Abstract
Purpose
Maternal diet during pregnancy impacts foetal growth and development. In particular, dietary levels of methylating micronutrients (methionine, folate, choline, vitamins B6, and B12) interfere with the availability and allocation of methyl groups for methylation reactions, thereby influencing normal transcription. However, the currently recommended methylating micronutrient supplementation regimen is haphazard and arbitrary at best.
Methods
To investigate the effects of a methylating micronutrient-rich maternal diet, pregnant Pietrain sows were fed either a standard diet (CON) or a diet supplemented with methionine, folate, choline, B6, B12, and zinc (MET). Foetal liver and muscle (M. longissimus dorsi) tissues were collected at 35, 63, and 91 days post-conception. Transcriptional responses to diet were assessed in foetal liver. Altered insulin-like growth factor (IGF) signalling in transcriptome analyses prompted investigation of IGF-2 and insulin-like growth factor binding proteins (IGFBPs) levels in muscle and liver.
Results
Maternal diet enriched with methylating micronutrients was associated with increased foetal weight in late gestation. Hepatic transcriptional patterns also revealed differences in vitamin B6 and folate metabolism between the two diets, suggesting that supplementation was effective. Additionally, shifts in growth-supporting metabolic routes of the lipid and energy metabolism, including IGF signalling, and of cell cycle-related pathways were found to occur in liver tissue in supplemented individuals. Weight differences and modulated IGF pathways were also reflected in the muscle content of IGF-2 (increased in MET) and IGFBP-2 (decreased in MET).
Conclusions
Maternal dietary challenges provoke stage-dependent and tissue-specific transcriptomic modulations in the liver pointing to molecular routes contributing to the organismal adaptation. Subtle effects on late foetal growth are associated with changes in the IGF signalling mainly in skeletal muscle tissue that is less resilient to dietary stimuli than liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutrient availability during pregnancy affects foetal development in a number of ways. For example, dietary exposures at various foetal stages are thought to impact long-term metabolism and health [1–4] in accordance with the concept of genetic plasticity [5]. However, our understanding of the mechanisms driving foetal programming is limited, despite on-going debate [6, 7]. Nutritional programming has been linked to epigenetic modifications reflecting maternal macro- and micronutrient supply [8, 9], but the detailed knowledge of such adaptive mechanisms is rather fragmentary [10–12].
Methyl donor nutrients often interfere with the availability and allocation of methyl groups for methylation reactions (Fig. 1). The one-carbon metabolism involves the passing of methyl groups from choline, folate, and methionine to S-adenosyl methionine (SAM), acting as a key methyl donor to methylate DNA, RNA, and proteins; B vitamins serve as coenzymes in this process. Additionally, the trace element zinc is required. As a result, the expression of many genes is affected by dietary levels of methylating micronutrients [13, 14]. The metabolism of methylating micronutrients is common among numerous species, including rats [15] and humans [16], suggesting that dietary folate, methionine, and choline are fungible sources of methyl groups [17]. A deficiency of methyl group donors during pregnancy can lead to severe complications [18], which has generated the now-commonplace practice of supplementation among pregnant women living in the developed world. Importantly, the consequences of chronic supplementation with methylating micronutrients are not fully clear. To date, several studies have shown that an excessive methylating micronutrient intake during pregnancy can have adverse effects on embryonic development, glucose homoeostasis, and health [19–22].
According to the commonly accepted phylogeny of mammals [23], mice (Muridae, Rodentia) are more closely related to humans (Hominidae, Primates) than pigs (Suidae, Cetartiodactyla). However, pigs have become a prominent animal model to study dietary effects [24–29], because porcine metabolism more closely resembles human requirements for methylating micronutrients during pregnancy compared to the murine one [21] (Table 1). Pig models have been used to investigate the impact of paternal diets enriched with methylating micronutrients down the male line [37, 38], highlighting genes related to lipid metabolism as diet-dependent.
We conducted a longitudinal study in pigs examining foetal development in response to maternal diets varying in methylating micronutrients. The dietary intake of folate, choline, methionine, vitamin B6, vitamin B12, and zinc was altered to mimic de facto mandatory fortification and supplementation in an experimental setting. According to histological studies [39, 40], prenatal development in porcine liver is characterised by three periods of stage-specific organismal demands: (1) a period of cell differentiation [18–40 days post-conception (dpc)], (2) a period of metabolic activity and haematopoiesis (40–80 dpc), and (3) a period of increased growth and glycogen accumulation (80–113 dpc). These stages show distinctive expression profiles that link to postnatal development [41]. The aim of this study was to evaluate whether porcine foetuses are affected due to an overloaded one-carbon cycle following excess dietary supplementation with methylating micronutrients. In particular, hepatic expression patterns were monitored for long-term consequences. Moreover, growth-promoting effects were deduced from the diet and stage-specific levels of insulin-like growth factor-2 and its binding proteins that were determined in liver and skeletal muscle tissue.
Materials and methods
Animals, diets, and sample collection
This study was approved by the Scientific Committee of the Leibniz Institute for Farm Animal Biology (FBN), and the experimental setup was approved by the ethics committee of the federal state of Mecklenburg-Western Pomerania, Germany. Pietrain gilts (N = 18; 3 sows × 2 diets × 3 stages) were randomly assigned to either a standard diet (CON) or a standard diet supplemented with one-carbon cycle substrates and associated cofactors (MET). Specifically, the diets differed in levels of methionine, choline, folic acid, vitamin B6, vitamin B12, and zinc ( Table 2). The doses of the altered micronutrients match about 80 % of their estimated toxicity. Gilts were artificially inseminated with semen from pure-bred sires. Experimental diets began 10 days before insemination and lasted until slaughtering at the time that foetuses were sampled (Fig. 2). At three prenatal time points [35 days post-conception (dpc), 63 dpc, and 91 dpc], gilts were exsanguinated and uteri were quickly removed, weighed, and dissected. Foetal liver and muscle samples were immediately collected, frozen in liquid nitrogen, and stored at −80 °C until further analyses. At stage 35 dpc, precursor tissue from the back muscle was dissected from the area along the spine.
Experimental design. In three individual experiments, Pietrain gilts were fed either a control gestation diet (CON) or a gestation diet supplemented with substrates and co-factors of the one-carbon-cycle (MET). Foetal liver tissue (N = 138) was collected at three sampling points (35, 63, and 91 dpc). CON control diet, MET methyl-supplemented diet, IN insemination, dpc days post-conception
RNA isolation, target preparation, and hybridisation
Foetal liver tissue was crushed with a mortar and pestle in liquid nitrogen. Total RNA was isolated from individual liver samples (N = 138) using Tri-Reagent (Sigma-Aldrich, Taufkirchen, Germany) as per the manufacturer’s instructions. DNase treatment and subsequent purification steps were performed using a column-based system (RNeasy Mini Kit, Qiagen, Hilden, Germany). RNA integrity and quantity were checked by agarose gel electrophoresis and spectrophotometer (ND1000, PEQLAB, Erlangen, Germany). Absence of genomic DNA was verified by a PCR amplification of the porcine GAPDH gene (forward primer: 5′-AAGCAGGGATGATGTTCTGG-3′; reverse primer: 5′-ATGCCTCCTGTACCACCAAC-3′). All RNA samples were stored at −80 °C until downstream analysis was performed.
The number of foetuses per sow did not differ significantly due to maternal diet (Table 3). However, in order to average positional effects regarding growth performance within the uterus, foetuses were analysed in a pooling design. The foetuses originating from each litter were allocated to constitute two foetal pools per litter. In total, 36 pools (2 pools × 3 litters × 2 diets × 3 stages) were assigned, resulting in six pools per dietary group and ontogenetic stage. For the microarray experiments, biotin-labelled cRNA samples were hybridised on Affymetrix GeneChip Porcine Genome Arrays (Affymetrix, Santa Clara, CA, USA).
Data analyses
In total, 32 arrays passed the appropriate quality control criteria as proposed by Kauffmann et al. [42] (35 dpc CON: 5 pools; 35 dpc MET: 5 pools; 63 dpc CON: 6 pools; 63 dpc MET: 6 pools; 91 dpc CON: 5 pools; 91 dpc MET: 5 pools). The data were GC-RMA normalised (Log2) and filtered by three criteria: the present rate (>50 % per dietary group and stage), the standard deviation (SD > 0.4), and the mean (m > 2.5). Relative changes in mRNA abundance were estimated via multi-factorial variance analyses (SAS Institute, Cary, NC, USA) considering dietary group, ontogenetic stage, and gilt [V ijk = µ + dieti + stagej + (diet × stage)ij + giltk(dieti, × stagej) + eijk]. The interaction between dietary treatment and ontogenetic stage refers to the longitudinal experimental design as presented in studies dealing with nutritional insults in a longitudinal experiment [26, 27]. Due to multiple testing, p values were converted to a set of q values [43]. p values ≤0.05 and q values ≤0.25 were considered statistically significant. Raw data were deposited in a MIAME-compliant database [44], the National Center for Biotechnology Information Gene Expression Omnibus (accession number: GSE59299).
Pathway analysis based on IPA
The probe-sets were annotated according to EnsEMBL Sus scrofa Build 9 [45]. Gene lists obtained from the microarray analyses were evaluated with IPA (Ingenuity Pathway Analysis, Ingenuity Systems, Redwood City, CA, USA). Statistical significance was set at p ≤ 0.05.
Quantitative real-time PCR
Total transcript levels of selected targets (IGFBP1, IGFBP2, IGFBP3, IGF2, IGF2R, DNMT1, DNMT3A, DNMT3B, BHMT, MAT2B) and reference genes (RPL32) were quantified by real-time qPCR (Table S1) as previously described [27]. In brief, individual liver mRNA samples (N = 12 per dietary group per stage) were analysed in duplicate on a LightCycler 480 system using LightCycler 480 SYBR Green I Master (Roche, Mannheim, Germany). Data were factorial normalised and correlated to corresponding microarray data (Pearson’s product-moment correlation).
Western ligand blot (WLB) analyses of IGFBP
IGFBPs were measured in extracts of liver and skeletal muscle tissue from porcine foetuses by Western ligand blot (WLB) analysis as described previously [46, 47]. Liver and skeletal muscle tissue from five individual foetuses from each dietary group, ontogenetic stage, and sex were analysed (N = 120). In brief, 50 mg tissue was homogenised in RIPA buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 0.1 % SDS (w/v), 1 % Triton X-100 (w/v)] using Precellys 24 (PEQLAB Biotechnologie GmbH, Erlangen, Germany) and incubated on ice for 30 min. After centrifugation at 14,000g for 10 min, the supernatants were isolated and analysed. Protein concentrations in the extracts were quantified by BCA assay. Extracts were diluted in sample buffer [312.5 mM Tris (pH 6.8), 50 % (w/v) glycerol, 5 mM EDTA (pH 8), 1 % (w/v) SDS, and 0.02 % bromphenol blue] and heated for five minutes. Then, 20 µg of total protein per lane was applied to SDS-PAGE [48]. Gels were blotted on polyvinylidene fluoride membranes (Milipore Corp., Bedford, MA). Blots were blocked and incubated with biotinylated human IGF-II (1:500; BioIGF2-10; Ibt Systems GmbH, Binzwangen, Germany). Membranes were washed and incubated with horseradish peroxidase-conjugated streptavidin (1:2500; Ibt Systems GmbH, Binzwangen, Germany) and washed again. All washing and incubation steps were performed at room temperature. The binding proteins were detected by enhanced chemiluminescence using Luminata Forte (Milipore Corp., Bedford, MA) on KODAK IMAGE Station 4000MM (Molecular Imaging Systems; Carestream Health Inc., New Haven, CT) and semi-quantified using GelAnalyzer 2010a. Signal intensities were corrected for background and normalised according to Ponceau staining (liver samples) and stain-free technique (muscle samples). Diet- and stage-mediated effects were analysed by ANOVA considering dietary group, ontogenetic stage, gilt, and sex. The level of significance was set at p ≤ 0.05.
Muscular IGF1 and IGF2 levels
Foetal IGF1 and IGF2 levels were analysed in extracts of skeletal muscle tissue with a commercial enzyme-linked immunosorbent assay (ELISA) purchased from Mediagnost, Reutlingen, Hamburg, Germany. Five individual foetuses per dietary group, ontogenetic stage, and sex (N = 60) were analysed. Diet- and stage-mediated effects were analysed by ANOVA considering dietary group, ontogenetic stage, gilt, and sex. The level of significance was set at p ≤ 0.05.
Results
The current study investigated foetal weight and global transcript abundances as well as appearance of IGF-related components of porcine foetuses in response to either a standard or an excessive amount of methylating micronutrients fed to their mothers during gestation.
Increased foetal weight at 35 and 91 dpc
Individual foetal weight was recorded at three prenatal stages (Table 3). Foetuses obtained from gilts fed the MET diet showed increased foetal weight (p < 0.0001). Stage-specifically, foetal weight was increased at 35 dpc in MET samples but unaltered at 63 dpc. At stage 91 dpc, both male and female MET foetuses were heavier than their age-matched controls. Here, the difference in foetal weight was approximately 28 %.
Microarray experiment
Initial microarray analyses of porcine liver tissue identified 14,853 expressed probe-sets (~61 % present calls). Further analyses left 9363 probe-sets after filtering, representing 6697 genes.
Transcriptional responses between MET and CON foetuses within stages
At 35 dpc, 204 transcripts (96 probe-sets MET > CON) were found to be altered between the dietary groups (Table S2). Ingenuity Pathway Analyses (IPA) indicated enrichment of molecular routes related to fatty acid β-oxidation and oleate biosynthesis (Table 4). When CON and MET foetuses were compared at stage 63 dpc, only 13 transcripts (11 probe-sets MET > CON) showed altered mRNA levels. No associated pathways were assigned. At stage 91 dpc, 514 transcripts (185 probe-sets MET > CON) differed significantly between the dietary groups. According to IPA, a set of metabolic pathways was found to differ due to the maternal dietary treatment; in particular, these included transcripts associated with GADD45 signalling, folate polyglutamylation, IGF signalling, the pyridoxal 5-phosphate salvage pathway, and Wnt/β-catenin signalling. Notably, no transcript was found to be altered at all of the examined foetal stages.
Longitudinal transcriptional responses in MET and CON foetuses
Expression patterns at different stages of gestation and dietary backgrounds were visualised by a hierarchical clustering (Fig. 3). The three clusters corresponded to the distinct foetal stages, indicating that effects mediated by stage dominated effects mediated by diet. To highlight transcriptional changes between the developmental stages that were diet-specific and occurred only in one of the diet groups, comparisons were first made within diet groups between 35–63, 63–91, and 35–91 dpc. The resulting gene lists were compared between MET and CON foetuses at the corresponding developmental periods to identify commonly altered transcripts normally associated with physiological maturation processes. Consequently, the analyses focused on transcripts representing longitudinal transcriptional patterns specific to either MET or CON foetuses (Table S3 and Table S4).
Hierarchical cluster analyses of CON and MET samples at different prenatal stages. Least-squares means derived from mixed-model analyses are displayed as a heat map, including all subgroups from the interaction between diet and stage. Columns indicate subgroups and rows indicate probe-sets. Colouring reflects mRNA abundance (see key)
When diet-specific alterations in CON foetuses were compared between 35 and 63 dpc, 487 probe-sets were found to be different (258 probe-sets 35 dpc < 63 dpc). Of those, transcripts associated with fatty acid β-oxidation and oleate biosynthesis were pronounced. Hence, along ontogenesis, the obtained differences referring to lipid metabolism appeared in CON samples but not in MET samples. At 63–91 dpc, foetuses showed differences in mRNA expression represented by 966 probe-sets (732 probe-sets 63 dpc < 91 dpc). In particular, molecular routes related to AMPK signalling, IGF signalling, and Wnt/β-catenin signalling were enriched. Between 35 and 91 dpc, 374 probe-sets showed an altered mRNA abundance (237 probe-sets 35 dpc < 91 dpc). Transcripts participating in glutamate removal from folates and oleate biosynthesis exhibited diet-specific expression.
The analyses of MET foetuses at 35 dpc compared to 63 dpc revealed 1018 probe-sets as diet-specific (750 probe-sets 35 dpc < 63 dpc). Of those, transcripts associated with AMPK signalling, cell cycle control of chromosomal replication, cyclins and cell cycle regulation, mitotic roles of Polo-like kinase, and Wnt/β-catenin signalling were altered. Hence, along ontogenesis, the obtained differences in energy metabolism and growth control appeared in MET samples but not in CON samples. Diet-related effects during the developmental interval 63–91 dpc revealed 897 probe-sets (331 probe-sets 63 dpc < 91 dpc) and highlighted transcripts associated with tRNA charging. Between 35 and 91 dpc, 519 probe-sets were altered in MET foetuses (282 probe-sets 35 dpc < 91 dpc). Of those, transcripts related to folate polyglutamylation were highlighted.
qPCR experiment
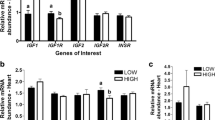
To assess the reproducibility of the microarray experiment, selected transcripts (IGFBP1, IGFBP2, IGFBP3, IGF2, IGF2R, DNMT1, DNMT3a, DNMT3b, BHMT, MAT2B) were analysed by qPCR at multiple sampling points in liver tissue (Table S1). The degrees of correlation indicate reliable results.
IGFBP and IGF appearance in liver and skeletal muscle tissue
Since foetal weight differed and IGF signalling, a major driver of growth was altered in liver at later stages of foetal development, we also profiled the expression of IGF-binding proteins on the protein level. Liver, a key metabolic organ, and muscle, a major growth-related tissue, were selected for investigation. In foetal liver tissue, intact IGFBP-1 (30 kDa), IGFBP-2 (35 kDa), IGFBP-3 (44 kDa), and IGFBP-6 (40 kDa) were detectable (Table S5). IGFBP-2 was the dominant IGFBP and was present in both intact and fragmented forms. IGFBP-6 was below the detection limit at stages 35 and 63 dpc but appeared at stage 91 dpc. In liver tissue, no overall diet effects were observed for IGFBP1, IGFBP2, IGFBP3, and IGFBP6 (p = 0.1043, p = 0.0685, p = 0.4538, and p = 0.1549, respectively). Further, no interaction between diet and stage on IGFBPs was observed.
In skeletal muscle tissue, intact IGFBP-2 (31 kDa) and IGFBP-5 (27 kDa) were detectable (Table 5). Again, IGFBP-2 appeared to be the dominant IGFBP. No overall diet effects were observed for IGFBP2 and IGFBP5 (p = 0.3275, p = 0.1049, respectively) nor for IGF1 and IGF2 (p = 0.8790, p = 0.0514, respectively). However, the interaction between diet and stage revealed decreased IGFBP-2 level and increased IGF2 level in MET muscle tissue at 91 dpc compared to age-matching CON samples.
Discussion
Foetuses exposed to maternal MET diet were heavier than age-matched controls at stage 91 dpc, but not at stages 35 and 63 dpc, suggesting accelerated growth during mid and late gestation when compared to regular foetal development [49]. Obviously, dietary effects cumulated at stage 91 dpc. There are numerous studies investigating complex phenotypes programmed by various nutritional challenges, reporting correlations between maternal diet and foetal weight in pigs [50], sheep [51], and rats [2, 52]. In particular, maternal intake of methylating micronutrients was identified to act on both foetal and neonatal weight, although inconsistencies appeared due to either single or combined effects of such nutrients [10, 53–55]. Interestingly, previous meta-analysis suggested that a twofold increase in the amount of dietary methylating micronutrients during pregnancy (i.e. folate) was followed by a 2 % increase in birth weight in humans [56]. Our results are in line with these findings, as the maternal folate intake was elevated by a factor of 31 (Table 2), and foetal weight was increased by 28 % at stage 91 dpc (Table 3).
The link between IGF signalling and growth is well known. In our study, the increased levels of IGF2 and the decreased levels of IGFBP-2 (Table 5) in muscle tissue likely contribute to the weight differences obtained in MET foetuses at 91 dpc. However, in liver tissue, the decreased transcript abundances of IGFBP1 and IGFBP2 were not observed on the protein level in MET foetuses. Sophisticated patterns of components related to the IGF system were reported previously when mice exhibiting high growth rates showed decreased muscular IGFBP3 and IGFBP6 transcript abundances [57]. Conversely, porcine intra-uterine growth retarded (IUGR) foetuses and newborns showed increased expression of IGFBP2, IGFBP3, and IGFBP5 in liver [58] and muscle tissue [59], respectively. Moreover, overexpression of both hepatic IGFBP-1 and muscular IGFBP-2 in adult transgenic mice resulted in reduced body weight [60, 61]. Hence, the expression of distinct IGFBPs is thought to contribute to growth rate modulations by altering the bioavailability of insulin-like growth factors [61, 62], which are known regulators of foetal development and differentiation. The protein expression of IGFBPs revealed stage-specific patterns in accordance with previous experiments investigating foetal muscular tissue [63], maternal serum, and milk [64, 65]. Notably, in our study, IGFBP-2 appeared to be the dominant IGFBP in both foetal liver and skeletal muscle tissue, in contrast to porcine pancreas, where IGFBP3 levels were pronounced [66].
In liver tissue, our analysis of transcripts encoding enzymes involved in the one-carbon cycle revealed a sophisticated pattern including both diet-responsive (Table 4; MTHFD1L, SHMT2) and diet-unresponsive genes (e.g. AHCYL1, BHMT, MAT2B; data not shown). As stated recently, methyl donor supply and placental transfer to the foetus comprise complex mechanisms involving compensatory mechanisms and feedback loops if deficiency occurs [67]. It is conceivable that the decreased mRNA levels of MTHFD1L and SHMT2 (encoding enzymes utilising tetrahydrofolate and 10-formyltetrahydrofolate) account for reduced folate derivatives in the foetal circulation due to maternal buffer capacity, or for substrate-dependent inhibition due to unmetabolised folate equivalents in the foetus itself [68]. These findings indicate that the maternal diet was effective. The transcriptional pattern reflects that liver tissue is much more resilient during early and mid-gestation in order to contribute adapting to the metabolic requirements.
At early and mid-gestation, hepatic expression patterns were widely similar and revealed moderate dietary effects. Regarding liver tissue, this observation may reflect the minor challenging potential of an excessive maternal supply of methylating micronutrients in a short term. However, the resilient character of foetal liver tissue excluded parts of the lipid metabolism, indicating an increased demand for acyl components for both synthesis and β-oxidation (Table 4). Indeed, alterations in lipid metabolism have been highlighted previously as a result of parental methyl-deficient [69, 70] and methyl-supplemented diets [37]. Furthermore, alterations of the energy sensing and energy utilising AMPK signalling were specifically pronounced in MET foetuses between 35 and 63 dpc (Table S4). Such processes may serve to meet the metabolic requirements associated with cell proliferation and cell differentiation. Notably, one of the few transcripts highlighted at early and mid-gestation (MET < CON), follistatin (FST), is known to impact cell proliferation and cell differentiation during embryogenesis via Wnt/β-catenin signalling and myostatin signalling [71].
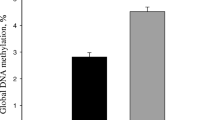
Despite results describing the methylating potential of methylating micronutrients on DNA and histones [11, 72, 73], insight regarding their impact on gene expression remains elusive. In fact, concerns about chronic supplementation of methylating micronutrients during pregnancy have been raised since contribution to metabolic disorders was discussed [74, 75]. In our study, liver tissue appeared to be neither positively nor negatively affected in gene expression at foetal stages. However, foetal overgrowth during the second half of pregnancy has been associated with postnatal obesity [76], suggesting hepatic alterations of metabolic relevant genes in the long term.
According to hierarchical cluster analyses (Fig. 3), stage-specific effects on transcription were more pronounced than those mediated by diet. These results are in line with previous findings in which porcine foetuses and offspring were examined at various ontogenetic stages following exposure to maternal diets varying in protein content [7].
Conclusions
In summary, a maternal diet enriched with methylating micronutrients was associated with an increased foetal weight in late gestation. Hepatic expression patterns reflected moderate effects from supplementation. The transcriptomic response maps pathways undergoing stage-specific modulation in order to warrant metabolic adaptation to nutritional factors. Moreover, our data insinuate that alterations of late foetal growth may be a result of adjustments of IGF signalling (IGF2; IGFBP-2) in the liver but mainly in the skeletal muscle that is less resilient to dietary effects than the liver.
References
Snoeck A, Remacle C, Reusens B, Hoet J (1990) Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate 57:107–118
Bertin E, Gangnerau M, Bellon G, Bailb D, Arbelot De Vacqueur A, Portha B (2002) Development of beta-cell mass in fetuses of rats deprived of protein and/or energy in last trimester of pregnancy. Am J Physiol Regul Integr Comp Physiol 283:R623–R630
Du M, Zhu M, Means W, Hess B, Ford S (2005) Nutrient restriction differentially modulates the mammalian target of rapamycin signalling and the ubiquitin-proteasome system in skeletal muscle of cows and their fetuses. J Anim Sci 83:117–123
Hyatt M, Gardner D, Sebert S, Wilson V, Davidson N, Nigmatullina Y, Chan L, Budge H, Symonds M (2011) Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction 141:119–126
McMillen I, Robinson J (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85:571–633
McMullen S, Langley-Evans S, Gambling L, Lang C, Swali A, McArdle H (2012) A common cause for a common phenotype: the gatekeeper hypothesis in fetal programming. Med Hypotheses 78:88–94
Oster M, Muráni E, Metges C, Ponsuksili S, Wimmers K (2014) High and low protein gestation diets do not provoke common transcriptional responses representing universal target-pathways in muscle and liver of porcine progeny. Acta Physiol (Oxf) 210:202–214
Burdge G, Lillycrop K, Phillips E, Slater-Jefferies J, Jackson A, Hanson M (2009) Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr 139:1054–1060
Sebert S, Sharkey D, Budge H, Symonds M (2011) The early programming of metabolic health: is epigenetic setting the missing link? Am J Clin Nutr 94:1953S–1958S
Maloney C, Hay S, Rees W (2007) Folate deficiency during pregnancy impacts on methyl metabolism without affecting global DNA methylation in the rat fetus. Br J Nutr 97:1090–1098
Lillycrop K, Phillips E, Jackson A, Hanson M, Burdge G (2005) Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135:1382–1386
Sie K, Li J, Ly A, Sohn KJ, Croxford R, Kim YI (2013) Effect of maternal and postweaning folic acid supplementation on global and gene-specific dna methylation in the liver of the rat offspring. Mol Nutr Food Res 57:677685
Waterland R, Dolinoy D, Lin J, Smith C, Shi X et al (2006) Maternal methyl supplements increase offspring dna methylation at axin fused. Genesis 44:401–406
Wolff G, Kodell R, Moore S, Cooney C (1998) Maternal epigenetics and methyl supplements affect agouti gene expression in avy/a mice. FASEB J 12:949–957
Varela-Moreiras G, Selhub J, da Costa K, Zeisel S (1992) Effect of chronic choline deficiency in rats on liver folate content and distribution. J Nutr Biochem 3:519–522
Jacob R, Jenden D, Allman-Farinelli M, Swendseid M (1999) Folate nutriture alters choline status of women and men fed low choline diets. J Nutr 129:712–717
Niculescu M, Zeisel S (2002) Diet, methyl donors and dna methylation: interactions between dietary folate, methionine and choline. J Nutr 132:2333S–2335S
Shaw G, Lammer E, Wasserman C, O’Malley C, Tolarova M (1995) Risks of orofacial clefts in children born to women using multivitamins containing folic acid periconceptionally. Lancet 346(8972):393–396
Hoile S, Lillycrop K, Grenfell L, Hanson M, Burdge G (2012) Increasing the folic acid content of maternal or post-weaning diets induces differential changes in phosphoenolpyruvate carboxykinase mRNA expression and promoter methylation in rats. Br J Nutr 108:852–857
Schaible T, Harris R, Dowd S, Smith C, Kellermayer R (2011) Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum Mol Genet 20:1687–1696
Mikael L, Deng L, Paul L, Selhub J, Rozen R (2013) Moderately high intake of folic acid has a negative impact on mouse embryonic development. Birth Defects Res A Clin Mol Teratol 97:47–52
Pickell L, Brown K, Li D, Wang X, Deng L, Wu Q, Selhub J, Luo L, Jerome-Majewska L, Rozen R (2011) High intake of folic acid disrupts embryonic development in mice. Birth Defects Res A Clin Mol Teratol 91:8–19
Bininda-Emonds O, Cardillo M, Jones K, MacPhee R, Beck R, Grenyer R, Price S, Vos R, Gittleman J, Purvis A (2007) The delayed rise of present-day mammals. Nature 446:507–512
Lunney J (2007) Advances in swine biomedical model genomics. Int J Biol Sci 3:179–184
Guilloteau P, Zabielski R, Hammon H, Metges C (2010) Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr Res Rev 23:4–22
Oster M, Muráni E, Metges C, Ponsuksili S, Wimmers K (2011) A high protein diet during pregnancy affects hepatic gene expression of energy sensing pathways along ontogenesis in a porcine model. PLoS One 6:e21691
Oster M, Muráni E, Metges C, Ponsuksili S, Wimmers K (2012) A low protein diet during pregnancy provokes a lasting shift of hepatic expression of genes related to cell cycle throughout ontogenesis in a porcine model. BMC Genomics 13:93
Valle M, Guay F, Beaudry D, Matte J, Blouin R et al (2002) Effects of breed, parity, and folic acid supplement on the expression of folate metabolism genes in endometrial and embryonic tissues from sows in early pregnancy. Biol Reprod 67:1259–1267
Liu J, Chen D, Yu B, Mao X (2011) Effect of maternal folic acid supplementation on hepatic one-carbon unit associated gene expressions in newborn piglets. Mol Biol Rep 38:3849–3856
Rucker RB (2007) Allometric scaling, metabolic body size and interspecies comparison of basal nutritional requirements. J Anim Physiol Anim Nutr 91:148–156
Zeyner A, Harris P (2013) Vitamins. In: Geor R, Harris P, Coenen M (eds) Equine applied and clinical nutrition. Saunders Elsevier, Philadelphia, pp 168–189
AfBN (2014) Empfehlungen zur Energie- und Nährstoffversorgung von Pferden. DLG-Verlag, Frankfurt (Main)
AfBN (2005) Communications of the Committee for Requirement Standards of the Society of Nutrition Physiology: standardised precaecal digestibility of amino acids in feedstuffs for pigs—methods and concepts. Proc Soc Nutr Physiol 14:185–205
Deutsche Gesellschaft für Ernährung e.V. (DGE) (2013) Referenzwerte für die Nährstoffzufuhr. Umschau Braus GmbH Verlagsgesellschaft, Frankfurt (Main)
Reeves P, Nielsen F, Fahey G (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
AfBN (2006) Energie- und Nährstoffbedarf landwirtschaftlicher Nutztiere. 10: Empfehlungen zur Energie- und Nährstoffversorgung von Schweinen. Ausschuss für Bedarfsnormen der Gesellschaft für Ernährungsphysiologie. DLG-Verlag, Frankfurt (Main)
Braunschweig M, Jagannathan V, Gutzwiller A, Bee G (2012) Investigations on transgenerational epigenetic response down the male line in f2 pigs. PLoS One 7:e30583
Bruggmann R, Jagannathan V, Braunschweig M (2013) In search of epigenetic marks in testes and sperm cells of differentially fed boars. PLoS One 8:e78691
Bielanska-Osuchowska Z, Krzynwek-Wojciechowska J (1990) Morphometric investigations of the pig developing liver during the prenatal period. Pol Arch Weter 30:7–16
Bielanska-Osuchowska Z (1996) Ultrastructural and stereological studies of hepatocytes in prenatal development of swine. Folia Morphol (Warsz) 55:1–19
Ponsuksili S, Muráni E, Walz C, Schwerin M, Wimmers K (2007) Pre- and postnatal hepatic gene expression profiles of two pig breeds differing in body composition: insight into pathways of metabolic regulation. Physiol Genomics 29:267–279
Kauffmann A, Gentleman R, Huber W (2009) arrayqualitymetrics—a bioconductor package for quality assessment of microarray data. Bioinformatics 25:415–416
Storey J, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100:9440–9445
Edgar R, Domrachev M, Lash A (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210
Naraballobh W, Chomdej S, Muráni E, Wimmers K, Ponsuksili S (2010) Annotation and in silico localization of the affymetrix genechip porcine genome array. Arch Anim Breed 53:230–238
Hossenlopp P, Seurin D, Segovia-Quinson B, Hardouin S, Binoux M (1986) Analysis of serum insulin-like growth factor binding proteins using western blotting: use of the method for titration of the binding proteins and competitive binding studies. Anal Biochem 154:138–143
Laeger T, Wirthgen E, Piechotta M, Metzger F, Metges C, Kuhla B, Hoeflich A (2014) Effects of parturition and feed restriction on concentrations and distribution of the insulin-like growth factor-binding proteins in plasma and cerebrospinal fluid of dairy cows. J Dairy Sci 97:2876–2885
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Ullrey D, Sprague J, Becker D, Miller E (1965) Growth of the swine fetus. J Anim Sci 24:711–717
Rehfeldt C, Lang I, Goers S, Hennig U, Kalbe C, Stabenow B, Bruessow K, Pfuhl R, Bellmann O, Nuernberg G, Otten W, Metges C (2011) Low and excess dietary protein levels during gestation affect growth and compositional traits in gilts and impair offspring fetal growth. J Anim Sci 89:329–341
Osgerby J, Wathes D, Howard D, Gadd T (2002) The effect of maternal undernutrition on ovine fetal growth. J Endocrinol 173:131–141
Rees W, Hay S, Brown D, Antipatis C, Palmer R (2000) Maternal protein deficiency causes hypermethylation of dna in the livers of rat fetuses. J Nutr 130:1821–1826
Rees W, Hay S, Cruickshank M (2006) An imbalance in the methionine content of the maternal diet reduces postnatal growth in the rat. Metabolism 55:763–770
Maloney C, Hay S, Rees W (2009) The effects of feeding rats diets deficient in folic acid and related methyl donors on the blood pressure and glucose tolerance of the offspring. Br J Nutr 101:1333–1340
Roberfroid D, Huybregts L, Lanou H, Habicht J, Henry M, Meda N, Kolsteren P (2012) Prenatal micronutrient supplements cumulatively increase fetal growth. J Nutr 142:548–554
Fekete K, Berti C, Trovato M, Lohner S, Dullemeijer C, Souverein O, Cetin I, Decsi T (2012) Effect of folate intake on health outcomes in pregnancy: a systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr J 11:75
Hoeflich A, Bünger L, Nedbal S, Renne U, Elmlinger M, Blum W, Bruley C, Kolb H, Wolf E (2004) Growth selection in mice reveals conserved and redundant expression patterns of the insulin-like growth factor system. Gen Comp Endocrinol 136:248–259
Kampman K, Ramsay T, White M (1993) Developmental changes in hepatic IGF-2 and IGFBP-2 mRNA levels in intrauterine growth-retarded and control swine. Comp Biochem Physiol B 104:415–421
Tilley R, McNeil C, Ashworth C, Page K, McArdle H (2007) Altered muscle development and expression of the insulin-like growth factor system in growth retarded fetal pigs. Domest Anim Endocrinol 32:167–177
Gay E, Seurin D, Babajko S, Doublier S, Cazillis M, Binoux M (1997) Liver-specific expression of human insulin-like growth factor binding protein-1 in transgenic mice: repercussions on reproduction, ante- and perinatal mortality and postnatal growth. Endocrinology 138:2937–2947
Hoeflich A, Wu M, Mohan S, Föll J, Wanke R, Froehlich T, Arnold G, Lahm H, Kolb H, Wolf E (1999) Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology 140:5488–5496
D’Ercole A, Dai Z, Xing Y, Boney C, Wilkie M, Lauder J, Han V, Clemmons D (1994) Brain growth retardation due to the expression of human insulin like growth factor binding protein-1 in transgenic mice: an in vivo model for the analysis of igf function in the brain. Brain Res Dev Brain Res 82:213–222
Hausman G, Campion D, Buonomo F (1991) Concentration of insulin-like growth factors (IGF-I and IGF-II) in tissues of developing lean and obese pig fetuses. Growth Dev Aging 55:43–52
Sohlström A, Katsman A, Kind K, Roberts C, Owens P, Robinson J, Owens J (1998) Food restriction alters pregnancy-associated changes in IGF and IGFBP in the guinea pig. Am J Physiol 274:E410–E416
Donovan S, McNeil L, Jiménez-Flores R, Odle J (1994) Insulin-like growth factors and insulin-like growth factor binding proteins in porcine serum and milk throughout lactation. Pediatr Res 36:159–168
Peng M, Abribat T, Calvo E, LeBel D, Palin M, Bernatchez G, Morisset J, Pelletier G (1998) Ontogeny of insulin-like growth factors (IGF), IGF binding proteins, IGF receptors, and growth hormone receptor mRNA levels in porcine pancreas. J Anim Sci 76:1178–1188
Wyrwoll C, Kerrigan D, Holmes M, Seckl J, Drake A (2012) Altered placental methyl donor transport in the dexamethasone programmed rat. Placenta 33:220–223
Nijhout H, Reed M, Budu P, Ulrich C (2004) A mathematical model of the folate cycle: new insights into folate homeostasis. J Biol Chem 279:55008–55016
McNeil C, Hay S, Rucklidge G, Reid M, Duncan G, Rees W (2009) Maternal diets deficient in folic acid and related methyl donors modify mechanisms associated with lipid metabolism in the fetal liver of the rat. Br J Nutr 102:1445–1452
Pooya S, Blaise S, Moreno Garcia M, Giudicelli J, Alberto J, Guéant-Rodriguez R, Jeannesson E, Gueguen N, Bressenot A, Nicolas B, Malthiery Y, Daval J, Peyrin-Biroulet L, Bronowicki J, Guéant J (2012) Methyl donor deficiency impairs fatty acid oxidation through pgc-1alpha hypomethylation and decreased er-alpha, err-alpha, and hnf-4alpha in the rat liver. J Hepatol 57:344–351
Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K (2004) Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol 279:19–30
Lillycrop K, Rodford J, Garratt E, Slater-Jefferies J, Godfrey K et al (2010) Maternal protein restriction with or without folic acid supplementation during pregnancy alters the hepatic transcriptome in adult male rats. Br J Nutr 103:1711–1719
Burdge G, Hoile S, Lillycrop K (2012) Epigenetics: are there implications for personalised nutrition? Curr Opin Clin Nutr Metab Care 15:442–447
Rees W, Wilson F, Maloney C (2006) Sulfur amino acid metabolism in pregnancy: the impact of methionine in the maternal diet. J Nutr 136:1701S–1705S
Ciappio E, Mason J, Crott J (2011) Maternal one-carbon nutrient intake and cancer risk in offspring. Nutr Rev 69:561–571
Parker M, Rifas-Shiman SL, Oken E, Belfort MB, Jaddoe VW, Gillman MW (2012) Second trimester estimated fetal weight and fetal weight gain predict childhood obesity. J Pediatr 161:864–870
Acknowledgments
The authors thank Hannelore Tychsen, Angela Garve, Annette Jugert, and Kerstin Jahnke for their excellent technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was partially funded by the 6th Research Framework Programme of the European Union as part of the SABRE project (cutting-edge genomics for sustainable animal breeding).
Conflict of interest
The authors have declared that no competing interests exist. The manuscript does not contain clinical studies or patient data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table S1
Selected transcripts used for qPCR. Primers and correlation between microarray and qPCR are displayed (XLSX 293 kb)
Supplemental Table S2
Transcripts differing in mRNA levels between MET and CON liver samples (XLSX 89 kb)
Supplemental Table S3
Pathways altered between two ontogenetic stages within CON group in liver tissue (XLSX 14 kb)
Supplemental Table S4
Pathways altered between two ontogenetic stages within MET group in liver tissue (XLSX 14 kb)
Supplemental Table S5
IGFBP-1, IGFBP-2, IGFBP-3, and IGFBP-6 levels in foetal liver tissue (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Oster, M., Nuchchanart, W., Trakooljul, N. et al. Methylating micronutrient supplementation during pregnancy influences foetal hepatic gene expression and IGF signalling and increases foetal weight. Eur J Nutr 55, 1717–1727 (2016). https://doi.org/10.1007/s00394-015-0990-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0990-2