in China from 1995 to 2011 [25]. The results of the last three national IDD survey (median UIC of SAC was 241.2, 246.3 and 238.6 μg/L in 2002, 2005 and 2011, respectively) noted that China has more than adequate iodine nutrition. However, variable iodine intake persists in a country as large and geographically diverse as China [26]. Regional iodine status surveys are very important.
Our results indicated more than adequate iodine status in Shandong adults (median UIC 248.5 μg/L), in agreement with the results of a survey conducted on SAC in 2007 (median UIC 240.5 μg/L) [27]. In our survey, we found that 8.5 % of the adults surveyed had a UIC below 100 μg/L, while 40.1 % had a UIC above 300 μg/L. A similar distribution was found in all analytical stratifications (data not shown). These results indicated that most people in Shandong Province had optimal or excessive iodine intake, and, in particular, women of reproductive age exhibited excessive iodine intake. However, the results were slightly different when considering daily iodine intake because daily iodine intake is a more accurate indicator of iodine nutrition status. The median daily iodine intake of Shandong adults was 368.2 μg/day, with 3.2 % of the population below the EAR (120 μg/day) and 12.6 % above the UL (1000 μg/day). Even though the proportion of adults with deficient and excessive iodine intake were declined, respectively, the median daily iodine intake in Shandong was high relative to Chinese and international dietary recommendations.
Excessive iodine status and its major epidemiologic consequences are attracting increasing amounts of attention. In the context of USI, salt is the main iodine source in China. The standard for salt iodization was modified several times since the commencement of this prophylaxis, according to the results of national IDD survey. It is noteworthy that the salt iodization level was reduced from the initial 50 to 35 ppm in 2000, because of 14 provinces with a median UIC over 300 μg/L in 1999. However, the median urinary iodine excretion among Chinese children was still high in 2005 (246.3 μg/L). Based on this finding, China has been described as “at risk of iodine-induced hyperthyroidism in susceptible groups” by the WHO [28]. Whether the salt iodization content should be adjusted was discussed widely [15, 29]. Subsequently, a new standard for salt iodization was issued by the Chinese Ministry of Health in September, 2011, and implemented from March, 2012 [30]. The iodine content in edible salt declined to 20–30 ppm (permitted fluctuation ±30 %). It could be revised in each province according to local specific conditions such as daily salt intake, iodine content of drinking water, climate and living habits. In Shandong Province, the salt iodization standard was adjusted to 18–33 ppm.
Wu et al. [25] demonstrated that salt (iodization level 28.3 ppm) contributed 63.5 % of food iodine. Although the present survey did not collect information about iodized salt consumption, we found a positive correlation between iodine intake and salt intake (r = 0.350, p < 0.001). And even stratified by residence and geographical region, the correlation persisted. Considering the major source of sodium intake was salt added during food preparation and processed food accounted for the minority of sodium intake in Shandong [19], we speculate that a decrease in salt intake may reduce iodine intake. The SMASH campaign was launched in Shandong Province in 2011, with the aim of preventing hypertension by reducing salt consumption. As a consequence, salt consumption may be expected to fall in Shandong in the near future. In addition, the iodized salt produced by new iodization standard captured the Shandong market completely in September, 2013. In this context, regular, reliable, effective and representative monitoring of iodine status is critical.
Iodine is widely distributed in the environment, including rock, soil, water, plants, animals and air. Iodine in drinking water is an important natural source of iodine intake, and the iodine concentration of drinking water is regarded as an indicator of the iodine content of the external environment. A significantly positive correlation between daily iodine intake and water iodine concentration was found in our analysis. And this positive correlation existed mainly in high water iodine areas. In areas where the iodine content of drinking water was above 300 μg/L, the median daily iodine intake was above the UL, up to 1200.7 μg/day. It is generally known that iodine intake above the UL may pose a progressively increasing risk of thyroid disease [22]. Several recommendations, including changing the water supply to provide drinking water with appropriate iodine content, have been proposed and adopted by the Chinese government to correct high water iodine. In Shandong, the water supply source in some counties with high water iodine changed after the present survey. For example, the water iodine concentration at one of our survey sites fell from 798.1 μg/L in 2011 to 20.1 μg/L in 2013, attributed to the South–North Water Diversion. Furthermore, a separate analysis of high iodine areas (water iodine concentration above 150 μg/L) found that although the iodine concentrations of the drinking water in some areas were lower, the median iodine intakes were higher. One possible reason for this finding is that iodized salt plays an important role in these areas, and prohibition of the supply of iodized salt has been proposed for such regions [17, 31, 32]. Thus, monitoring of drinking water iodine and iodize salt consumption is also important.
We also found that urinary iodine excretion and daily iodine intake levels varied across different geographical regions and types of residential areas. In particular, inland areas (especially rural inland areas) had the highest iodine intakes, compared with coastal areas. Even though surveys in other countries demonstrated that UIC values were highest in coastal urban areas as opposed to the inland rural areas because of more consumption of aquatic foods; however, foods of sea origin contributed minimally to iodine intake in coastal areas in China [20, 26]. According to the water iodine content analysis, we believe that variations in the iodine content in drinking water also play an important role in variations of iodine intake. It is also possible that people in the eastern coastal areas where naturally occurring iodine is deficient may voluntarily consume non-iodized salt, because of concerns about the adverse effects of excessive iodine intake on thyroid health.
Recently, the international medical literature and local media have paid more attention to Chinese thyroid health in the context of USI [33–35]. In particular, the incidence of thyroid diseases has appeared to increase in recent years. The question of whether excessive iodine intake contributes to the high incidence of thyroid diseases has been raised and discussed widely. In some regions where the sale of salt without iodine formerly requires a prescription, non-iodized salt counters were added in supermarkets by salt industry aiming to be convenient for some patients with thyroid disease. However, non-iodized salt is also available to healthy people. Our results showed that iodine intake progressively fell with educational level and annual household income level, perhaps because respondents with higher level of education and/or with a higher economic status pay more attention to thyroid health and prefer to consume non-iodized salt. Another explanation is that these groups have more health awareness and prefer to have healthier dietary patterns such as low salt diet. Comprehensive study of this topic is necessary in future efforts.
The main strength of the present study is that the estimation of iodine status takes into account iodine levels in drinking water. The iodine content of drinking water varies considerably in different terrain areas. And water iodine content plays an important role in high water iodine areas. Another advantage is the timing for assessment of iodine status, i.e., before the initiatives of salt reduction campaign and the new salt iodization standard. It will provide background information to investigate whether salt reduction strategies have impact on the population iodine intake by comparison with the data of surveys in the future. The third advantage is the use of 24-h urine samples for the evaluation of iodine intake, which is regarded as the “reference standard” because it could make a precise estimate of the individual iodine excretion [36]. Although the collection of 24-h urine is difficult, it could reduce the population dispersion by 24 %. While spot urine samples tend to overestimate low and high iodine intakes in population studies [37]. Regrettably, using a single 24-h urine collection will result a wider distribution of iodine intake because of the variation in day-to-day 24-h urinary iodine excretion. Moreover, complete 24-h urine collection is important and necessary to evaluate urinary excretion of iodine reliably. Currently, there are two objective methods to assess the completeness of the 24-h urine collection, i.e., the creatinine and p-amino benzoic acid check tests. Compared with the latter, the use of creatinine is more common in epidemiological studies, because of the simplicity and feasibility. Although the constancy of daily creatinine excretion was controversial, several studies indicated that if urine is collected seriously, creatinine excretion is a reliable marker. There are many judgement standards using creatinine excretion, and we used the simplest one in the analysis which may result in false-negative or false-positive results. Further limitation of this study is that we did not refer to information about iodized salt consumption, so how much effect iodized salt has on iodine intake cannot be analyzed, which needs to investigate comprehensively in the near future.
Conclusion
The present paper demonstrated that the iodine nutritional status of the adult population of Shandong Province, China, exceeded the minimum requirements. Currently, the dietary iodine intake of Shandong adults was generally appropriate and safe, except residents in high water iodine areas. In the context of the implementation of a salt reduction program and a new salt iodization standard, the iodine status of high water iodine areas may remain in the recommended level, but in low water iodine areas, the risk of inadequate iodine intake may increase, needing monitoring of urinary iodine excretion, dietary iodine intake and iodized salt consumption regularly. Further studies are necessary to investigate whether reductions in salt intake result in inadequate iodine intake.
References
Wass JAH, Stewart P (2011) Oxford textbook of endocrinology and diabetes. In: Arnott R, Laurberg P, Andersen S (eds) Part I: principles of international endocrine practice, 2nd edn. Oxford University Press, New York, pp 3–8
Hetzel BS (1983) Iodine deficiency disorders (IDD) and their eradication. Lancet 8359:1126–1129
Zimmermann MB, Jooste PL, Pandav CS (2008) Iodine-deficiency disorders. Lancet 372:1251–1262
Zimmermann MB (2009) Iodine deficiency. Endocr Rev 30:376–408
Zimmermann MB, Andersson M (2012) Update on iodine status worldwide. Curr Opin Endocrinol Diabetes Obes 19(5):382–387
Pearce EN, Andersson M, Zimmermann MB (2013) Global iodine nutrition: Where do we stand in 2013? Thyroid 23(5):523–528
Zimmermann MB (2013) Iodine deficiency and excess in children: worldwide status in 2013. Endocr Pract 19(5):839–846
Carvalho AL, de Souza Meirelles CJC, Oliveira LA, Costa TMB, Navarro AM (2012) Excessive iodine intake in schoolchildren. Eur J Nutr 51:557–562
Institute of Medicine Food and Nutrition Board (2006) Dietary Reference Intakes. National Academy Press, Washington
WHO Unicef ICCIDD (2007) Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. WHO Unicef ICCIDD, Geneva
Venturi S, Venturi M (2009) Iodine, thymus, and immunity. Nutrition 25:977–999
Markou K, Georgopoulos N, Kyriazopoulou V et al (2001) Iodine-induced hypothyroidism. Thyroid 11:501–510
Pedersen IB, Knudsen N, Carlé A et al (2011) A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol (Oxf) 75:120–126
Delange F, Bürgi H, Chen ZP et al (2002) World status of monitoring of iodine deficiency disorders control programs. Thyroid 12:915–924
Li S, Zheng Q, Xu J et al (2011) Iodine excess or not: analysis on the necessity of reducing the iodine content in edible salt based on the national monitoring results. Asia Pac J Clin Nutr 20(4):501–506
Chen Z (2006) Sustained elimination of IDD in China: an update. IDD Newsl 24:14–16
Shen H, Liu S, Sun D et al (2011) Geographical distribution of drinking-water with high iodine level and association between high iodine level in drinking-water and goiter: a Chinese national investigation. Br J Nutr 106:243–247
Lv S, Wang Y, Xu D et al (2013) Drinking water contributes to excessive iodine intake among children in Hebei, China. Eur J Clin Nutr 67(9):961–965
Bi Z, Liang X, Xu A et al (2014) Hypertension prevalence, awareness, treatment, and control and sodium intake in Shandong province, China: baseline results form Shandong-Ministry of Health Action on Salt reduction and Hypertension (SMASH), 2011. Prev Chronic Dis 11:130423. doi:10.5888/pcd11.130423
Baxter JR, Riddell L, Huggins CE et al (2011) Iodine status in Melbourne adults in the early 1990s and 2007-08. Aust N Z J Public Health 35(5):408–411
Ge KY (2004) An overview of nutrition sciences, 1st edn. People’s Health Publishing House, pp 138–139 (in Chinese)
Institute of Medicine of the National Academies of Science (2006) Dietary reference intakes: the essential guide to nutrient requirements. The National Academies Press, Washington
Shen H, Su X, Ge X et al (2009) Delimitation for the endemic areas of iodine deficiency disorders (IDD). National Standard of China, GB 16005-2009
Liu D, Zhao J, Zhu H et al (2003) Determination and classification of the areas of high water iodine and the endemic areas of iodine excess goiter. National Standard of China, GB/T 19380-2003
Shen H (2013) The problems and challenges for China after achieving the national elimination IDD standard. Chin J Prev Med 47:5–7 (in Chinese)
Wu Y, Li X, Chang S et al (2012) Variable iodine intake persists in the context of universal salt iodization in China. J Nutr 142(9):1728–1734
Wang LF, Wang JB, Wang XM et al (2009) Investigation of iodine nutrition of school aged children after universal salt iodization in Shandong province. Chin Prev Med 8:713–715 (in Chinese)
Anderson M, Takkouche B, Egli I et al (2005) Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ 83:518–525
Li S, Fan Y, Chen H et al (2010) Is the current iodine content in edible salt appropriate for eliminating iodine deficiency in China. Asia Pac J Clin Nutr 19(2):231–235
Ministry of Health (2011) Announcement on food safety national standard “iodine content in edible salt”. Available at http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohwsjdj/s7891/201109/53064.htm
Guo XW, Liu Y, Zhai LP et al (2005) Distribution and characteristic of the areas with high water iodine in Shandong, China. Thyroid 15(Supplement 1):S-171
Zhao J, Chen Z, Maberly G (1998) Iodine-rich drinking water of natural origin in China. Lancet 352:1519
Teng W, Shan Z, Teng X et al (2006) Effect of iodine intake on thyroid diseases in China. N Engl J Med 354:2783–2793
Teng X, Shan Z, Chen Y et al (2011) More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 164:943–950
Juan S (2010) Nation sees more thyroid problems. China Daily. Available at http://www.chinadaily.com.cn/usa/2010-09/03/content-11253269.htm
Vejbjerg P, Knudsen N, Perrild H et al (2009) Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 19:1281–1286
Andersen S, Pedersen KM, Pedersen IB et al (2001) Variations in urinary iodine excretion and thyroid function. A 1-year study in healthy men. Eur J Endocrinol 144(5):461–465
Acknowledgments
We would like to thank all investigators and experts from national, Shandong provincial and county level CDC and all respondents of the survey. This work was supported by funds from the Technical Development Plan in Shandong (implemented by Shandong CDC, Grant Number: 2012GSF11828).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Chunxiao Xu and Xiaolei Guo who have contributed equally to this work should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Xu, C., Guo, X., Tang, J. et al. Iodine nutritional status in the adult population of Shandong Province (China) prior to salt reduction program. Eur J Nutr 55, 1933–1941 (2016). https://doi.org/10.1007/s00394-015-1009-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1009-8