Abstract
Introduction
A sedentary lifestyle and high-fat feeding are risk factors for cardiometabolic disorders. This study determined whether moderate exercise training prevents the cardiometabolic changes induced by a high-fat diet (HFD).
Materials and methods
Sixty-day-old rats were subjected to moderate exercise three times a week for 30 days. After that, trained rats received a HFD (EXE-HFD) or a commercial normal diet (EXE-NFD) for 30 more days. Sedentary animals also received the diets (SED-HFD and SED-NFD). Food intake and body weight were measured weekly. After 120 days of life, analyses were performed. Data were analysed with two-way ANOVA and the Tukey post-test.
Results
Body weight gain induced by HFD was attenuated in trained animals. HFD reduced food intake by approximately 30 % and increased body fat stores by approximately 75 %. Exercise attenuated 80 % of the increase in fat pads and increased 24 % of soleus muscle mass in NFD animals. HFD induced a hyper-response to glucose injection, and exercise attenuated this response by 50 %. Blood pressure was increased by HFD, and the beneficial effect of exercise in reducing blood pressure was inhibited by HFD. HFD increased vagal activity by 65 % in SED-HFD compared with SED-NFD rats, and exercise blocked this increase. HFD reduced sympathetic activity and inhibited the beneficial effect of exercise on ameliorating sympathetic activity.
Conclusion
Four weeks of moderate exercise at low frequency was able to prevent the metabolic changes induced by a HFD but not the deleterious effects of diet on the cardiovascular system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a major public health problem that can be related to a sedentary lifestyle and a western diet, which is rich in calories including fatty acids [1]. One consequence of obesity is metabolic syndrome, which is defined as a cluster of dysfunctions including central adiposity, dyslipidemia, elevated blood pressure and impaired glucose homeostasis. The clustering of risks factors for metabolic syndrome may depend on stressful factors in early life [2]. The prevalence of metabolic syndrome in young adults, which covers the transition period from adolescence to adulthood, has increased in recent years [3] and may depend on shifts in dietary and lifestyle patterns due to environmental and social pressure [4]. Therefore, strategies for risk factor management are needed in early adulthood, but the preventive effect of physical activity on cardiometabolic outcomes remains unclear.
A high-fat diet (HFD) is linked to many of the metabolic and cardiovascular changes that are characteristic of obesity and related to metabolic syndrome [5, 6]. Animals readily accumulate adipose tissue and develop higher blood pressure and insulin resistance when they consume a short-term HFD [7, 8]. These changes may involve the autonomic nervous system (ANS) [9–11]. Studies have shown that HFD increases plasma norepinephrine, renal sympathetic nerve activity and norepinephrine turnover in the heart, which may lead to cardiovascular changes [8, 12, 13]. Interestingly, vagus nerve activity is increase by HFD [7, 14] suggesting an increase in the activity of the parasympathetic nervous system which may be related to impairment of parasympathetic-dependent insulin action in animals exposed to HFD [15].
Studies have reported the beneficial effects of moderate exercise in preventing obesity and metabolic syndrome. Moderate exercise that is started after weaning appears to induce better protection against metabolic disorders than exercise that is started later in life [16], emphasising the importance of interventions during the period of active brain development [17]. Furthermore, it has been shown that moderate exercise stimulates neuronal plasticity, which may contribute to its protective effect [18–20]. Recently, we demonstrated that the early adulthood period may be susceptible to dietary insults, such as protein restriction which induces long-lasting hallmarks of metabolic malfunctions leading to changes in fat deposition, glucose homeostasis and ANS later in life in rats [21]. Additionally, spontaneous exercise that is performed in early adulthood has also been shown to have a protective effect on metabolism [22].
Most of the studies on the mechanisms underlying the protective effect of exercise evaluate exercise that is performed concurrently with the obesity-inducing factor. However, the long-lasting protective effect of exercise remains controversial [23, 24]. Furthermore, post-weaning exercise benefits are still present when the exercise intensity is low [25]. It is important to consider the duration, frequency and type of exercise that are necessary to obtain the protective and beneficial effects of exercise [26]. We hypothesised that short-term moderate exercise at low frequency performed in young adult rats may provide long-lasting protection against the cardiometabolic changes induced by HFD.
Materials and methods
Experimental model and diet
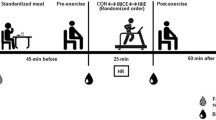
Fifty-day-old male Wistar rats were housed three animals per cage and were provided water and food ad libitum in a room that was maintained at 22 ± 2 °C with a 12/12 h light/dark cycle. After 10 days of environmental adaptation, a group of 60-day-old rats underwent an exercise training protocol for 30 days. The control rats remained sedentary. During this period, all animals were fed a normal-fat diet (NFD). After this period (from 90 to 120 days of life), the animals were fed with a NFD or HFD and remained sedentary. The protocol design is shown in Fig. 1. Four groups were obtained: sedentary rats subjected to a NFD (SED-NFD), sedentary rats subjected to a HFD (SED-HFD), exercised rats subjected to a NFD (EXE-NFD) and exercised rats subjected to a HFD (EXE-HFD). The four groups in the present study included a total of 80 rats: 20 rats for each of the two groups that were fed with a NFD and 20 rats for each of the two groups that were fed with a HFD. All protocols were approved by the Ethics Committee of the State University of Maringá.
The NFD was a commercial diet with 3.801 kcal/g (AIN 93 M, Nuvital-Curitiba, PR), and the HFD was a hypercaloric home-made diet with 5.817 kcal/g and 35 % lard. The composition of the NFD and HFD was described previously [14].
Short-term moderate exercise treadmill protocol
The rats were trained on an animal treadmill (model ET-2000 Insight; RibeirãoPreto, SP, Brazil) three times a week for 4 weeks (12 sessions from 60 to 90 days of life), always in the morning. A plastic ball that was 10 cm in diameter was placed on the back end of the treadmill as a contact stimulus to keep the animal moving. The training started with sessions of 10 m/min for 10 min and finished with sessions of 16 m/min for 60 min by the end of the fourth week. This protocol was modified from a moderate physical exercise protocol proposed by Negrão et al. [27]. The intensity of the training was confirmed using a maximum effort test performed at 58, 75 and 94 days of life, corresponding to the times before, at the middle and at the end of the exercise period. The velocity proposed by the exercise protocol was considered as a percentage of the peak velocity obtained in the maximum effort test for each age. At the three tested ages, 58, 75 and 94 days of life, the proposed protocol velocity was 50, 66 and 68 %, respectively, of the peak velocity obtained in the maximum effort test, confirming the moderate intensity of the training.
Caloric intake and body weight gain
The food was weighed once a week from 60 to 120 days of life. The food intake was calculated as the difference between the amount of food remaining and the total provided, which was divided by the number of days and the number of rats in the box [28]. Because the energetic values between the diets were different, the values in grams were converted into caloric values. The animals were weighed once a week during the experimental period.
Intravenous glucose tolerance test
A silicone cannula was implanted into the right jugular vein of 120-day-old rats, which were under anaesthesia (ketamine/xylazine, 0.5 mg/100 g of body weight each). After 12 h of fasting (from 8 PM to 8 AM), the animals received a bolus of glucose (1 g/kg of body weight). Blood samples were collected via the implanted cannula 0 min prior to the glucose infusion and 5, 15, 30 and 45 min after the infusion. Plasma was used to determine glycaemia via the glucose-oxidase technique (Gold Analisa® Belo Horizonte, MG, Brazil). The glucose responses during the glucose tolerance test were calculated by estimating the total area under the glucose curve using the trapezoidal method [29].
Cardiovascular parameters
After 12 h of fasting, catheters (PE-10) filled with 5 % heparinised saline were implanted into the femoral artery of rats that were anesthetised with thiopental (45 mg/kg of body weight). The arterial cannula was connected to a fluid-filled blood pressure transducer (MLT0699, ADInstruments, Dunedin, New Zealand), which was connected to a signal amplifier (Insight, RibeirãoPreto, SP, Brazil). Direct recordings of the arterial pressure (AP) were performed over 10 min using a microcomputer equipped with an analogue-to-digital converter board (CODAS, 1-kHz sampling frequency, Dataq Instruments, Inc., Akron, OH). Each recording was visualised to select segments without erratic fluctuations and with sufficient duration (1 min) [30]. Analyses were performed on a beat-to-beat basis over two periods of one min per animal to quantify the changes in the mean AP and heart rate (HR) [31].
Parasympathetic and sympathetic activity
After the blood pressure recordings, a longitudinal surgical incision was made on the anterior cervical region of the animals. The left vagus superior branch was isolated and placed over a silver electrode inside a faraday cage, as previously described [6]. After 12 min of vagus nerve electrical recordings, a laparotomy was performed to isolate the branch of the sympathetic nerve that is located in the splanchnic region; this branch originates in the lumbar plexus at the level of L2 and extends to the retroperitoneal adipose tissue.
The neural signal output was acquired using the Insight interface (Insight®, RiberãoPreto, SP, Brazil) for 12 min, from which 20 recorded frames of 5 s from each animal were randomly chosen for spike counting. Spikes >0 mV were considered. The average number of spikes was used as the nerve firing rate.
Evaluation of obesity and muscle mass
After the exercise period, 90-day-old animals were euthanised by decapitation. Blood samples were collected, and the fat pads (retroperitoneal and periepididymal) were removed and weighed. After autonomic nerve recording, 120-day-old anaesthetised animals were euthanised by decapitation. Retroperitoneal and periepididymal fat pads and the right soleus muscle were removed and weighed. The percentage of fat relative to the total animal body weight was used as an estimation of the total fat accumulation; the weight of the soleus muscle was used to determine muscle mass.
Statistical analysis
Data were expressed as the mean ± SEM. GraphPad Prism version 6.01 for Windows (GraphPadSoftware, La Jolla, CA, USA) was used for statistical analyses and developing graphs. Statistical analysis was performed using Student’s t test for animals at 90 days of life, and two-way analysis of variance (ANOVA) followed by the Tukey multiple comparisons test was used for 120-day-old animals. A p value <0.05 was considered significant when considering the main effect of diet (D), exercise (E), their interaction (I; diet vs exercise) and the differences between groups.
Results
Caloric intake and body weight
During the physical exercise period, from 60 to 90 days of life, exercise did not impact caloric consumption. After this period, the HFD animals showed a significant decrease in caloric intake (p d < 0.001; Fig. 2a). At the first week of HFD, the weekly average caloric intake was decreased by 10 %, and this effect persisted until the end of the HFD exposure, at 120 days of life. The physical exercise performed prior to the HFD exposure did not influence energy consumption.
Caloric intake (a) and body weight gain (b) from 60 to 120 days of life (n = 7–13 and 22–30, respectively). SED-NFD sedentary rats subjected to a normal-fat diet, SED-HFD sedentary rats subjected to a high-fat diet, EXE-NFD exercised animals subjected to a normal-fat diet and EXE-HFD exercised animals subjected to a high-fat diet. E exercise factor, D diet factor and I interaction between exercise and diet factors. +++ p < 0.001, ++ p < 0.01 and + p < 0.05 for the probability based on a two-way analysis of variance
Body weight gain was attenuated by exercise only at the last week of the training period, finishing at 90 days of life (Fig. 2b). Furthermore, the HFD induced a greater body weight gain, which was continually attenuated by the prior exercise in EXE-HFD rats after the second week of the diet resulting in a significant diet versus exercise interaction (p i < 0.001).
Fat deposition and muscle mass
Fat deposition was evaluated in 90- and 120-day-old animals (Table 1). At 90 days of life, before the start of the HFD, the EXE animals showed approximately 15 % lower retroperitoneal and periepididymal fat deposition than the SED rats (p < 0.05). At 120 days of life, HFD induced approximately 75 % greater retroperitoneal fat deposition in the SED-HFD and EXE-HFD animals than in those exposed to NFD (p d < 0.001). Interestingly, the previous exercise attenuated the body fat gain in approximately 20 % of the EXE-NFD and EXE-HFD animals compared with the sedentary animals (p e < 0.001). No interaction was observed between diet and exercise (p i = 0.47). The periepididymal fat showed a similar profile. The HFD reduced muscle mass in the sedentary and exercised animals (13 and 26 % in SED-HFD and EXE-HFD compared with SED-NFD and EXE-NFD, respectively, p d < 0.001). Interestingly, exercise increased only the muscle mass in the EXE-NFD animals (24 % increase compared with SED-NFD, p < 0.01), leading to a significant diet versus exercise interaction (p i < 0.05).
Glycaemia and insulinaemia
Fasting glycaemia was not affected by exercise or HFD (Table 1). During the ivGTT, the SED-HFD animals showed greater glycaemia (Fig. 3a) and insulinaemia (Fig. 3c) over the 45 min after the glucose bolus injection. This pattern led to an increase in the AUC of glycaemia (SED-HFD vs SED-NFD: 41 %; EXE-HFD vs EXE-NFD: 22 %; p d < 0.001; Fig. 3b) and insulinaemia (SED-HFD vs SED-NFD: 69 %; EXE-HFD vs EXE-NFD: 13 %; p d < 0.001; Fig. 3d). Interestingly, the previous exercise attenuated the AUC of glycaemia by 16 % and insulinaemia by 50 % in the EXE-HFD compared with the SED-HFD animals, which led to a significant exercise versus diet interaction (glycaemia: p i < 0.05; insulinaemia: p i < 0.001).
Glucose curve and area under curve for glycaemia (a, b) and insulinaemia (c, d) evaluated during ivGTT in 120-day-old rats (n = 18–26 per group). SED-NFD sedentary rats subjected to a normal-fat diet, SED-HFD sedentary rats subjected to a high-fat diet, EXE-NFD exercised animals subjected to a normal-fat diet and EXE-HFD exercised animals subjected to a high-fat diet. E exercise factor, D diet factor and I interaction between exercise and diet factors. +++ p < 0.001, ++ p < 0.01 and + p < 0.05 for the probability based on a two-way analysis of variance. ***p < 0.001 and *p < 0.05 statistical significance of the differences between NFD and HFD, ## p < 0.01 and ### p < 0.001 statistical significance of sedentary versus exercised animals for the probability based on a Tukey multiple comparisons test
Blood pressure
The SED-HFD and EXE-HFD animals showed 7 and 26 % greater systolic blood pressure than the SED-NFD and EXE-HFD animals, respectively (Fig. 4a). A similar pattern was observed in diastolic blood pressure (Fig. 4b). This pattern was evidenced by the main effect of HFD on increased blood pressure (p d < 0.001). Interestingly, previous exercise reduced systolic and diastolic blood pressure by 12 % only in the EXE-NFD compared with the SED-NFD animals (Tukey post-test: p < 0.01), but the HFD inhibited this reduction in the EXE-HFD animals. This pattern was reflected by a significant diet versus exercise interaction (p i < 0.01; Fig. 4a, b).
Systolic blood pressure (a), diastolic blood pressure (b) and heart rate (c) in 120-day-old rats (n = 7–14 per group). NFD normal-fat diet, HFD high-fat diet, EXE exercised animals, SED sedentary animals, E exercise factor, D diet factor and I interaction between exercise and diet factors. +++ p < 0.001, ++ p < 0.01 and ns (not significant) for the probability based on a two-way analysis of variance. ***p < 0.001 and **p < 0.01 statistical significance of the differences between NFD and HFD. ### p < 0.001 and ## p < 0.01 statistical significance of sedentary versus exercised animals for the probability based on a Tukey multiple comparisons test
The HFD increased HR by 18 % (p d < 0.01). The previous physical exercise did not affect the HR (Fig. 4c).
Parasympathetic and sympathetic activity
The HFD increased the vagal tone by 65 % in the SED-HFD animals (Tukey post-test: p < 0.001; Fig. 5a). However, previous exercise blocked the HFD-induced increase in vagal activity in the EXE-HFD animals leading to a significant diet versus exercise interaction (p i < 0.01).
Parasympathetic (a) and sympathetic (b) nerve activity evaluated in 120-day-old rats (n = 15–17 and 12–17, respectively, per group). NFD normal-fat diet, HFD high-fat diet, EXE exercised animals, SED sedentary animals, E exercise factor, D diet factor and I interaction between exercise and diet factors. ***p < 0.001 statistical significance of the differences between NFD and HFD. ### p < 0.001 and ## p < 0.01 statistical significance of sedentary versus exercised animals for the probability based on a Tukey multiple comparisons test. +++ p < 0.001, ++ p < 0.01, + p < 0.05 and ns (not significant) for the probability based on a two-way analysis of variance
The sympathetic nerve activity was reduced in the HFD-fed animals (p d < 0.001; Fig. 5b) and increased in the exercised animals (p e < 0.001; Fig. 5b). The exercise-induced increase in sympathetic activity was attenuated by 50 % in the EXE-HFD animals leading to a significant diet versus exercise interaction (p i < 0.05).
Discussion
The major finding of this study was that previous exercise that was performed during young adulthood protected metabolism but not the cardiovascular system against the deleterious effects of a HFD. Earlier studies have shown that performing exercise during the post-weaning period protects animals from developing the full obesity phenotype [32, 33]. These and other studies suggested that the long-lasting protective effect of exercise performed early in life on energy homeostasis may implicate the nervous system via both their function and their organisation [7, 18]. Interestingly, the present findings suggest that the young adulthood period may also offer long-lasting beneficial effects from exercise.
The rats that were pre-exposed to exercise during their young adult life showed attenuated body weight gain and reduced body fat deposition after consuming a HFD. These findings contrast with a study that used a genetic model of obesity with congenital deficiency of the cholecystokinin 1 receptor (implicated in satiety), the Otsuka Long-Evans Tokushima fatty (OLETF) rats, which are already overweight at 8 weeks of life [22]. In that study, Chao et al. [22] showed that the long-lasting protective effect of spontaneous exercise, which was performed during a period similar to that of the present study, was no longer evident when the animals consumed a HFD. The animals that consumed a HFD after exercise regained their body weight and fat deposits; this effect may depend on hyperphagic behaviour that is modulated centrally [22]. Conversely, in the present study, the HFD animals showed reduced food intake (data not shown) and reduced energy intake, which is consistent with previous studies from our group and others [14, 34]. It has been proposed that rats fed with a HFD gain more weight even if energetic intake is not high, and the high amount of lipids in the diet are arguably one of the most influential factors for the induction of obesity [35, 36]. The balance between intake and oxidation is not accurate for fat [37], which may contribute to body weight gain as well. Interestingly, the present results show a greater body weight gain and fat deposition in animals exposed to HFD. Furthermore, the present reduction in energy intake observed in the HFD animals was independent of previous exercise, suggesting that the present beneficial effect of previous exercise on body weight and fat deposition may depend on factors other than energy intake.
Rinaldi et al. [38] described the chronic effect of exercise on increases lean mass by skeletal muscle hypertrophy. In this context, the current work shows that previous exercise in animals exposed to NFD increases muscle mass but that HFD exposure reduced the muscle mass increased by exercise. This pattern was associated with reduced fat deposits in EXE-HFD animals and may contribute to their reduced body weight gain compared with SED-HFD animals. In contrast, the control EXE-NFD animals did not show changes in body weight gain, with reductions in fat deposits and increased soleus mass. These findings suggest that the balance between lean and fat mass may be determinant of body weight gain. Sasaki et al. [39] also evaluated the best combination of daily timing of HFD feeding and aerobic exercise. They showed that HFD eating followed by exercise minimised increases in body and fat weight while increases in skeletal muscle weight were maximised [39]. The divergent findings in both studies should take into consideration animal age and duration of exercise and diet, as well the order of treatment introduction.
Previous studies show that 2 days of HFD-induced insulin hyper-secretion partially related to a decreased sympathetic tone, which was followed by glucose intolerance 7 days after the diet and persisted until 8 weeks of HFD exposure [40]. The present results are in accordance with those of that study as 4 weeks of HFD induced greater glycaemia and insulinaemia in response to glucose bolus at 4 weeks with HFD. Interestingly, the present findings show that the exercise programming protected against HFD-induced changes in glucose tolerance and hyperinsulinaemia. These findings are consistent with a recent study showing that mice subjected to exercise from 9 weeks of life had improved glucose tolerance at 20 weeks of life [41]. Furthermore, the preventive effect against the development of non-insulin-dependent diabetes mellitus has been shown to last for at least 3 months after the cessation of exercise in OLETF rats [42]. Conversely, exercised obese OLETF rats that consumed a HFD became glucose intolerant and insulin insensitive to the same degree as the sedentary OLETF rats on a HFD [22]. Together, these studies suggest that the protective effect of exercise is more evident when the exercise exposure happens before obesity. Early life exercise training in rats that were born small, which suggests a tendency for developing diabetes, restored beta-cell mass in adulthood [43]. Additionally, previous studies from our group have shown that the beneficial effect of exercise on glucose metabolism may depend on an improvement in pancreatic islet function and ANS activity in different models of metabolic syndrome [7, 25, 44, 45].
Previously, we showed that exercise performed early in life ameliorates the balance between autonomic nerve activity and catecholamine content in the adrenal gland in a model of programmed metabolic syndrome with monosodium l-glutamate (MSG) [44, 45]. Additionally, this benefit of exercise on the ANS may last for several weeks [16] with increased sympathetic and reduced parasympathetic nerve activity related to metabolism regulation in obese MSG rats that performed exercise during post-weaning and the pubertal period (unpublished data). This pattern is also observed in the present study in which Wistar rats exposed to exercise training during the young adulthood period showed a long-lasting protective effect against HFD-induced autonomic changes. Barella et al. [14] showed that a HFD increases vagus nerve activity independent of the period of life when the diet is offered. Recently, we showed that the present exercise protocol starting post-weaning and lasting until early adulthood restores vagus nerve activity in animals exposed to HFD [7]. Interestingly, the present findings show that this pattern is maintained when the exercise training is performed in early adulthood and followed by a HFD, reinforcing the long-lasting beneficial effect of this exercise training on glucose metabolism.
It is important to consider that in contrast to the present findings, several studies focused on the implication of the ANS on hypertension related to obesity have suggested that the sympathetic nervous system is increased and the parasympathetic nervous system inhibited by HFD [8, 12, 46, 47]. Interestingly, previous studies suggest that obesity induced by HFD is associated with the preservation of leptin’s ability to increase blood pressure despite the resistance to the metabolic effects of leptin [48, 49]. They showed that the renal sympathetic nervous activity response to intracerebroventricular leptin was preserved, whereas lumbar and brown adipose tissue sympathetic nervous activity responses were attenuated in rats fed a HFD, suggesting a regional sympathetic sensitivity to leptin [48, 49] which may implicate the brain renin angiotensin system to selectively facilitate renal sympathetic nerve responses to leptin while sparing effects on food intake [50].
The current study also shows that a HFD increases blood pressure and HR; these findings were previously demonstrated in different animal models [6, 8, 12]. In contrast to the present findings on metabolism and the data from the literature [51], exercise did not protect the animals from the HFD-induced deleterious effect on cardiovascular parameters. Recent studies have shown that performing exercise concomitant with the consumption of a high-fructose diet, which leads to hypertension, prevented cardiovascular disorders [52–54]. Furthermore, long-term exercise (up to 10 weeks) showed a long-lasting protective effect on the cardiovascular and autonomic dysfunction observed in streptozotocin-diabetic or infarcted rats, thus also reducing mortality [55–57]. This beneficial effect of exercise on cardiovascular parameters may indicate a role of central mechanisms and their modulation of the ANS, mainly the parasympathetic nervous system [56, 58, 59]. Neurotropic factors, mainly brain-derived neurotrophic factor, whose levels are increased centrally by exercise and remain elevated [60, 61], may modulate the ANS control of blood pressure and HR via central baroreflex pathways [19, 59]. The present study showed a beneficial effect of exercise on blood pressure only in the control animals. However, a protective effect of exercise on the HFD-induced disturbance of blood pressure and HR was not observed. The deleterious HFD-induced effect may indicate a role of central pathways and their capacity to stimulate the kidney and muscles through the sympathetic nervous system [10, 12, 62]; this effect may predominate over the protective cardiovascular pathways modulated by exercise. Furthermore, 4 weeks of moderate exercise at low frequency may not be sufficient to initiate the long-lasting cardiovascular protection mechanism as most benefits of exercise have been associated with longer-term training [63].
In conclusion, a HFD disturbs metabolism and the cardiovascular system, but 4 weeks of moderate exercise at low frequency is able to protect against metabolic dysfunctions induced by a dietary insult. The mechanism underlying the long-lasting protection of glucose metabolism and body weight may depend, at least in part, on the ANS. The benefits of this exercise pattern are not sufficiently strong to protect the cardiovascular system against the HFD insult. Thus, the ideal physical exercise that will lead to a long-lasting protective effect against cardiometabolic syndrome remains unclear; however, early adult life may be a susceptible period during which permanent preventive actions can be established.
References
Casazza K, Dulin-Keita A, Gower BA, Fernandez JR (2009) Differential influence of diet and physical activity on components of metabolic syndrome in a multiethnic sample of children. J Am Diet Assoc 109(2):236–244
Lopez-Jaramillo P, Lahera V, Lopez-Lopez J (2011) Epidemic of cardiometabolic diseases: a Latin American point of view. Ther Adv Cardiovasc Dis 5(2):119–131
Ervin RB (2009) Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Rep 5(13):1–7
Deshmukh-Taskar PR, O’Neil CE, Nicklas TA, Yang SJ, Liu Y, Gustat J et al (2009) Dietary patterns associated with metabolic syndrome, sociodemographic and lifestyle factors in young adults: the Bogalusa heart study. Public Health Nutr 12(12):2493–2503
Buettner R, Scholmerich J, Bollheimer LC (2007) High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity 15(4):798–808
pBarnes MJ, Lapanowski K, Conley A, Rafols JA, Jen KL, Dunbar JC (2003) High fat feeding is associated with increased blood pressure, sympathetic nerve activity and hypothalamic mu opioid receptors. Brain Res Bull 61(5):511–519
Gomes RM, Tofolo LP, Rinaldi W, Scomparin DX, Grassiolli S, Barella LF et al (2013) Moderate exercise restores pancreatic beta-cell function and autonomic nervous system activity in obese rats induced by high-fat diet. Cell Physiol Biochem 32(2):310–321
Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP et al (2010) Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 55(4):862–868
Wang H, Storlien LH, Huang XF (1999) Influence of dietary fats on c-Fos-like immunoreactivity in mouse hypothalamus. Brain Res 843(1–2):184–192
Chen F, Cham JL, Badoer E (2010) High-fat feeding alters the cardiovascular role of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 298(3):R799–R807
Matsuo T, Iwashita S, Komuro M, Suzuki M (1999) Effects of high-fat diet intake on glucose uptake in central and peripheral tissues of non-obese rats. J Nutr Sci Vitaminol 45(5):667–673
Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K et al (2012) Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension 60(1):163–171
Schwartz JH, Young JB, Landsberg L (1983) Effect of dietary fat on sympathetic nervous system activity in the rat. J Clin Investig 72(1):361–370
Barella LF, de Oliveira JC, Branco RC, Camargo RL, Gomes RM, Mendes FC et al (2012) Early exposure to a high-fat diet has more drastic consequences on metabolism compared with exposure during adulthood in rats. Horm Metab Res 44(6):458–464
Afonso RA, Lautt WW, Schafer J, Legare DJ, Oliveira AG, Macedo MP (2010) High-fat diet results in postprandial insulin resistance that involves parasympathetic dysfunction. Br J Nutr 104(10):1450–1459
Scomparin DX, Grassiolli S, Marcal AC, Gravena C, Andreazzi AE, Mathias PC (2006) Swim training applied at early age is critical to adrenal medulla catecholamine content and to attenuate monosodium l-glutamate-obesity onset in mice. Life Sci 79(22):2151–2156
Levin BE (2005) Factors promoting and ameliorating the development of obesity. Physiol Behav 86(5):633–639
Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR et al (2006) Neurobiology of exercise. Obesity 14(3):345–356
Rothman SM, Griffioen KJ, Wan R, Mattson MP (2012) Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann NY Acad Sci 1264(1):49–63
Wang Q, Wang M, Whim MD (2013) Neuropeptide y gates a stress-induced, long-lasting plasticity in the sympathetic nervous system. J Neurosci 33(31):12705–12717
Malta A, de Oliveira JC, Ribeiro TA, Tofolo LP, Barella LF, Prates KV, et al. (2014). Low protein diet in adult male rats has long term effects on metabolism. J Endocrinol 221(2):285–295
Chao PT, Terrillion CE, Moran TH, Bi S (2011) High-fat diet offsets the long-lasting effects of running-wheel access on food intake and body weight in OLETF rats. Am J Physiol Regul Integr Comp Physiol 300(6):R1459–R1467
Yasari S, Paquette A, Charbonneau A, Gauthier MS, Savard R, Lavoie JM (2006) Effects of ingesting a high-fat diet upon exercise-training cessation on fat accretion in the liver and adipose tissue of rats. Appl Physiol Nutr Metab 31(4):367–375
Applegate EA, Upton DE, Stern JS (1984) Exercise and detraining: effect on food intake, adiposity and lipogenesis in Osborne–Mendel rats made obese by a high fat diet. J Nutr 114(2):447–459
Scomparin DX, Grassiolli S, Gomes RM, Torrezan R, de Oliveira JC, Gravena C et al (2011) Low-Intensity swimming training after weaning improves glucose and lipid homeostasis in MSG hypothalamic obese mice. Endocr Res 36(2):83–90
Achten J, Jeukendrup AE (2004) Optimizing fat oxidation through exercise and diet. Nutrition 20(7–8):716–727
Negrao CE, Moreira ED, Santos MC, Farah VM, Krieger EM (1992) Vagal function impairment after exercise training. J Appl Physiol 72(5):1749–1753
Vicente LL, de Moura EG, Lisboa PC, Monte Alto Costa A, Amadeu T, Mandarim-de-Lacerda CA et al (2004) Malnutrition during lactation in rats is associated with higher expression of leptin receptor in the pituitary of adult offspring. Nutrition 20(10):924–928
Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA et al (2012) Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes 61(6):1455–1462
Baudrie V, Laude D, Elghozi JL (2007) Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am J Physiol Regul Integr Comp Physiol 292(2):R904–R912
Palma-Rigo K, Baudrie V, Laude D, Elghozi JL (2010) Cardiovascular rhythms and cardiac baroreflex sensitivity in AT1A receptor gain-of function mutant mice. Hypertension 55:1507–1508
Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH (2005) Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146(4):1676–1685
Patterson CM, Dunn-Meynell AA, Levin BE (2008) Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol 294(2):R290–R301
Borst SE, Conover CF (2005) High-fat diet induces increased tissue expression of TNF-alpha. Life Sci 77(17):2156–2165
Sclafani A (2001) Psychobiology of food preferences. Int J Obes Relat Metab Disor 25(Suppl 5):S13–S16
Warwick ZS, Schiffman SS (1992) Role of dietary fat in calorie intake and weight gain. Neurosci Biobehav Rev 16(4):585–596
Schutz Y, Flatt JP, Jequier E (1989) Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Am J Clin Nutr 50(2):307–314
Rinaldi B, Donniacuo M, Sodano L, Gritti G, Signoriello S, Parretta E et al (2013) Effects of sildenafil on the gastrocnemius and cardiac muscles of rats in a model of prolonged moderate exercise training. PLoS One 8(7):e69954
Sasaki H, Ohtsu T, Ikeda Y, Tsubosaka M, Shibata S (2014) Combination of meal and exercise timing with a high-fat diet influences energy expenditure and obesity in mice. Chronobiol Int 9:1–17
Cruciani-Guglielmacci C, Vincent-Lamon M, Rouch C, Orosco M, Ktorza A, Magnan C (2005) Early changes in insulin secretion and action induced by high-fat diet are related to a decreased sympathetic tone. Am J Physiol Endocrinol Metab 288(1):E148–E154
Wagener A, Schmitt AO, Brockmann GA (2012) Early and late onset of voluntary exercise have differential effects on the metabolic syndrome in an obese mouse model. Exp Clin Endocrinol Diabetes 120(10):591–597
Shima K, Shi K, Mizuno A, Sano T, Ishida K, Noma Y (1996) Exercise training has a long-lasting effect on prevention of non-insulin-dependent diabetes mellitus in Otsuka-Long-Evans-Tokushima Fatty rats. Metabolism 45(4):475–480
Laker RC, Gallo LA, Wlodek ME, Siebel AL, Wadley GD, McConell GK (2011) Short-term exercise training early in life restores deficits in pancreatic beta-cell mass associated with growth restriction in adult male rats. Am J Physiol Endocrinol Metab 301(5):E931–E940
Andreazzi AE, Scomparin DX, Mesquita FP, Balbo SL, Gravena C, De Oliveira JC et al (2009) Swimming exercise at weaning improves glycemic control and inhibits the onset of monosodium l-glutamate-obesity in mice. J Endocrinol 201(3):351–359
Scomparin DX, Gomes RM, Grassiolli S, Rinaldi W, Martins AG, de Oliveira JC et al (2009) Autonomic activity and glycemic homeostasis are maintained by precocious and low intensity training exercises in MSG-programmed obese mice. Endocrine 36(3):510–517
Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ Jr (1995) Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol 269(2 Pt 2):H629–H637
Pelat M, Verwaerde P, Lazartiques E, Cabrol P, Galitzky J, Berlan M et al (1998) Variabilite temporelle et frequentielle de la pression arterielle et de la frequence cardiaque au cours du nycthemere dans un modele experimental d’hypertension arterielle avec obesite [Twenty-four hour time and frequency domain variability of systolic blood pressure and heart rate in an experimental model of arterial hypertension plus obesity]. Arch Mal Coeur Vaiss 91(8):999–1002
Morgan DA, Thedens DR, Weiss R, Rahmouni K (2008) Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol 295(6):R1730–R1736
Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG (2005) Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54(7):2012–2018
Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL et al (2012) A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol 303(2):H197–H206
Himeno E, Nishino K, Okazaki T, Nanri H, Ikeda M (1999) A weight reduction and weight maintenance program with long-lasting improvement in left ventricular mass and blood pressure. Am J Hypertens 12(7):682–690
Moraes-Silva IC, Mostarda C, Moreira ED, Silva KA, dos Santos F, de Angelis K et al (2013) Preventive role of exercise training in autonomic, hemodynamic, and metabolic parameters in rats under high risk of metabolic syndrome development. J Appl Physiol 114(6):786–791
Morvan E, Lima NE, Machi JF, Mostarda C, De Angelis K, Irigoyen MC et al (2013) Metabolic, hemodynamic and structural adjustments to low intensity exercise training in a metabolic syndrome model. Cardiovasc Diabetol 12(1):89
Mostarda C, Moraes-Silva IC, Salemi VM, Machi JF, Rodrigues B, De Angelis K et al (2012) Exercise training prevents diastolic dysfunction induced by metabolic syndrome in rats. Clinics 67(7):815–820
Barboza CA, Rocha LY, Mostarda CT, Figueroa D, Caperuto EC, De Angelis K et al (2013) Ventricular and autonomic benefits of exercise training persist after detraining in infarcted rats. Eur J Appl Physiol 113(5):1137–1146
Mostarda C, Rogow A, Silva IC, De La Fuente RN, Jorge L, Rodrigues B et al (2009) Benefits of exercise training in diabetic rats persist after three weeks of detraining. Auton Neurosci 145(1–2):11–16
Silva KA, da Silva Luiz R, Rampaso RR, de Abreu NP, Moreira ED, Mostarda CT et al (2012) Previous exercise training has a beneficial effect on renal and cardiovascular function in a model of diabetes. PLoS One 7(11):e48826
Agarwal D, Dange RB, Vila J, Otamendi AJ, Francis J (2012) Detraining differentially preserved beneficial effects of exercise on hypertension: effects on blood pressure, cardiac function, brain inflammatory cytokines and oxidative stress. PLoS One 7(12):e52569
Mattson MP, Wan R (2008) Neurotrophic factors in autonomic nervous system plasticity and dysfunction. Neuromolecular Med 10(3):157–168
Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW (1996) Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726(1–2):49–56
Russo-Neustadt A, Beard RC, Cotman CW (1999) Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology 21(5):679–682
Hasegawa A (1991) Innervation of skeletal muscle by the lumbar sympathetic nervous system. Nihon Seikeigeka Gakkai zasshi 65(5):368–381
Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30(9):464–472
Acknowledgments
This research was supported by Grants from the Brazilian Research National Foundation: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) by receipt of the scholarship from Ciências em Fronteiras Program (Process No. 10040/13-7) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPQ.
Conflict of interest
The author declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tófolo, L.P., da Silva Ribeiro, T.A., Malta, A. et al. Short-term moderate exercise provides long-lasting protective effects against metabolic dysfunction in rats fed a high-fat diet. Eur J Nutr 54, 1353–1362 (2015). https://doi.org/10.1007/s00394-014-0816-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0816-7