Abstract
Objective
This study investigated whether circulating cold-inducible RNA-binding protein (CIRP) could be a biomarker to reflect the current activity, function, and damage status in patients with microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA).
Methods
This study selected 39 MPA and 26 GPA patients. Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV)-specific indices include the Birmingham Vasculitis Activity Index (BVAS), five-factor score (FFS), the Korean version of the Short-Form 36-Item Health Survey (SF-36) physical component summary (PCS) and mental component summary (MCS), and the vasculitis damage index (VDI). The highest tertile of BVAS was defined as high activity of AAV.
Results
The median age of the study subjects was 65.0 years and 53.8% were women. The median BVAS, FFS, SF-36 PCS, MCS, and VDI scores were 12.0, 2.0, 47.5, 50.3, and 3.0, respectively. The median circulating CIRP level was 6.4 ng/mL. Among the four AAV-specific indices, circulating CIRP was significantly correlated with BVAS (r = 0.256). Using the receiver operator characteristic curve, the cut-off of circulating CIRP for high activity of AAV was 6.16 ng/mL. High activity of AAV was identified more frequently in patients with circulating CIRP ≥ 6.16 ng/mL than in those with circulating CIRP < 6.16 ng/mL (48.6% vs. 21.4%). In addition, patients with circulating CIRP ≥ 6.16 ng/mL exhibited a significantly higher risk for high activity of AAV than those with circulating CIRP < 6.16 ng/mL (relative risk 3.474).
Conclusion
This study suggests the clinical potential of circulating CIRP as a biomarker for reflecting the current BVAS and predicting high activity of AAV in patients with MPA and GPA.
Zusammenfassung
Ziel
In der vorliegenden Studie wurde untersucht, ob zirkulierendes kälteinduzierbares RNA-bindendes Protein (CIRP) ein Biomarker sein könnte, der die aktuelle Aktivität, Funktionsfähigkeit und den Schädigungsstatus bei Patienten mit einer mikroskopischen Polyangiitis (MPA) und Granulomatose mit Polyangiitis (GPA) widerspiegelt.
Methoden
Für diese Studie wurden 39 MPA- und 26 GPA-Patienten ausgewählt. Zu den Indizes, die für eine mit antineutrophilen zytoplasmatischen Antikörpern (ANCA-)assoziierte Vaskulitis (AAV-)spezifisch sind, gehören der Birmingham Vasculitis Activity Index (BVAS), Fünf-Faktor-Score (FFS), die koreanische Version des Short-Form 36-Item Health Survey (SF-36) mit seiner körperlichen Komponente („physical component summary“, PCS) und seiner psychischen Komponente („mental component summary“, MCS) und der Vasculitis Damage Index (VDI). Das höchste Terzil des BVAS wurde definiert als höchste Aktivität der AAV.
Ergebnisse
Das Alter der Studienteilnehmer lag im Median bei 65,0 Jahren; 53,8 % der Teilnehmer waren Frauen. Die medianen Scores für BVAS, FFS, SF-36 PCS, MCS und VDI betrugen 12,0; 2,0; 47,5; 50,3 bzw. 3,0. Der mediane Wert für zirkulierendes CIRP lag bei 6,4 ng/ml. Bei den 4 AAV-spezifischen Indizes war zirkulierendes CIRP signifikant mit dem BVAS korreliert (r = 0,256). Unter Verwendung der ROC-Kurve („receiver operator characteristic curve“) betrug der Grenzwert des zirkulierenden CIRP für hohe Aktivität der AAV 6,16 ng/ml. Eine hohe Aktivität der AAV wurde häufiger bei Patienten mit Werten ≥ 6,16 ng/ml für zirkulierendes CIRP als bei Patienten mit Werten < 6,16 ng/ml für zirkulierendes CIRP festgestellt (48,6 vs. 21,4 %). Darüber hinaus wiesen Patienten mit Werten ≥ 6,16 ng/ml für zirkulierendes CIRP ein signifikant höheres Risiko für eine hohe Aktivität der AAV auf als Patienten mit Werten < 6,16 ng/ml für zirkulierendes CIRP (relatives Risiko 3,474).
Schlussfolgerung
Die vorliegende Studie liefert Hinweise auf das klinische Potenzial von zirkulierendem CIRP als Biomarker, der den aktuellen BVAS widerspiegelt und eine hohe Aktivität der AAV bei Patienten mit MPA and GPA vorhersagt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold-inducible RNA-binding protein (CIRP) is a universal stress-response protein, and its expression may be regulated by hypoxia, ultraviolet radiation, glucose starvation, and oxygen radicals in addition to cold shock [1]. Intracellular CIRP may play an important role in anti-apoptotic processes and cell protection in response to stress through stabilizing mRNAs and facilitating their translation [2]. Concurrently, secreted extracellular CIRP (circulating CIRP) participates in the proinflammatory process via Toll-like receptor 4/myeloid differentiation 2 (TLR4/MD2)-mediated and triggering receptor expressed on myeloid cell‑1 (TREM-1)-mediated intracellular inflammatory signaling [3]. To date, roles of circulating CIRP in various inflammatory diseases such as rheumatoid arthritis, ulcerative colitis, sepsis, acute lung injury, and cardiac ischemia have been reported [4]. Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a representative small-vessel vasculitis with histopathological features exhibiting necrotizing vasculitis affecting mainly small-sized vessels including capillaries, adjacent arterioles, and venules. In addition, AAV may occasionally initiate inflammatory processes in medium-sized arteries [5, 6]. It can be assumed that circulating CIRP may be associated with the activity, function, and damage status of AAV, given that TLR4 and TREM‑1 have been reported to be involved in the pathogenesis of AAV [7, 8]. However, there have been no studies on the clinical role of circulating CIRP in patients with AAV to date. Hence, this study investigated whether circulating CIRP could be a biomarker to reflect the current activity, function, and damage status in patients with microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA).
Materials and methods
Study population
This study selected 65 microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA) patients who met the inclusion criteria described below from the Severance Hospital ANCA-Associated Vasculitides (SHAVE) cohort (a prospective observational cohort of patients with MPA, GPA, and (eosinophilic granulomatosis with polyangiitis) EGPA). The inclusion criteria were as follows: i) the first classification of MPA and GPA at the Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, and Severance Hospital; ii) fulfilment of both the 2007 European Medicines Agency algorithms for AAV and polyarteritis nodosa, and the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides [5, 6]; iii) reclassified as having MPA and GPA according to the 2022 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) classification criteria for MPA and GPA [9, 10]; iv) well-documented medical records regarding clinical and laboratory data at diagnosis including AAV subtype, ANCA positivity, AAV-specific indices, the systemic items belonging to Birmingham Vasculitis Activity Score (BVAS), and routine laboratory results; v) absence of serious medical conditions such as malignancies, infectious diseases requiring hospitalization, and systemic and autoimmune diseases other than AAV; and vi) no exposure to glucocorticoids more than 20 mg/day or immunosuppressive drugs for AAV treatment within the first year prior to AAV diagnosis. This study was approved by the Institutional Review Board (IRB) of Severance Hospital (4-2016-0901) and, when required, written informed consent was obtained from patients at the time of blood sampling. The IRB waived the need for written informed consent when it had been previously obtained at entry into the SHAVE cohort.
AAV-specific indices and blood collection
AAV-specific indices include BVAS version 3 [11], five-factor score (FFS) [12], the Korean version of the Short-Form 36-Item Health Survey (SF-36) physical component summary (PCS) and mental component summary (MCS) [13], and the vasculitis damage index (VDI) [14]. Whole blood was obtained from patients who provided consent on the same day that the assessments of AAV-specific indices were completed. Serum was immediately isolated from whole blood and stored at −80 °C. In this study, AAV-specific indices and serum stored at AAV diagnosis were used.
Clinical and laboratory data
Clinical and laboratory data were collected as described in Table 1. The titers of myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA were measured using an immunoassay, the novel anchor-coated highly sensitive (hs) Phadia ELiA (ThermoFisher Scientific/Phadia, Freiburg, Germany) and human native antigens, on the Phadia250 analyzer. An immunoassay for MPO-ANCA and PR3-ANCA is recommended as the first screening method for ANCA [15]; however, perinuclear (P)-ANCA or cytoplasmic (C)-ANCA positivity was also accepted as ANCA positivity according to the 2022 ACR/EULAR classification criteria for MPA and GPA [9, 10].
High activity of AAV
The highest tertile of BVAS was defined as high activity of AAV.
Measurement of circulating CIRP
Circulating CIRP was quantified in stored sera using enzyme-linked immunosorbent assay kits (MBL, Nagoya, Japan) according to the manufacturer’s instructions.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as medians with interquartile ranges, whereas categorical variables were expressed as numbers (percentages). The correlation coefficient (r) between the two variables was obtained using Pearson correlation analysis. The significant area under the curve (AUC) was confirmed and the optimal cut-off was extrapolated by performing a receiver operator characteristic (ROC) curve analysis. The value of circulating CIRP with the maximized sum of sensitivity and specificity for the lower limit of the highest tertile of BVAS was selected as its cut-off for high activity of AAV. The relative risk (RR) of the cut-off for high activity of AAV was analyzed using contingency tables and the chi-square test. P-values ≤ 0.05 were considered statistically significant.
Results
Characteristics
The median age of the study subjects was 65.0 years and 53.8% were women. Among the 65 patients, 39 and 26 had MPA and GPA, respectively. MPO-ANCA (or P‑ANCA) and PR3-ANCA (or C‑ANCA) were positive in 47 and 13 patients, respectively. The median BVAS, FFS, SF-36 PCS, MCS, and VDI scores were 12.0, 2.0, 47.5, 50.3, and 3.0, respectively. The most common systemic item defined by the form of BVAS was pulmonary manifestation (66.2%), followed by renal manifestation (61.5%). Median erythrocyte sedimentation rate (ESR), C‑reactive protein (CRP) level, and circulating CIRP were 57.0 mm/h, 7.0 mg/L, and 6.4 ng/mL, respectively (Table 1).
Correlations
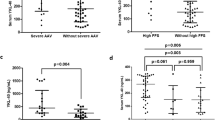
Circulating CIRP was significantly correlated with BVAS (r = 0.256, P = 0.039). However, circulating CIRP was not correlated with other AAV-specific indices or acute-phase reactants, including ESR and CRP (Fig. 1).
Cut-off and relative risk
Using the ROC curve, the cut-off of circulating CIRP for high activity of AAV was 6.16 ng/mL; the sensitivity and specificity were 75.0 and 53.7%, respectively. When patients were divided into two groups according to circulating CIRP ≥ 6.16 ng/mL, 37 of the 65 patients were assigned to the group with circulating CIRP ≥ 6.16 ng/mL. High activity of AAV was identified more frequently in patients with circulating CIRP ≥ 6.16 ng/mL than in those with circulating CIRP < 6.16 ng/mL (48.6% vs. 21.4%, P = 0.024). In addition, patients with circulating CIRP ≥ 6.16 ng/mL exhibited a significantly higher risk for high activity of AAV than those with circulating CIRP < 6.16 ng/mL (RR 3.474, 95% confidence interval 1.146, 10.533; Fig. 2).
ROC curve and relative risk. a ROC curve of circulating CIRP based on high activity of AAV (BVAS ≥ 16), b Relative risk of the cut-off of CIRP for high activity of AAV (BVAS ≥ 16). ROC receiver operator characteristic, CIRP cold-inducible RNA-binding protein, AAV antineutrophil cytoplasmic antibody-associated vasculitis, BVAS Birmingham vasculitis activity score
Discussion
This study is the first to demonstrate that circulating CIRP could reflect the current BVAS and predict high activity of AAV in patients with MPA and GPA. However, circulating CIRP was not significantly correlated with other AAV-specific indices or acute-phase reactants, whereas BVAS was significantly correlated with SF-36 PCS (r = −0.340, P = 0.006), SF-36 MCS (r = −0.344, P = 0.05), ESR (r = 0.472, P < 0.001), and CRP (r = 0.342, P = 0.005). These results suggest that circulating CIRP reflects BVAS through rather complex and diverse mechanisms.
This study suggests several possibilities given several shared roles in the inflammatory processes between circulating CIRP and AAV pathophysiology, although the direct mechanism by which circulating CIRP is correlated with BVAS remains uncertain. The first shared mechanism is the upregulated production of various proinflammatory cytokines and chemokines [16]. Circulating CIRP was reported to promote the production of tumor necrosis factor‑α, interleukin-1β, interleukin‑6, and C‑X‑C motif chemokine ligand 2, which initiate and aggravate the inflammatory response and recruit neutrophils into inflamed tissues [17]. These cytokines may play an important role in not only ANCA formation but also in neutrophil priming in AAV pathogenesis [16]. The second shared mechanism is neutrophil extracellular trap (NET) formation [18]. Circulating CIRP has been reported to induce neutrophil extracellular trap (NET) formation in patients with sepsis and acute pancreatitis [19, 20]. Subsequently, circulating CIRP-induced NET formation might deteriorate phagocytic clearance of apoptotic cells by macrophages [21], leading to a vicious circle. The third shared mechanism is endothelial dysfunction [16]. It was reported that circulating CIRP can increase the formation of reactive oxygen species and caspase‑1 activation via nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome activation [17, 22]. Based on these shared mechanisms, it was concluded that circulating CIRP has a reasonable background supporting its clinical significance as a biomarker to reflect the activity of MPA and GPA.
This study performed subgroup analyses according to the subtype of AAV, ANCA type, and systemic manifestations based on the items of BVAS, and obtained several interesting findings. First, regarding the subtype of AAV, MPA patients exhibited a significantly higher median circulating CIRP than GPA patients (7.6 ng/mL vs. 5.4 ng/mL, P = 0.001). Second, regarding AAV type, circulating CIRP was not significantly correlated with MPO-ANCA or PR3-ANCA titers (r = 0.183 and r = −0.082, respectively). In addition, no significant differences in circulating CIRP were noted based on the presence of MPO-ANCA (or P‑ANCA) or PR3-ANCA (or C‑ANCA) positivity. Third, regarding systemic manifestations based on BVAS, circulating CIRP exhibited a significant correlation with the total score of renal manifestation (r = 0.264, P = 0.033). Concurrently, MPA patients showed renal manifestation more frequently than GPA patients (84.6% vs. 26.9%, P < 0.001). Therefore, it was concluded that the subgroup analyses elucidated that circulating CIRP serves as a more significant biomarker in MPA patients through a higher frequency of renal manifestations.
This study has several limitations. Firstly, the size of this study was not large enough to provide potentially reliable information on the clinical significance of CIRP in MPA and GPA patients. Secondly, the results of this study could not be verified in a validation cohort consisting of patients from other hospitals because there is no additional AAV cohort other than our cohort for which clinical data of BVAS, FFS, and SF-36 and blood samples of Korean patients who have been newly diagnosed with AAV are immediately available for use. Thirdly, this study could not provide evidence supporting the hypothetical mechanisms of the correlation between circulating CIRP and the current BVAS. Lastly, the absence of paired results for the same patients was also a critical limitation. However, this study has clinical implications in that it is the first pilot study to demonstrate the possibility that circulating CIRP could reflect the current activity of MPA and GPA. A prospective future study with more patients, in particular by including patients from other hospitals, will provide serial, dynamic, and reliable data regarding the clinical potential of circulating CIRP in MPA and GPA.
Conclusion
This study suggests the clinical potential of circulating CIRP as a biomarker for reflecting the current BVAS and predicting high activity of AAV in patients with MPA and GPA.
References
Zhong P, Huang H (2017) Recent progress in the research of cold-inducible RNA-binding protein. Future Sci OA 3(4):FSO246
Liao Y, Tong L, Tang L, Wu S (2017) The role of cold-inducible RNA binding protein in cell stress response. Int J Cancer 141(11):2164–2173
Zhong P, Peng J, Yuan M, Kong B, Huang H (2021) Cold-inducible RNA-binding protein (CIRP) in inflammatory diseases: molecular insights of its associated signalling pathways. Scand J Immunol 93(1):e12949
Aziz M, Brenner M, Wang P (2019) Extracellular CIRP (eCIRP) and inflammation. J Leukoc Biol 106(1):133–146
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC et al (2013) 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65(1):1–11
Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, Mahr A, Segelmark M, Cohen-Tervaert JW, Scott D (2007) Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 66(2):222–227
O’Sullivan KM, Ford SL, Longano A, Kitching AR, Holdsworth SR (2018) Intrarenal Toll-like receptor 4 and Toll-like receptor 2 expression correlates with injury in antineutrophil cytoplasmic antibody-associated vasculitis. Am J Physiol Renal Physiol 315(5):F1283–F1294
Ajmani S, Singh H, Chaturvedi S, Mishra R, Rai MK, Jain A, Misra DP, Agarwal V (2019) Utility of neutrophil CD64 and serum TREM‑1 in distinguishing bacterial infection from disease flare in SLE and ANCA-associated vasculitis. Clin Rheumatol 38(4):997–1005
Suppiah R, Robson JC, Grayson PC, Ponte C, Craven A, Khalid S, Judge A, Hutchings A, Merkel PA, Luqmani RA et al (2022) 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann Rheum Dis 81(3):321–326
Robson JC, Grayson PC, Ponte C, Suppiah R, Craven A, Judge A, Khalid S, Hutchings A, Watts RA, Merkel PA et al (2022) 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis 81(3):315–320
Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, Flossmann O, Hall C, Hollywood J, Jayne D et al (2009) Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 68(12):1827–1832
Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Toumelin PL, French Vasculitis Study Group (FVSG) (2011) The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 90(1):19–27
Han CW, Lee EJ, Iwaya T, Kataoka H, Kohzuki M (2004) Development of the Korean version of Short-Form 36-Item Health Survey: health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J Exp Med 203(3):189–194
Bhamra K, Luqmani R (2012) Damage assessment in ANCA-associated vasculitis. Curr Rheumatol Rep 14(6):494–500
Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suárez LF, Guillevin L, Hellmich B, Jayne D, Jennette JC, Kallenberg CGM et al (2017) Position paper: Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 13(11):683–692
Choi CB, Park YB, Lee SW (2019) Antineutrophil cytoplasmic antibody-associated vasculitis in Korea: a narrative review. Yonsei Med J 60(1):10–21
Zhong P, Zhou M, Zhang J, Peng J, Zeng G, Huang H (2022) The role of Cold-Inducible RNA-binding protein in respiratory diseases. J Cell Mol Med 26(4):957–965
Söderberg D, Segelmark M (2016) Neutrophil extracellular traps in ANCA-associated vasculitis. Front Immunol 7:256
Ode Y, Aziz M, Jin H, Arif A, Nicastro JG, Wang P (2019) Cold-inducible RNA-binding protein induces neutrophil extracellular traps in the lungs during sepsis. Sci Rep 9(1):6252
Linders J, Madhi R, Rahman M, Mörgelin M, Regner S, Brenner M, Wang P, Thorlacius H (2020) Extracellular cold-inducible RNA-binding protein regulates neutrophil extracellular trap formation and tissue damage in acute pancreatitis. Lab Invest 100(12):1618–1630
Chen K, Murao A, Arif A, Takizawa S, Jin H, Jiang J, Aziz M, Wang P (2021) Inhibition of efferocytosis by extracellular CIRP-induced neutrophil extracellular traps. J Immunol 206(4):797–806
Yang WL, Sharma A, Wang Z, Li Z, Fan J, Wang P (2016) Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci Rep 6:26571
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare (HI14C1324), the Handok Inc., Seoul, Republic of Korea (HANDOK 2021-006), and CELLTRION PHARM, Inc. Chungcheongbuk-do, Republic of Korea (NCR 2019-6).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by TY, JWH, SSA, and SWL. The first draft of the manuscript was written by TY and JWH, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
T. Yoon, J.W. Ha, J.Y. Pyo, J.J. Song, Y.-B. Park, S.S. Ahn, and S.-W. Lee declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
Redaktion
Ulf Müller-Ladner, Bad Nauheim
Uwe Lange, Bad Nauheim
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Co-first authors: Taejun Yoon and Jang Woo Ha contributed equally to this work.
Co-corresponding authors: Sung Soo Ahn and Sang-Won Lee contributed equally to this work.

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Yoon, T., Ha, J.W., Pyo, J.Y. et al. Circulating cold-inducible RNA-binding protein levels in microscopic polyangiitis and granulomatosis with polyangiitis. Z Rheumatol 83 (Suppl 1), 230–235 (2024). https://doi.org/10.1007/s00393-023-01320-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-023-01320-x