Abstract
Purpose
Using a diet-induced obesity (DIO) mouse model, we investigated the antidiabetic effect of Labrador tea [Rhododendron groenlandicum (Oeder) Kron and Judd], a beverage and medicinal tea used by the Cree Nations of northern Quebec.
Methods
C57BL6 mice were divided into five groups and given standard chow (~4 % of lipids) or high-fat diet (~35 % of lipids) for 8 weeks until they became obese and insulin resistant. Treatment began by adding the plant extract at three doses (125, 250 and 500 mg/kg) to the high-fat diet for another 8 weeks. At the end of the study, insulin-sensitive tissues (liver, skeletal muscle, adipose tissue) were collected to investigate the plant’s molecular mechanisms.
Results
Labrador tea significantly reduced blood glucose (13 %), the response to an oral glucose tolerance test (18.2 %) and plasma insulin (65 %) while preventing hepatic steatosis (42 % reduction in hepatic triglyceride levels) in DIO mice. It stimulated insulin-dependent Akt pathway (55 %) and increased the expression of GLUT4 (53 %) in skeletal muscle. In the liver, Labrador tea stimulated the insulin-dependent Akt and the insulin-independent AMP-activated protein kinase pathways. The improvement in hepatic steatosis observed in DIO-treated mice was associated with a reduction in inflammation (through the IKK α/β) and a decrease in the hepatic content of SREBP-1 (39 %).
Conclusions
Labrador tea exerts potential antidiabetic action by improving insulin sensitivity and mitigating high-fat diet-induced obesity and hyperglycemia. They validate the safety and efficacy of this plant, a promising candidate for culturally relevant complementary treatment in Cree diabetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of diabetes among the Canadian aboriginal populations is 3–5 times higher than that of non-aboriginals. Risk factors such as sedentary lifestyle and genetic predisposition are major causes behind the alarmingly high rates of diabetes in these populations [1]. They are also more susceptible to diabetes complications in part due to low treatment compliance caused by the disconnection between traditional and modern therapeutic approaches [2]. Our team has thus been working with the Cree of Eeyou Istchii (Eastern James Bay area of Quebec, Canada) to document the potential of their plants to mitigate type 2 diabetes and its precursor, obesity. Obesity is indeed a major risk factor for insulin resistance and diabetes. Insulin resistance is characterized by a decrease in the ability of target tissues, especially muscle, liver and fat, to respond to insulin effects.
Rhododendron groenlandicum (Oeder) Kron and Judd, commonly known as Labrador tea, is a plant of the Heath family (Ericaceae). The Eastern James Bay Cree use the leaves to make a beverage tea and to treat diseases such as asthma, diabetes and kidney infections. It is as popular among them as is green tea for Western culture [3]. The Europeans adopted the tea for its distinctive flavor and aroma, and it was used to make a tea substitute during the Revolutionary War [4]. Concerning the identification and quantification of the components of the Labrador tea leaf extract, quantitative analysis was performed in our laboratories using an Agilent 1100 series RP-HPLC–DAD-APCI/MSD system. The principal chemical constituents are mainly flavonoids: quercetin-3-galactoside [6.49 mg/g dry weight (DW)], (+) catechin (4.98 mg/g DW), (−) epicatechin (2.73 mg/g DW), quercetin-3-glucoside (1.81 mg/g DW), quercetin-3-rhamnoside (0.38 mg/g DW) and quercetin (0.12 mg/g DW). The major phenolic acid is chlorogenic acid (3.17 mg/g DW). The polyphenols procyanidin B2 and procyanidin A1 were also detected [5, 6]. R. groenlandicum was previously reported to possess promising antidiabetic effects on several insulin-sensitive cell lines, notably skeletal muscle C2C12 and 3T3-L1 adipocyte cells [7]. The mechanisms of action appear to involve a metformin-like activity [8]. We therefore sought to confirm the plant’s antidiabetic potential in vivo and to further investigate the mechanisms by which this plant can improve systemic glucose and lipid homeostasis. For this purpose, we chose the diet-induced obese (DIO) mouse model. Indeed, mice fed chronically with a high-fat diet develop obesity, hyperglycemia and hyperlipidemia [9], much as such dietary habits cause overweight and participate in the metabolic syndrome in humans [10].
Approximately 80 % of total body glucose uptake occurs in skeletal muscle through insulin- and exercise-sensitive glucose transporters, GLUT4, whose translocation to the cell surface involves Akt and AMPK pathways, respectively [11, 12]. In addition, transgenic mice revealed that specific elevation in muscle GLUT4 expression prevents insulin resistance [13]. Finally, AMPK activation also upregulates skeletal muscle GLUT4 expression, thus leading to increased insulin sensitivity and glucose uptake [14]. We therefore assessed Akt, AMPK and GLUT4 components of muscle glucose homeostasis in skeletal tissues of obese and insulin-resistant mice treated with R. groenlandicum.
Adipose tissue is a major site of fatty acid synthesis and storage. Peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer binding protein (C/EBPs) are two key factors affecting the proliferation and differentiation of adipose cells and inducing lipid accumulation into white adipose tissue (WAT) [15]. Thiazolidinediones, a class of oral hypoglycemic drugs that act through PPARγ agonism, can improve insulin sensitivity and glucose tolerance by promoting WAT storage of free fatty acids, thereby preventing ectopic fat storage in muscle and liver. WAT also plays an important role in regulating systemic insulin sensitivity by secreting adipokines such as adiponectin and leptin, which influence whole-body metabolism [16]. Adiponectin modulates glucose and fatty acid handling [17]. It plays a role in the suppression of the metabolic disturbances that may result in type 2 diabetes, obesity, atherosclerosis nonalcoholic fatty liver disease (NAFLD) and metabolic syndrome [17, 18]. Leptin plays a key role in regulating energy intake and energy expenditure. Obesity is often associated with leptin resistance, deregulating satiety signals and an elevated leptin/adiponectin ratio [19]. In the present study, we therefore paid attention to PPARγ, C/EBP, adiponectin and leptin parameters.
The liver plays an equally important role in glucose homeostasis through the production and storage of glucose. The liver also regulates lipid homeostasis through a process implicating key lipogenic enzymes such as acetyl-CoA carboxylase (ACC) and the transcription factor sterol response element-binding protein-1 (SREBP-1). The latter controls the synthesis of cholesterol, fatty acids and triglycerides [20] and may be involved in the pathogenesis of hepatic insulin resistance when overexpressed [21]. This leads to intracellular triglyceride accumulation and hence hepatosteatosis [22]. Hyperinsulinemia and inflammatory cytokine pathways implicating the inhibitor of kappa B kinase inhibitor (IKK) usually cause increased SREBP-1 expression [23, 24]. Hence, hepatic ACC, AMPK, SREBP-1 and IKK have been assessed in this study to determine the effects of R. groenlandicum on liver in vivo. These animal studies are a first step in the assessment of the therapeutic potential of R. groenlandicum in the context of metabolic syndrome and type 2 diabetes.
Materials and methods
Plant materials
The leaves of R. groenlandicum were harvested in the Eeyou Istchii territory, QC, Canada, dried and kept in dry cool conditions until use. Taxonomist Dr. A. Cuerrier identified that the plant and voucher specimens were deposited at the Marie-Victorin Herbarium of the Montreal Botanic Garden. The 80 % ethanol extract was prepared as described previously [5–7].
Animals and diets
Four-week-old male C57BL/6 mice were purchased from Charles River (St-Constant, QC), housed in separate cages and kept in a temperature- and humidity-controlled room with a 12-h light–dark cycle. After acclimation, mice were randomly divided into five groups (n = 12) including CHOW control group (16 weeks on standard chow diet—Charles River) and DIO control group (16 weeks on a high-fat diet (HFD) providing 35 % of lipids (Table 1); Bio-Serv, Frenchtown, NJ, USA). The other three groups received HFD for 8 weeks until they became obese and insulin resistant. The treatment protocol was then initiated by adding the R. groenlandicum extract into the HFD at three doses (125, 250, 500 mg/kg) for another 8 weeks. The doses were chosen based on pilot animal experiments and on mouse–human interspecies dose extrapolation according to Reagan-Shaw et al. (2008) [25]. After extrapolation, the equivalent human doses are 10.1, 20.2 and 40.5 mg/kg, respectively, which equates to 0.6, 1.2 and 2.4 g of recovered alcoholic extracts for an adult human of 60 kg body weight. To obtain the mass of the dry plant material, the mass of recovered alcoholic extracts is divided by the yield (31 %) to give 1.9, 3.8 and 7.7 g, respectively, for a 60-kg person, which is close to the amount of dry plant material used in herbal tea preparations (about 5 g of dry tea leaves/cup of Labrador tea).
During the study, body weight, food intake, water intake and blood glucose level were measured two or three times a week. Tail vein glycemia was measured with a commercial glucometer (Accu-Check Roche, Montreal, QC). At study end, the mice were anesthetized (intraperitoneal sodium pentobarbital, 45 mg/kg), killed by complete blood sampling and organs such as liver, skeletal muscle, WAT (epididimal and retroperitoneal fat pads) and kidney were immediately collected, weighted and stored at −80 °C for further analysis.
Livers from each mouse were harvested, and dissected sections were fixed in 10 % formalin solution and embedded in paraffin. Each section (2) was cut, mounted on glass slides and stained with hematoxylin phloxine saffron (HPS) by the Institut de Recherche en Immunologie et en Cancérologie (IRIC), Department of Histology, Université de Montréal, Montreal, QC, Canada.
Oral glucose tolerant test (OGTT)
A second study was conducted to assess the effect of R. groenlandicum on glucose tolerance. Thirty-three C57/BL/6 mice were acclimated to the animal facilities as previously described in the treatment study. Twelve of these animals were then randomly assigned to CHOW control group and were provided with standard chow diet for 6 weeks. The remaining 21 mice were fed HFD for the same period of time. Food intake, water intake and blood glucose levels were measured once a week. Blood glucose levels were measured in tail vein blood as previously mentioned in the treatment study. After 8 weeks on HFD, the animals were randomly segregated into two groups: one received HFD alone, while the other had 250 mg/kg of R. groenlandicum incorporated in the HFD for an additional 8 weeks. This dose was chosen for the OGTT study because it induced the best metabolic response in the first study (reduction in hepatic triglycerides levels, enhancement of muscle GLUT4 expression, stimulation of Akt pathway in both liver and muscle, and restoration of AMPK activity in the liver). At the end of the study, the mice were fasted for 5.5 h and were given 2 g/kg glucose by oral gavage. Tail vein glucose readings were taken at 0, 15, 30, 60 and 120 min after glucose administration.
All animal experimental protocols were approved by Université de Montréal Animal Experimentation Ethics Committee and respected guidelines of the Canadian Council for the Care and Protection of Animals.
Biochemical assays
Plasma insulin (Linco; St-Charles, MO), adiponectin and leptin (Millipore; St-Charles, MO) were determined using radioimmunoassay kits according to manufacturer’s specifications. Insulin sensitivity was estimated using Homeostasis Model Assessment-estimated insulin resistance (HOMA-IR); HOMA-IR = [(plasma insulin (µg/mL)) × plasma glucose (mM/L)]/22.5 [26]. Circulating lipids (triglycerides, total cholesterol, LDL, HDL) as well as liver and kidney functional parameters (ALT, AST, creatinine, alkaline phosphatase) were assessed by standard clinical biochemistry protocols at Sainte-Justine’s Children Hospital (Montreal, Quebec).
Measurement of tissue triglyceride content
Liver samples (about 100 mg each) were powdered under liquid nitrogen and extracted with Folch’s chloroform/methanol (2:1) reagent [27]. Triglycerides (TG) content was determined by using a commercial kit (Randox Laboratories Ltd., UK).
Western blot analysis
Frozen tissue (muscle, liver and WAT) samples were homogenized in RIPA lysis buffer (50 mM Hepes, 150 mM NaCl, 5 mM EGTA, 2 mM MgCl2, 5 % glycerol, 1 % Triton-X 100, 0.1 % SDS, pH 7.4) containing protease and phosphatase inhibitors. To determine GLUT4 protein levels, muscles were lysed in sucrose buffer instead (Tris buffer pH 7.4, 20 mM Tris–HCl, 255 mM sucrose, 1 mM EDTA). After homogenization, samples were centrifuged and supernatant removed for analysis. Equal amounts of protein (50 μg) were subjected to electrophosphoresis on 10 % SDS–polyacrylamide gels and transferred to nitrocellulose membrane (Millipore, Bedford, MA). Membranes were probed with the following antibodies: p-Akt (Ser 473), Akt, p-ACC (Ser 79), ACC, p-AMPKα (Thr 172), AMPK, p-IKK αβ (Ser176/180), ß-actin (1:1000 dilution, 5 % BSA, Cell Signaling Technology, Danvers, MA); GLUT 4 (1:1000 dilution, 5 % milk, Cell Signaling Technology, Danvers, MA); PPARα, PPARγ, SREBP-1, C/EBPα, C/EBPß (1:200 dilution; 5 % milk, Santa Cruz Biotechnology, Inc. Santa Cruz, CA). Anti-rabbit IgG or anti-mouse IgG HRP-conjugated secondary antibodies were used at 1:4000 dilutions in 5 % milk in TBST (Cell Signaling Technology).
PPARγ activation experiments
Using a nuclear receptor reporter assay, R. groenlandicum extract was tested for agonist and antagonist activity against PPARγ (Indigo Biosciences, State College, PA, USA). Briefly, HEK 293-T cells were transfected with Gal4-human PPARγ, Gal 4-luciferase and pRL [28]. Cells were incubated with different concentrations of R. groenlandicum (100, 25, 6.25, 1.56 and 0.39 μg/mL), of the PPARγ agonist rosiglitazone (20, 2, 0.2, 0.02 and 0.002 μM) or of the PPARγ antagonist GW9662 (10, 1, 0.1 and 0.01 μM) for 14 h. Luciferase activity was then determined, and the EC50 and IC50 values were calculated by Prism® 4.0 software (GraphPad Software, Inc., San Diego, CA).
Statistical analysis
Results are presented as mean ± SEM. Data analyses were performed using SigmaStat 3.1 software (Jandel Scientific, San Rafael, CA). Statistically significant differences between group means were assessed by ANOVA. P values <0.05 were considered statistically significant. Values of AUC for OGTT are calculated for the intervals 0–120 min and presented as the mean ± SEM of AUCs from single curves using Prism® 4 software.
Results
Diet-induced obesity (DIO) model
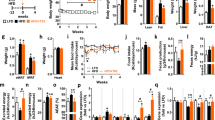
To induce obesity and a prediabetic state, the DIO group mice were fed HFD for 16 weeks. This resulted in a significant weight gain as compared to standard CHOW fed group (47.8 ± 0.6 vs. 36.5 ± 0.9 g, respectively; p < 0.05; Table 2; Fig. 1a). Similarly, significant increases in liver, WAT (retroperitoneal fat pad) and brown adipose tissue weights were observed (p < 0.05; Table 2). This occurred without any change in food intake between the DIO and CHOW groups (data not illustrated). In addition, blood lipid profile reflected the high-fat intake since LDL, HDL as well as total cholesterol were doubled in DIO animals when compared to CHOW congeners (Table 3).
R. groenlandicum treatment for 8 weeks decreases blood glucose levels without affecting body weight of DIO mice. The area under the weight-versus-time (a) and the glycemia-versus-time (b) curves was assessed for the first and second 4 weeks of treatment. These parameters were monitored three times a week. Asterisk indicates a p value <0.05 significantly different from CHOW group, and dagger indicates a p value <0.05 significantly different from DIO control group (n = 12 for each group)
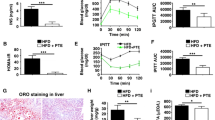
In parallel, a state of insulin resistance was established as evidenced by mild hyperglycemia and major hyperinsulinemia in DIO mice (33 % and eightfold increases, respectively, vs. CHOW; p < 0.05; Table 3). The high level of hepatic accumulation of triglycerides (20.3 ± 1.8 for DIO vs. 4.71 ± 0.57 for CHOW mg/g liver; p < 0.05; Fig. 2) corroborated the existence of an insulin-resistant state. Finally, circulating leptin/adiponectin ratio increased nearly 1.8-fold (p < 0.05; Table 3).
Treatment with R. groenlandicum improves hepatic steatosis induced by HFD in DIO mouse model. The colorimetric dosage of TG levels a was determined using a commercial kit (Randox), and liver sections b were prepared and stained with HSP. Data are presented as the mean ± SE of 12 mice. Asterisk indicates a p value <0.05 significantly different from CHOW group, and dagger indicates a p value <0.05 significantly different from DIO control group. TG triglycerides
R. groenlandicum significantly decreases glycemia, hepatic steatosis and insulin resistance of DIO mice after 8 weeks of treatment
When administered for the last half of a 16-week HFD protocol, R. groenlandicum significantly affected glycemia. Indeed, when the area under the glycemia-versus-time curve (AUC) was assessed for the last four treatment weeks, the two highest doses of R. groenlandicum diminished blood glucose levels by about 12.5 and 13.3 %, respectively, as compared to DIO controls (p < 0.05; Fig. 1b). This effect was more pronounced at killing where glycemia in plant-treated animals was between 25 and 30 % lower than that of DIO controls (p < 0.05; Table 3), without being significantly different from CHOW mice. R. groenlandicum treatment also had a major impact on insulin serum levels, reducing them by 62–66 % when compared to DIO controls (p < 0.05 for 250 mg/kg/d group; Table 3) and improved HOMA index by up to 75 % (p < 0.05; Table 3). However, insulin serum levels and HOMA index were still significantly higher than those of the CHOW group (p < 0.05).
Since the HOMA index is only an approximation of insulin sensitivity, we did additional experiments where an OGTT test was carried out using the most effective dose of R. groenlandicum, namely 250 mg/kg/d. As shown in Fig. 3a, DIO controls had a much larger glucose response to the OGTT as compared to CHOW animals. Mice ingesting HFD containing 250 mg/kg/d of R. groenlandicum had a significantly reduced response compared to DIO controls and lay intermediate between the latter and the CHOW controls. These results were corroborated by AUC measurements showing R. groenlandicum group (2158.0 ± 161.0) significantly inferior to DIO mice (2638.7 ± 149.7) and superior to CHOW controls (1447.5 ± 57.0; p < 0.05 for both; Fig. 3b).
R. groenlandicum improves glucose tolerance in DIO mice. a Shown are mean blood glucose levels following OGTT test. The test was performed after 16 weeks on HFD, the last eight with or without R. groenlandicum (RG) at 250 mg/kg/d. Blood glucose levels were measured at 0, 15, 30, 60 and 120 min after glucose administration. b AUC for OGTT in A. AUC, area under the curve, OGTT, oral glucose tolerance test. Asterisk indicates a p value <0.05 significantly different from CHOW group, and dagger indicates a p value <0.05 significantly different from DIO control group
Liver steatosis was significantly improved when the animals ingested R. groenlandicum alongside the HFD (Fig. 2). Liver TG levels decreased significantly under the influence of plant extract. The 250 mg/kg/d group showed the best outcome with a decrease of 42 % (p < 0.05) in TG levels when compared to DIO control group. These results were fully compatible with the changes observed at the level of liver weight, where mice treated with 250 mg/kg/d of R. groenlandicum had significantly smaller livers than DIO control animals (1.9 ± 0.7 vs. 2.4 ± 0.1 g, respectively; p < 0.05; Table 2).
In contrast, R. groenlandicum treatment did not decrease weight gain (6 % reduction in AUC; Fig. 1a; N.S.). When body weight at the end of treatments was considered, R. groenlandicum treatment also failed to induce a statistically significant difference (11 % difference between 250 mg/kg/d and DIO control groups; Table 2, N.S.). Moreover, R. groenlandicum did not significantly affect food intake in all DIO groups (data not illustrated). Similarly, circulating lipids (TG, LDL, HDL and total cholesterol), leptin, adiponectin as well as their ratio were not significantly modified by plant extract administration (Table 3). Finally, R. groenlandicum treatment in DIO mice lacked toxicity as shown by unaltered liver (ALT, AST) or renal (creatinine and alkaline phosphatase) functional parameters (Table 2).
To begin elucidating the mechanisms of action of R. groenlandicum in the DIO mouse model, we analyzed key signaling pathways in muscle, liver and adipose tissue.
R. groenlandicum enhances glucose transporter GLUT4 expression through an Akt-dependent pathway in the muscle
Figure 4 demonstrates that HFD does not alter the phosphorylation of Akt or GLUT protein content. Interestingly, R. groenlandicum 250 mg/kg/d treatment increases Akt phosphorylation levels when compared with CHOW and DIO control mice [0.45 ± 0.09 vs. 0.20 ± 0.02 and 0.20 ± 0.04 arbitrary units (a.u.) for CHOW and DIO control mice respectively; p < 0.05; Fig. 4a]. Similarly, muscle GLUT4 protein levels significantly increased in mice fed with the same dose of R. groenlandicum (250 mg/kg/d) reaching values double that of DIO control (0.78 ± 0.10 vs. 0.37 ± 0.07 a.u.; p < 0.05; Fig. 4b). This occurred without any significant effect on AMPK or its substrate ACC (N.S.; Fig. 4, panels c, d).
R. groenlandicum increases GLUT4 protein content by stimulating the Akt pathway but not the AMPK pathway in skeletal muscle of treated mice. Samples of skeletal muscle (50 µg protein) from mice fed CHOW, DIO and DIO + R. groenlandicum (125, 250, 500 mg/kg) were homogenized and analyzed by immunoblotting. Blots were quantified by densitometry, and data are expressed as mean ± SEM from 12 animals in each group. Representative immunoblots and their quantification are shown for samples probed with a p-Akt (Ser 473)/Akt, b GLUT 4/β-actin, c p-AMPKα (Thr 172)/AMPK, d p-ACC (Ser 79)/ACC. Asterisk indicates a p value <0.05 significantly different from CHOW group, and dagger indicates a p value <0.05 significantly different from DIO control group
R. groenlandicum stimulates Akt and AMPK pathways in the liver
As illustrated in Fig. 5, treatment with 250 mg/kg/d of R. groenlandicum activated insulin-dependent Akt and restored insulin-independent AMPK; an activity that was inhibited by HFD feeding. Again, compared to control DIO mice, the 250 mg/kg/d group yielded the largest (over twofold) increase in the phosphorylation level of Akt (p < 0.05; Fig. 5a) and AMPK (p < 0.05; Fig. 5b). However, the phosphorylation of ACC was not significantly affected by R. groenlandicum treatment (Fig. 5c). Further investigations will be necessary to clarify the reasons for this lack of effect.
R. groenlandicum activates the Akt and AMPK pathways in the liver of DIO mice. Samples of liver tissue (50 µg protein) from mice fed CHOW, DIO and DIO + R groenlandicum (125, 250, 500 mg/kg) were homogenized and analyzed by immunoblotting. Blots were quantified by densitometry, and data are expressed as mean ± SEM from 12 animals in each group. Representative immunoblots and their quantification are shown for samples probed with a p-Akt (Ser 473)/Akt, b p-AMPKα (Thr 172)/AMPK, c p-ACC (Ser 79)/ACC, d PPARα/β-actin, e SREBP-1 and f p-IKKαβ (Ser173/180). Asterisk indicates a p value <0.05 significantly different from CHOW group, and dagger indicates a p value <0.05 significantly different from DIO control group
Liver PPARα content was significantly increased in DIO control animals as compared to CHOW (0.55 ± 0.12 vs. 0.32 ± 0.10 a.u., respectively; p < 0.05; Fig. 5d), suggesting compensatory mechanisms in the face of enhanced fatty acid intake. Hepatic PPARα levels remained elevated in R. groenlandicum treatment groups (p < 0.05 vs. CHOW). The levels of the 68 kDa active fragments of SREBP-1 were not statistically different in DIO versus CHOW animals (p = 0.08; Fig. 5e). Conversely, R. groenlandicum at the dose of 250 mg/kg/d decreased p68 SREBP-1 levels by 39 % compared to DIO controls.
Finally, consistent with the role of IKKαβ phosphorylation in inflammatory components of hepatic metabolic diseases [23, 24], DIO animals exhibited phosphorylated IKK levels that were double those observed in CHOW (p < 0.05; Fig. 5f). Conversely, R. groenlandicum treatment with 250 and 500 mg/kg/d groups yielded phosphorylated IKK values that were statistically similar to CHOW controls (Fig. 5f).
R. groenlandicum has weak effects on adipogenesis in white adipose tissue
In view of previously reported [7] strong in vitro glitazone-like adipogenic activity of R. groenlandicum, studies on PPARγ ligand binding activity were conducted using a gene reporter assay in the HEK 293-T cell line. Crude R. groenlandicum extract was found to exhibit weak partial agonistic activity (EC50 = 2678 μg/mL; Fig. 6a), as compared to rosiglitazone, a well-known PPARγ agonist (EC50 = 0.2896 μg/mL; Fig. 6b—inset). Conversely, when antagonistic studies were carried out against rosiglitazone (800 nM) in the same assay, R. groenlandicum extract showed much weaker antagonist activity than GW9662, a typical PPARγ antagonist (IC50 of 11,285 vs. 0.2896 μg/mL, respectively; data not illustrated). However, further studies are required to fully validate these in vitro findings in an in vivo setting since active molecules in vivo could be very different from parent compounds in extracts. As presented in Fig. 7, neither the DIO protocol alone nor with R. groenlandicum intake had a significant impact on expression levels of PPARγ (Fig. 7a), SREBP-1 (Fig. 7b), C/EBP α (Fig. 7c) and C/EBP β (Fig. 7d).
R. groenlandicum is a weak activator of PPARγ. HEK 293-T cells were transfected with Gal4-human PPARγ, Gal 4-luciferase and pRL; then, cells were incubated with different concentrations of R. groenlandicum (a) or PPARγ agonist rosiglitazone (b). Luciferase activity was then determined, and the EC50 and IC50 values were calculated. Asterisk indicates significantly different (p < 0.05) from vehicle control
R. groenlandicum has weak effects on adipogenesis in WAT of DIO mice. Samples of adipose tissue (50 µg protein) from mice fed CHOW, DIO and DIO + R groenlandicum (125, 250, 500 mg/kg) were homogenized and analyzed by immunoblotting. Blots were quantified by densitometry, and data are expressed as mean ± SEM from 12 animals in each group. Representative immunoblots and their quantification are shown for samples probed with a PPAR γ, b SREBP-1, c C/EBPα and d C/EBPβ. Asterisk indicates a p value <0.05 significantly different from CHOW group, and dagger indicates a p value <0.05 significantly different from DIO control group
Discussion
Modern lifestyle that promotes overconsumption of calorie-dense food contributes to visceral obesity, now a leading cause of metabolic disorders such as type 2 diabetes. The diet-induced obesity mouse model, based on high-fat diet feeding, therefore represents a valuable tool to investigate and validate new therapeutic avenues in the treatment of obesity and diabetes. Indeed, our study confirmed components previously reported in the DIO mouse model, namely obesity, hyperglycemia, insulin resistance and fatty liver [9], reminiscent of the pathophysiological characteristics of the so-called metabolic syndrome continuum that spans from obesity to non-insulin-dependent diabetes mellitus [29].
Canadian aboriginals, as others worldwide, have been exposed to sudden lifestyle changes (reduced physical activity and increased intake of nontraditional “western” diets). Consequently, rates of diabetes and obesity rapidly escalated over the past several decades [1]. Our team has worked for several years with Eastern James Bay Cree to study Boreal forest plants with the purpose of providing evidence-based, culturally adapted, complementary and alternative antidiabetic therapeutic approaches.
R. groenlandicum, also known as katchichepukh in Cree, is a popular beverage and one of the highest ranking Cree plants used to treat diabetes symptoms [3]. Labrador tea is rich in dietary flavonoids including flavan-3-ol monomers such as catechin and epicatechin as well as the flavone quercetin and its glycosides. Several studies have reported the antidiabetic activities of dietary flavonoids and polyphenols in their nutritional doses [30]. In one of these studies, green tea extract containing 33.2 mg of catechin/350 mL was found to reduce postprandial glucose levels in healthy postmenopausal women [31]. Similarly, epicatechin (90 mg/day) administered to diabetic postmenopausal women in flavonoid-enriched chocolate resulted in an improvement in peripheral lipid parameters, biomarkers of insulin sensitivity and cardiovascular risk [32]. The doses of catechin and epicatechin in these studies are achievable through daily consumption of Labrador tea.
In the present study, the antidiabetic activity of Labrador tea was investigated in the DIO mouse model. R. groenlandicum was administered for 8 weeks, after obesity and mild hyperglycemia were established by an initial 8 weeks of HFD feeding. Such treatment clearly improved glucose homeostasis in the face of continued HFD feeding, most likely by an attenuation of insulin resistance. Indeed, R. groenlandicum treatment significantly countered hyperglycemia, hyperinsulinemia as well as hepatic steatosis (improved histological grade, reduced hepatic TG accumulation and smaller liver weight). This was confirmed by an OGTT in the best responding group receiving 250 mg/kg/d whose glycemic response lay halfway between those of DIO and CHOW controls. All these effects occurred without any significant change in food intake and body weight in all groups. Finally, there was no sign of toxicity from liver and kidney functional parameters, even with the highest dose of 500 mg/kg/d, consistent with traditional consumption of Labrador tea by Cree populations and GRAS status.
The activation and the expression of key proteins involved in glucose and lipid homeostasis were then assessed in major insulin-sensitive tissues, in order to begin elucidating the molecular mechanisms underlying the apparent systemic antidiabetic activity of R. groenlandicum.
Interestingly, the dose of 250 mg/k/d of R. groenlandicum, which gave the best improvement in systemic glucose and lipid homeostasis, also induced the best effect on the aforementioned proteins. Firstly, this dose increased the expression of muscle GLUT4 transporters up to twofold as compared to DIO control mice. This was associated with the activation of the insulin-dependent Akt pathway, supporting our previous in vitro findings, where R. groenlandicum stimulated glucose uptake in C2C12 cells without any synergistic effect with insulin [7]. Notably, specific elevation of GLUT4 expression in the muscle of normal mice prevents insulin resistance [13].
In the liver, R. groenlandicum at the same dose concomitantly activated the Akt pathway and restored the activity of the AMPK pathway. The latter was disrupted by HFD feeding consistent with previous findings using palmitate-rich HFD [33]. The two pathways are known to regulate hepatic glucose output, a major component of glucose homeostasis and type 2 diabetes pathogenesis [34, 35]. Indeed, activation of these phosphorylation cascades can reduce hepatic glucose output by stimulating glucose storage in the form of glycogen while inhibiting gluconeogenesis [36, 37]. In fact, we found that R. groenlandicum administered in vitro to hepatic cell lines significantly decreased glucose-6-phosphatase activity while increasing that of glycogen synthase, two key enzymes of gluconeogenesis and glycogen synthesis, respectively [38]. Hence, the significant reduction in hyperglycemia afforded in vivo by R. groenlandicum can likely be related to a combination of reduced hepatic glucose output and enhanced muscle glucose uptake.
On the other hand, liver fat accumulation is a major contributor to the development of insulin resistance in this organ and a crucial component in diabetes pathogenesis [39]. Moreover, liver insulin sensitivity is enhanced by stimulating hepatic fatty acid oxidation through AMPK/ACC [40–42] and/or PPARα [43, 44], on one hand, and by inhibiting cholesterol and triglyceride synthesis through regulation of SREBP-1, on the other hand [45, 46]. In the present study, R. groenlandicum treatment improved hepatic lipid homeostasis as evidenced by (1) a significant attenuation of liver steatosis and intracellular TG, (2) maintenance of high PPARα levels elevated by HFD feeding and (3) a strong tendency to reduce hepatic SREBP-1 levels as compared to DIO controls. In addition, the DIO-associated increase in IKK phosphorylation appeared to diminish as the dose of R. groenlandicum increased, suggesting that the plant may decrease hepatic inflammation. Taken altogether, these data suggest that R. groenlandicum treatment could tip the balance toward increased oxidation of fatty acids and less lipid storage in the liver, thus improving hepatic steatosis and insulin sensitivity.
Furthermore, it is well documented that thiazolidinediones (PPARγ agonists) improve insulin sensitivity and glucose tolerance by favoring the storage of lipids (free fatty acids) in adipose tissue as opposed to ectopic sites such as muscle and liver [47, 48]. As mentioned, R. groenlandicum previously demonstrated strong glitazone-like action in cultured 3T3-L1 adipocytes [7], suggesting PPARγ agonism. Using an in vitro gene reporter assay, the present study established R. groenlandicum as a weak partial PPARγ agonist at lower doses and a mild antagonist at higher doses. The plant extract also had weak effects on WAT in vivo, as attested by unaltered retroperitoneal fat pad weight. Similarly, the levels of PPARγ, another key transcription factors involved in adipogenesis in WAT and SREBP-1, a key factor of lipogenesis, were not altered by plant treatment; these results, therefore, suggest that Labrador tea extract acts primarily on the liver and muscle, rather than WAT, to exert its antidiabetic in DIO mice.
Finally, it was interesting to note that the highest dose of Labrador tea used often yielded results that were less than those of the 250 mg/kg/d dose and similar to those of the lowest dose of 125 mg/kg/d. In pharmacology, such bell-shaped (or inversed U-shaped) dose–response relationships can be encountered [49]. This pharmacological behavior will have to be confirmed in humans. On the other hand, certain parameters (e.g., liver IKKαβ phosphorylation, WAT C/EBPα/β) appeared to be modulated by Labrador tea in a more conventional dose–response relationship. This could be due to the complexity of plant extracts in which active compounds could exert different effects at different doses and in different sites.
In conclusions, R. groenlandicum exerts significant antidiabetic activity by improving glucose tolerance, systemic glucose and tissue lipid homeostasis through an attenuation of insulin resistance in DIO mice. Together with our previous in vitro data, the present studies lend credible, evidence-based support for the use of Labrador tea as a promising GRAS medicinal plant and culturally adapted therapeutic avenue for Cree diabetics. It is hoped that the inclusion of Labrador tea in Cree diabetes care will contribute to reduce the dramatic burden of type 2 diabetes in this population. The current animal studies are an encouraging first step in this direction; yet, they must be followed by further clinical studies in order to fully validate the antidiabetic effect of the plant preparations.
References
Hegele RA (2001) Genes and environment in type 2 diabetes and atherosclerosis in aboriginal Canadians. Curr Atheroscler Rep 3:216–221
Brassard P, Robinson E, Lavallee C (1993) Prevalence of diabetes mellitus among the James Bay Cree of northern Quebec. Can Med Assoc J 149:303–307
Leduc C, Coonishish J, Haddad P, Cuerrier A (2006) Plants used by the Cree Nation of Eeyou Istchee (Quebec, Canada) for the treatment of diabetes: a novel approach in quantitative ethnobotany. J Ethnopharmacol 105:55–63
Hedrick UP (1972) Sturtevant’s edible plants of the world. Dover, New York
Saleem A, Harris CS, Asim M et al (2010) A RP-HPLC–DAD-APCI/MSD method for the characterisation of medicinal Ericaceae used by the Eeyou Istchee Cree First Nations. Phytochem Anal 21:328–339
Rapinski M, Liu R, Saleem A, Arnason JT, Cuerrier A (2014) Environmental trends in the variation of biologically active phenolic compounds in Labrador tea, Rhododendron groenlandicum, from northern Quebec, Canada. Botany 92:783–794
Spoor DC, Martineau LC, Leduc C et al (2006) Selected plant species from the Cree pharmacopoeia of northern Quebec possess anti-diabetic potential. Can J Physiol Pharmacol 84:847–858
Martineau LC, Adeyiwola-Spoor DC, Vallerand D, Afshar A, Arnason JT, Haddad PS (2010) Enhancement of muscle cell glucose uptake by medicinal plant species of Canada’s native populations is mediated by a common, metformin-like mechanism. J Ethnopharmacol 127:396–406
Jiang T, Wang Z, Proctor G et al (2005) Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem 280:32317–32325
Bray GA, Lovejoy JC, Smith SR et al (2002) The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J Nutr 132:2488–2491
Wallberg-Henriksson H, Zierath JR (2001) GLUT4: a key player regulating glucose homeostasis? Insights from transgenic and knockout mice (review). Mol Membr Biol 18:205–211
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
Gibbs EM, Stock JL, McCoid SC et al (1995) Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J Clin Investig 95:1512–1518
Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW (1999) 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48:1667–1671
Farmer SR (2005) Regulation of PPARgamma activity during adipogenesis. Int J Obes 29(Suppl 1):S13–S16
Rasouli N, Kern PA (2008) Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93:S64–S73
Diez JJ, Iglesias P (2003) The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 148:293–300
Ukkola O, Santaniemi M (2002) Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med 80:696–702
Considine RV, Sinha MK, Heiman ML et al (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295
Shimano H (2001) Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res 40:439–452
Tobe K, Suzuki R, Aoyama M et al (2001) Increased expression of the sterol regulatory element-binding protein-1 gene in insulin receptor substrate-2(−/−) mouse liver. J Biol Chem 276:38337–38340
Postic C, Dentin R, Girard J (2004) Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab 30:398–408
Marchesini G, Brizi M, Bianchi G et al (2001) Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 50:1844–1850
Arkan MC, Hevener AL, Greten FR et al (2005) IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Benhaddou-Andaloussi A, Martineau LC, Vallerand D et al (2010) Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab 12:148–157
Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN (1988) Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–1167
Unnikrishnan MK, Veerapur V, Nayak Y, Mudgal PP, Mathew G (2013) Antidiabetic antihyperlipidemic and antioxidant effects of the flavonoids. In: Watson VRP, Zibadi S, Ronald R (eds) Polyphenols in human health and disease, 1st edn. Elsevier Inc., USA, pp 143–161
Takahashi M, Miyashita M, Suzuki K et al (2014) Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. Br J Nutr 112:1542–1550
Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A (2012) Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 35:226–232
Xie Y, Xie Z (2015) Experimental models of high fat obesity and leucine supplementation. In: Rajendram R, Preedy VR, Patel VB, Chain Branched (eds) Amino Acids in Clinical Nutrition. Springer, New York, pp 219–227
Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H (2005) Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54:1692–1697
Sanz P (2008) AMP-activated protein kinase: structure and regulation. Curr Protein Pept Sci 9:478–492
Haber BA, Chin S, Chuang E, Buikhuisen W, Naji A, Taub R (1995) High levels of glucose-6-phosphatase gene and protein expression reflect an adaptive response in proliferating liver and diabetes. J Clin Investig 95:832–841
Clore JN, Stillman J, Sugerman H (2000) Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes 49:969–974
Nachar A, Vallerand D, Musallam L et al (2013) The action of antidiabetic plants of the Canadian James bay Cree traditional pharmacopeia on key enzymes of hepatic glucose homeostasis. Evidence-based Complement Altern Med 2013:189819
Samuel VT, Liu ZX, Qu X et al (2004) Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279:32345–32353
Foretz M, Taleux N, Guigas B et al (2006) Regulation of energy metabolism by AMPK: a novel therapeutic approach for the treatment of metabolic and cardiovascular diseases. Med Sci M/S 22:381–388
Hardie DG, Scott JW, Pan DA, Hudson ER (2003) Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546:113–120
Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277:E1–E10
Fatehi-Hassanabad Z, Chan CB (2005) Transcriptional regulation of lipid metabolism by fatty acids: a key determinant of pancreatic beta-cell function. Nutr Metab 2:1
Poynter ME, Daynes RA (1998) Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem 273:32833–32841
Kohjima M, Higuchi N, Kato M et al (2008) SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med 21:507–511
Yahagi N, Shimano H, Hasty AH et al (2002) Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem 277:19353–19357
Lowell BB (1999) PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell 99:239–242
de Souza CJ, Eckhardt M, Gagen K et al (2001) Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 50:1863–1871
Schilling TM, Kolsch M, Larra MF et al (2013) For whom the bell (curve) tolls: cortisol rapidly affects memory retrieval by an inverted U-shaped dose–response relationship. Psychoneuroendocrinology 38:1565–1572
Acknowledgments
Very special thanks are due to E. Coon Come, M. Gunner, C. Husky Swallow, J. Husky Swallow, R. Loon and G. Loon from the Cree Nation of Mistissini as well as 27 other elders and healers, who kindly agreed to be interviewed. They made this article possible by allowing us to use, for the purposes of this research, their knowledge relating to medicinal plants, transmitted to them by their elders. Their trust has also enabled a useful exchange between indigenous knowledge and Western science. This work was supported by a Team Grant from the Canadian Institutes of Health Research (CIHR Team in Aboriginal Antidiabetic Medicines) to P.S.H. and J.T.A. It was conducted with the consent and support of the Cree Nation of Mistissini, of the Whapmagoostui First Nation, of the Cree Nation of Nemaska, of the Waskaganish First Nation and of the Cree Board of Health and Social Services of James Bay (Quebec, Canada).
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meriem Ouchfoun and Hoda M. Eid have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ouchfoun, M., Eid, H.M., Musallam, L. et al. Labrador tea (Rhododendron groenlandicum) attenuates insulin resistance in a diet-induced obesity mouse model. Eur J Nutr 55, 941–954 (2016). https://doi.org/10.1007/s00394-015-0908-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0908-z