Abstract
Objective
To assess safety, effectiveness and onset of effect of rituximab (RTX) in routine clinical treatment of severe, active rheumatoid arthritis (RA).
Methods
Prospective, multi-centre, non-interventional study in rheumatological outpatient clinics or private practices in Germany. RTX-naïve adult patients were to receive RTX according to marketing authorisation and at their physician’s discretion. Also according to their physician’s discretion, patients could receive a second cycle of RTX (re-treatment = treatment continuation). Major outcome was the change in Disease Activity Score based on 28-joints count and erythrocyte sedimentation rate (DAS28-ESR) over 24 weeks and during 6 months of re-treatment.
Results
Overall, 1653 patients received at least one cycle RTX; 99.2% of these had received disease-modifying antirheumatic drugs (DMARD) pre-treatment and 75.5% anti-tumor necrosis factor(TNF)‑α pre-treatment. After a mean interval of 8.0 months, 820 patients received RTX re-treatment. Mean DAS28-ESR decreased from 5.3 at baseline to 3.8 after 24 weeks (−1.5 [95% confidence interval, CI: −1.6; −1.4]), and from 4.1 at start of cycle 2 to 3.5 at study end (change from baseline: −1.8 [95% CI: −2.0; −1.7]). Improvements in DAS28-ESR and Health Assessment Questionnaire (HAQ) score occurred mainly during the first 12 weeks of RTX treatment, with further DAS28-ESR improvement until week 24 or month 6 of re-treatment. Improvements in DAS28-ESR and EULAR responses were more pronounced in seropositive patients. RF was a predictor of DAS28-ESR change to study end. Safety analysis showed the established profile of RTX.
Conclusion
RTX was safe and effective in a real-life setting with rapid and sustained improvement in RA signs and symptoms.
Zusammenfassung
Ziel der Arbeit
Ziel war die Untersuchung von Sicherheit, Wirksamkeit und Wirkeintritt von Rituximab (RTX) bei schwerer aktiver rheumatoider Arthritis (RA) im klinischen Alltag.
Methoden
Es handelt sich um eine prospektive multizentrische nichtinterventionelle Studie in rheumatologischen Praxen und Ambulanzen in Deutschland. Nicht mit RTX vorbehandelte erwachsene Patienten sollten gemäß Fachinformation und Entscheidung ihres Arztes eine Behandlung mit RTX erhalten, ggf. auch eine Retherapie (Therapiefortsetzung). Primärer Endpunkt war die Änderung des „Disease Activity Score“ nach Beurteilung von 28 Gelenken und der Blutsenkungsgeschwindigkeit (DAS28-BSG) von Therapiebeginn zu Woche 24 sowie ggf. von Beginn zu Monat 6 einer Retherapie.
Ergebnisse
Eine RTX-Primärtherapie erhielten 1653 Patienten, 99,2 % davon nach krankheitsmodifizierenden Antirheumatika („disease-modifying antirheumatic drugs“, DMARD) und 75,5 % nach Vortherapie mit einem Tumornekrosefaktor(TNF)-α-Inhibitor. Für 820 Patienten wurde nach einem mittleren Intervall von 8,0 Monaten eine Retherapie dokumentiert. Die DAS28-BSG sank von 5,3 vor Therapie auf 3,8 nach 24 Wochen (−1,5; 95%-Konfidenzintervall, 95%-KI: −1,6; −1,4) bzw. von 4,1 vor Beginn einer Retherapie auf 3,5 zu Studienende (Veränderung gegenüber dem Ausgangswert: −1,8; 95%-KI: −2,0; −1,7). Die Verbesserung der DAS28-BSG sowie der Gelenkfunktion (Health Assessment Questionnaire, HAQ) wurde überwiegend in den ersten 12 Wochen der Behandlung mit RTX erzielt; die DAS28-BSG fiel bis Woche 24 bzw. bis Monat 6 unter Retherapie weiter ab. Verbesserungen hinsichtlich DAS28-BSG sowie EULAR-Kriterien (European League Against Rheumatism) waren bei seropositiven Patienten stärker ausgeprägt, die Positivität für den Rheumafaktor (RF) war prädiktiv für die Veränderung der DAS28-BSG unter Retherapie. Die Behandlungssicherheit von RTX entsprach dem bekannten Profil.
Schlussfolgerung
Im klinischen Alltag erwies sich RTX als sicher und wirksam mit einer rasch einsetzenden und anhaltenden Besserung der Symptomatik der rheumatoiden Arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rituximab (RTX) is a monoclonal antibody targeting the CD20 antigen on the surface of B lymphocytes. Combined with methotrexate (MTX), RTX is approved for the treatment of adult patients with severe active RA who have had an inadequate response or intolerance to disease-modifying antirheumatic drugs (DMARDs), including one or more tumour necrosis factor-alpha (TNF-α) inhibitors.
In REFLEX [5], the pivotal trial evaluating RTX plus MTX in patients with active RA and an inadequate response to one or more TNF-α inhibitors, patients had a therapeutic response only 8 weeks after beginning RTX therapy, with a maximum effect at week 16. Although several non-interventional studies (NIS) report real-world effectiveness evidence for RTX in RA [1, 10, 12, 15, 19, 25], only interventional studies, which use selected patient populations, report onset of RTX effect. A good estimate of the expected course of RTX effect in clinical routine, however, may help to promptly adapt concomitant symptomatic treatment of the patient.
The present NIS therefore aimed to evaluate the onset and course of RTX effect in the first 24 weeks of primary therapy and during 6 months of re-treatment, and to investigate potential predictors of response on the effectiveness of RTX in this setting.
Methods
Study design and patients
This was a prospective, multi-centre NIS with the aims of evaluating the safety, effectiveness, and onset of RTX effect in adult patients with severe active RA in clinical practice. Patients were eligible for enrolment if the decision to start RTX treatment had been reached prior to, and independently of, the decision to include the patient in the study. No specific inclusion/exclusion criteria applied beyond the relative or absolute contraindications of the German Summary of Product Characteristics (SmPC). Patients were not enrolled if they had received prior RTX treatment, did not provide signed informed consent or if they had participated in an interventional study within the last 3 months before starting therapy with RTX. Participation was open to rheumatologists in outpatient clinics or private practices in Germany.
Patients were to be treated with RTX according to the German SmPC. Following an evaluation at week 24, patients could receive a second cycle of treatment (“re-treatment” = treatment continuation) at the physician’s discretion. Patients had a follow-up period of ~6 months, starting on the day of the first RTX infusion; patients receiving re-treatment were followed for an additional 6 months.
The study was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (Eth-14/09) and registered with the Paul Ehrlich Institute (study code ML22639) and ClinicalTrials.gov (NCT01071798).
Assessments
Baseline assessments included demographic and anamnestic data, comorbidities, prior therapies for RA and reasons for their discontinuation, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibody status.
Treatment data included pre-medication before each RTX infusion, time between cycles, and concomitant medication.
Effectiveness was assessed using the Disease Activity Score based on 28-joints count and erythrocyte sedimentation rate (DAS28-ESR), European League Against Rheumatism (EULAR) response criteria, and the Health Assessment Questionnaire (HAQ) score. The major outcomes were changes in DAS28-ESR between baseline and week 24 for primary treatment, and for re-treatment, between start and end of the re-treatment cycle (~6 months later). Safety data included adverse events (AEs) and laboratory data.
Data were documented on day 1 (baseline), day 15 (treatment visit) and after ~24 weeks (final visit); additional visits could be documented for week 6, 12 or 18. For patients receiving re-treatment, data were documented at day 1 and 15 of re-treatment and after ~3 and 6 months.
Statistical analysis
Patients were included in the main analysis set if they fulfilled all inclusion/exclusion criteria, received at least one RTX infusion, had at least one effectiveness assessment and their physician approved their data for analysis. Among these, patients receiving a second treatment cycle composed the re-treatment subpopulation.
Descriptive statistics were used for all parameters. For changes in DAS28-ESR over 24 weeks and during re-treatment (6 months), 95% confidence intervals (CI) were calculated. Missing values were not imputed. Differences between subgroups were analysed by t-tests for continuous variables or chi-square tests for categorical variables.
An analysis of variance (ANOVA) was performed to evaluate the effect of several factors on the changes of DAS28-ESR from baseline to end of cycle 1 and cycle 2 (age <65 years/≥65 years; comorbidities; RF and anti-CCP antibody status; prior treatment with TNF-α inhibitors or DMARDs).
For serotype subgroup analyses, patients positive for RF and/or anti-CCP antibodies at baseline were considered “seropositive”; patients negative for both were considered “seronegative” [11].
The effects of prior anti-TNF-α or concomitant DMARD treatment on changes in DAS28-ESR were analysed post-hoc.
Results
Patients and treatment
Between January 2010 and October 2014, 1834 patients receiving at least one treatment cycle of RTX were enrolled by rheumatologists in 264 outpatient clinics and private practices in Germany. Of these patients, 171 had incomplete data not approved for data analysis by their physicians, 10 were excluded due to violation of inclusion/exclusion criteria, leaving 1653 patients valid for analysis (main analysis set). RTX re-treatment was documented for 820 patients with a mean interval of 8.0 (±3.6) months between cycles. 357 patients were also enrolled in the German biologics registry RABBIT. Demographics and baseline characteristics of patients in the main analysis set and the re-treatment subpopulation are summarised in Table 1.
Among patients who had failed TNF-α inhibitor therapy, more than two thirds stopped treatment due to ineffectiveness. A higher proportion of anti-TNF-α-naïve patients presented with a history of solid tumours than TNF-α inhibitor pre-treatment patients (11.4% vs. 3.3%, respectively) or with a history of lymphoma/leukaemia (4.9% vs. 1.5%). Discontinuation of MTX pre-treatment was documented for 888/1400 patients (63.4%). There were no significant differences in demographic characteristics or duration of disease between seropositive and seronegative patients. However, seropositive patients had significantly more severe disease (Table 2). Baseline characteristics of the re-treatment subpopulation were similar to those of the main analysis set.
Prior to the RTX infusion, 95.6% of patients in the main analysis set received steroids and 85.6% received paracetamol; in re-treatment, 95.5% received steroids and 87.7% received paracetamol.
During primary treatment, 788 patients (47.7%) received RTX with concomitant MTX, 220 patients (13.3%) with leflunomide and 582 patients (35.2%) as monotherapy.
Treatment response
Data were available for 844 patients (51.1% of the main analysis set) at the end of treatment cycle 1 and for 671 patients (81.8% of the re-treatment subpopulation) at the end of cycle 2. For the ANOVA evaluating effects on DAS28-ESR change from baseline, data were available for 419 patients after cycle 1 and for 324 patients after cycle 2. Changes in variables of disease activity and functional status from baseline to week 24 of primary treatment and from start to end of re-treatment are summarised in Table 2.
Twenty-four weeks after starting RTX treatment, the mean change in DAS28-ESR from baseline was −1.5 [95% CI: −1.6; −1.4], with superior response in seropositive compared to seronegative patients (−1.6 [95% CI: −1.7; −1.5] vs. −0.9 [95% CI: −1.4; −0.4], respectively). In patients receiving two cycles, mean DAS28-ESR decrease at the end of re-treatment was −1.8 [95% CI: −2.0; −1.7], again with more pronounced effects in seropositive than in seronegative patients (−1.9 [95% CI: −2.1; −1.8] vs. −0.9 [95% CI: −1.6; −0.2], respectively). A greater proportion of seropositive than seronegative patients achieved moderate or good EULAR response at the end of treatment cycle 1 or 2 (Table 2). At week 24, changes in mean DAS28-ESR from baseline were similar in patients receiving RTX with concomitant MTX (−1.5 [95% CI: −1.7; −1.3]), leflunomide (−1.5 [95% CI: −1.8; −1.2]) or as monotherapy (−1.6 [95%CI: −1.8; −1.4]) and in patients with (−1.5 [95% CI: −1.6; −1.3]) or without TNF-α inhibitor pre-treatment (1.6 [95% CI: −1.8; −1.3]).
A major decrease of mean DAS28-ESR was already evident 6 and 12 weeks after the first RTX infusion, with the maximum effect reached at week 18 and sustained until week 24 (Fig. 1a and 2a). Compared to the levels reached at the end of cycle 1, patients receiving re-treatment presented with slightly worse parameters of disease activity before initiation of cycle 2 (Table 2). Re-treatment improved DAS28-ESR beyond the levels achieved after cycle 1, and the level reached after 3 months was sustained until the end of the observation period (Fig. 1b and 2b).
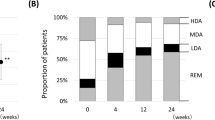
The swift onset of response to RTX was also reflected in the proportion of patients with high disease activity (DAS28-ESR >5.1) decreasing from 58.0% at baseline to 29.8% at week 6 and 17.6% at week 24. At the end of primary treatment, 20.7% of patients were in remission (DAS28-ESR <2.6; Table 2). Moderate or good EULAR response was documented for 69.8% of patients at week 24 and 78.8% at the end of re-treatment (Table 2).
The decrease in mean HAQ score achieved after 12 weeks of primary treatment was sustained with no further changes until week 24 (Table 2). In patients receiving re-treatment, the mean HAQ score remained at this level up to ~6 months later. A clinically relevant improvement in HAQ (≥0.3 points) was achieved in 38.8% of patients; only 12.7% of patients had a clinically relevant worsening.
Predictors of response
ANOVA demonstrated an effect on DAS28-ESR change from baseline for RF after cycle 2 (p = 0.001) but not after cycle 1 (p = 0.145). Anti-CCP status did not have an effect on DAS28-ESR change from baseline to either end of cycle 1 (p = 0.159) or cycle 2 (p = 0.934), neither did the separate factors of age, comorbidities, prior treatment with TNF-α inhibitors or DMARDs.
Safety
The total observation period was 1294.3 patient-years for the main analysis set and 953.0 patient-years for the re-treatment subpopulation. Overall, 1566 treatment-emergent adverse events (TEAEs) were observed in 575 patients (Table 3). Most TEAEs were mild or moderate and resolved by the end of the observation period. Safety laboratory parameters revealed no relevant changes.
The percentage of patients with TEAEs considered to be related to RTX treatment was similar in both cycles, with slightly more infusion-related reactions in cycle 1. Grouped by system organ classes (SOCs), the most frequently reported categories were infections/infestations (3.3%), general disorders/administration site conditions (1.9%; mainly fatigue and influenza-like illness), and skin and subcutaneous tissue disorders (1.3%; mainly pruritus). Malignancy was documented in 0.2% of patients.
Serious TEAEs occurred in 10.9% of patients. In 3.0% of patients these were considered to be related to RTX, with no difference in incidence between cycles.
Infections/infestations were the most frequently reported categories of TEAEs (24.9 per 100 patient-years [100 PY]), TEAEs related to treatment (6.26 per 100 PY), serious TEAEs (4.71 per 100 PY), and serious TEAEs related to treatment (1.39 per 100 PY; Table 3). For TEAEs considered to be related to treatment, infections were mainly bronchitis and nasopharyngitis; herpes zoster was documented in 0.2% of patients.
Premature discontinuation of the study due to TEAEs was documented for 43 patients. In 10 patients, TEAEs led to death. A causal relationship with RTX treatment was seen in one patient with septic arthritis and for one patient with pneumonia combined with respiratory insufficiency; four cases each were considered not related to RTX or assessed as unknown.
Discussion
The objective of our NIS was to assess the real-world safety and effectiveness of one or two cycles of RTX treatment in patients with severe active RA in Germany and to evaluate the onset of effect and potential predictors of response to RTX in this setting. As indicated by our results, RTX was effective in routine care, with a significant decrease in mean DAS28-ESR, a high rate of good/moderate EULAR responses, and a clinically meaningful change in mean HAQ at week 24. Re-treatment was associated with further improvement of disease activity, albeit no further gain in functional ability, possibly reflecting the long duration of disease, numerous comorbidities, and high age of the patients. Overall, our findings are consistent with results from clinical trials [4] and other NISs [2, 19, 21, 23, 25].
Patients responded rapidly to treatment, with an onset of effect evident at week 6, further improvement occurring until week 12 and stabilisation by week 24. Controlled clinical trials reported an onset of RTX effect at week 8 [5, 7]; the very early clinical response before 8 weeks, however, might be associated with glucocorticoid pre-medication [17]. Previous NISs and patient registries documented efficacy data no earlier than 3 months after the first RTX infusion [1, 2, 19, 21, 23, 25]. To our knowledge, this NIS is the first to collect data on the early phase and thus confirm a continuous clinical improvement from week 6 onwards in a real-world setting. The slight increase in disease activity that was apparent between end of the first treatment cycle at week 24 and start of the second cycle after a mean interval of 8.0 months (±3.6) may reflect a treatment-as-needed (PRN) approach to re-treatment in a sizeable number of patients. This observation would be in line with a retrospective pooled analysis of re-treatment strategies in RTX studies [9], adding further evidence showing the advantage of fixed treatment intervals of 6 months to maintain disease control.
Our analysis of DAS28-ESR change from baseline adds to the evidence that seropositive patients derive more benefit from RTX than seronegative patients, corroborating a meta-analysis of placebo-controlled studies [11] and several NISs [6, 8, 13, 16, 18] and registries [2, 19, 20]. In line with results from the British rheumatology registry BSRBR [19], RF positivity was a predictor of DAS28 response to RTX in the present study, albeit after re-treatment only. However, anti-CCP antibody status, a predictor of EULAR response in the CERERRA registries [2] and a French NIS [6], was not predictive in our cohort.
Although RTX is approved in combination with MTX for patients with severe active RA failing at least one TNF-α inhibitor, in clinical practice, varying forms of use—including the treatment of patients with less severe disease—are not uncommon. Other NISs report a considerable number of patients receiving RTX as monotherapy or in combination with leflunomide or other DMARDs [3, 15, 19, 25]; between 16.4% [19] and 36.6% of patients [2] received RTX as their first biological. Here, approximately one quarter of the patients were anti-TNF-α naïve, and a substantial number received RTX as monotherapy or in combination with leflunomide. Our findings of DAS28-ESR improvement compare well with previous research in subgroups with concomitant MTX, leflunomide or RTX monotherapy [15, 19, 25], but contrast with studies that report significantly better response in anti-TNF-α-naïve patients [2, 19].
Patients in the present study were older than patients in the controlled trials [4], had less severe disease and less functional disability. Longitudinal analysis of GERINIS, enrolling patients in Germany between 2006 and 2009 [25], suggested that, over time, physicians were using RTX in RA patients with less severe disease. This trend is also reflected in the RABBIT database [14] and is consistent with the present study’s baseline characteristics documenting DAS-ESR >5.1 for only 58% of our patients enrolled between 2010 and 2014.
The type of TEAEs observed with RTX in the present study were largely as expected. The incidence rates for related TEAEs of interest (e. g. serious infections, herpes zoster, malignancy and myocardial infarction) were similar to or lower than those reported in controlled clinical trials and recent NISs [4, 22, 24] and may reflect some underreporting.
The main strengths of the present study are the enrolment of a large number of patients and the broad inclusion criteria, reflecting current real-world clinical practice. Since registration started in 2007, 1457 RA patients receiving RTX have been enrolled in the German biologics registry RABBIT (July 2018 [5]). The 1653 patients documented in the present study add to this database.
Limitations of the present study are mostly related to its observational character and the lack of a control group. Compared to interventional studies, data collection was incomplete and a considerable number of patients were lost to follow-up, with no information on the reasons for discontinuing documentation. We thus cannot exclude the possibility that patients who dropped out had worse outcomes. As the option for documentation of re-treatment was only implemented more than a year after study commencement, the re-treatment subgroup does not reflect the proportion of patients in the main analysis set that were eligible for re-treatment. AEs may have been underreported and depletion of susceptible patients may have contributed to the decrease in infections/infestations and infusion-related reactions from cycle 1 to 2. Furthermore, from today’s perspective, we would aim to collect Clinical and/or Simplified Disease Activity Index (CDAI; SDAI) data to confirm the effectiveness seen in DAS28-ESR scores.
Overall, RTX was safe and effective in a real-life setting, with rapid and sustained improvement in the signs and symptoms of RA with superior results in seropositive patients.
References
Chatzidionysiou K, Lie E, Nasonov E et al (2016) Effectiveness of two different doses of rituximab for the treatment of rheumatoid arthritis in an international cohort: data from the CERERRA collaboration. Arthritis Res Ther 18:50
Chatzidionysiou K, Lie E, Nasonov E et al (2011) Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis 70:1575–1580
Chatzidionysiou K, Lie E, Nasonov E et al (2012) Effectiveness of disease-modifying antirheumatic drug co-therapy with methotrexate and leflunomide in rituximab-treated rheumatoid arthritis patients: results of a 1-year follow-up study from the CERERRA collaboration. Ann Rheum Dis 71:374–377
Cohen MD, Keystone E (2015) Rituximab for rheumatoid arthritis. Rheumatol Ther 2:99–111
Cohen SB, Emery P, Greenwald MW et al (2006) Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 54:2793–2806
Couderc M, Mathieu S, Pereira B et al (2013) Predictive factors of rituximab response in rheumatoid arthritis: results from a French university hospital. Arthritis Care Res (Hoboken) 65:648–652
Emery P, Deodhar A, Rigby WF et al (2010) Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 69:1629–1635 (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE))
Emery P, Gottenberg JE, Rubbert-Roth A et al (2015) Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis 74:979–984
Emery P, Mease PJ, Rubbert-Roth A et al (2011) Retreatment with rituximab based on a treatment-to-target approach provides better disease control than treatment as needed in patients with rheumatoid arthritis: a retrospective pooled analysis. Rheumatology (Oxf) 50:2223–2232
Harrold LR, Reed GW, Shewade A et al (2015) Effectiveness of Rituximab for the treatment of rheumatoid arthritis in patients with prior exposure to anti-TNF: results from the CORRONA Registry. J Rheumatol 42:1090–1098
Isaacs JD, Cohen SB, Emery P et al (2013) Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann Rheum Dis 72:329–336
Kekow J, Mueller-Ladner U, Schulze-Koops H (2012) Rituximab is more effective than second anti-TNF therapy in rheumatoid arthritis patients and previous TNFalpha blocker failure. Biologics 6:191–199
Quartuccio L, Fabris M, Salvin S et al (2009) Rheumatoid factor positivity rather than anti-CCP positivity, a lower disability and a lower number of anti-TNF agents failed are associated with response to rituximab in rheumatoid arthritis. Rheumatology (Oxf) 48:1557–1559
Richter A, Pattloch D, Manger B et al (2016) Initiation of biologic treatment over the past 15 years reflects changes in treatment strategies: results from the prospective cohort of the German Biologics Register Rabbit. Ann Rheum Dis 75:874–875
Richter A, Strangfeld A, Herzer P et al (2014) Sustainability of rituximab therapy in different treatment strategies: results of a 3-year followup of a German biologics register. Arthritis Care Res (Hoboken) 66:1627–1633
Sellam J, Hendel-Chavez H, Rouanet S et al (2011) B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label study. Arthritis Rheum 63:933–938
Smolen JS, Keystone EC, Emery P et al (2007) Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 66:143–150
Solau-Gervais E, Prudhomme C, Philippe P et al (2012) Efficacy of rituximab in the treatment of rheumatoid arthritis. Influence of serologic status, coprescription of methotrexate and prior TNF-alpha inhibitors exposure. Joint Bone Spine 79:281–284
Soliman MM, Hyrich KL, Lunt M et al (2012) Effectiveness of rituximab in patients with rheumatoid arthritis: observational study from the British Society for Rheumatology Biologics Register. J Rheumatol 39:240–246
Strangfeld A, Eveslage M, Kekow J (2009) Effectiveness of treatment with rituximab depends on autoantibody status: results from 2 years of experience in the German biologics register RABBIT. Arthritis Rheum 60:1695 (Abstract)
Valleala H, Korpela M, Mottonen T et al (2009) Rituximab therapy in patients with rheumatoid arthritis refractory or with contraindication to anti-tumour necrosis factor drugs: real-life experience in Finnish patients. Scand J Rheumatol 38:323–327
Van Vollenhoven RF, Fleischmann RM, Furst DE et al (2015) Longterm safety of Rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol 42:1761–1766
Vander Cruyssen B, Durez P, Westhovens R et al (2010) The Belgian MIRA (MabThera In Rheumatoid Arthritis) Registry: clues for the optimization of rituximab treatment strategies. Arthritis Res Ther 12:R169
Vassilopoulos D, Delicha EM, Settas L et al (2016) Safety profile of repeated rituximab cycles in unselected rheumatoid arthritis patients: a long-term, prospective real-life study. Clin Exp Rheumatol 34:893–900
Wendler J, Burmester GR, Sorensen H et al (2014) Rituximab in patients with rheumatoid arthritis in routine practice (GERINIS): six-year results from a prospective, multicentre, non-interventional study in 2,484 patients. Arthritis Res Ther 16:R80
Acknowledgements
The study was sponsored by Roche Pharma AG, Grenzach-Wyhlen, Germany. Medical writing assistance was provided by Physicians World Europe GmbH, supported by Roche Pharma AG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Krause: speakers’ bureau for Abbvie, Berlin-Chemie, BMS, Celgene, Chugai, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB; consultancy fees from Abbvie, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi; research grants from Abbvie, Ovamed, Roche. P.M. Aries: speakers’ bureau for Abbvie, BMS, Celgene, Chugai, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Mundipharma, consultancy fees from Abbvie, Celgene, Lilly, Medac, Novartis, Pfizer, Roche, Sanofi; Chugai, Roche. C. Fiehn: lecturer fees from Roche. H.-M. Lorenz: consultancy/lecturer fees, support for scientific projects, or travel reimbursements from Abbvie, Actelion, AstraZeneca, Bayer Vital, Baxter, Biogen, BMS, Celgene, Chugai, GSK, Janssen-Cilag, Lilly, Medac, MSD, Novartis, Octapharma, Pfizer, Roche, Shire, SOBI, UCB. L. Meier: consultancy/lecturer fees from Abbvie, BMS, Chugai-Roche, Lilly, Medac, MSD, Pfizer. U. Müller-Ladner: consultancy/lecturer fees from Roche, Chugai. A. Schwarting: honoraria from GSK, research grants from AbbVie, BMS, Pfizer. H.-P. Tony: consultancy/lecturer fees from Abbvie, AstraZeneca, BMS, Chugai, Janssen-Cilag, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi. M.A. Peters is an employee of Roche Pharma AG. J. Wendler: consultancy/lecturer fees from Roche. S. Berger, H. Kellner and G.A. Müller declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Redaktion
U. Müller-Ladner, Bad Nauheim
U. Lange, Bad Nauheim
Rights and permissions
About this article
Cite this article
Krause, A., Aries, P.M., Berger, S. et al. Rituximab in routine care of severe active rheumatoid arthritis. Z Rheumatol 78, 881–888 (2019). https://doi.org/10.1007/s00393-018-0552-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-018-0552-0