Abstract
Aims
Aortic stiffness, measured as aortic pulse wave velocity (PWV), is a powerful predictor of cardiovascular health but is difficult to accurately obtain non-invasively. This study sought to develop a novel CT aortic stiffness index (CTASI) which incorporates both anatomical (calcification) and physiological (distensibility) aspects of aortic health.
Methods
Invasive PWV and CT scans were obtained for 80 patients undergoing TAVI (cohort 1). CT data alone were obtained from an additional 238 patients (cohort 2). Aortic calcification was quantified using a modified Agatston’s methodology. Distensibility-PWV was calculated from minimum and maximum ascending aorta areas. Linear regression of these values was used to construct CTASI from cohort 1. CTASI was then calculated for cohort 2 who were prospectively followed-up.
Results
CTASI correlated with invasive PWV (rho = 0.47, p < 0.01) with a higher correlation coefficient than distensibility-PWV (rho = 0.35, p < 0.01) and aortic calcification (rho = 0.36, p < 0.01). Compared to invasive PWV, CTASI had a good accuracy as a diagnostic test (AOC 0.72 [95% CI 0.61–0.84]), superior to aortic calcification and distensibility-PWV alone (χ2 = 0.82, p = 0.02). There were 61 deaths during a median follow-up of 771 days (95% CI 751.4–790.5). CTASI was able to predict 1-year mortality (OR 2.58, 95% CI 1.18–5.61, p = 0.02) and Kaplan–Meier survival (log-rank p = 0.03).
Conclusion
CTASI is a stronger measure of aortic stiffness than aortic calcification or distensibility alone. Given the prolific use of CT scanning for assessing coronary and vascular disease, the additional calculation of CTASI during these scans could provide an important direct measurement of vascular health and guide pharmacological therapy.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arterial stiffness is an independent predictor of mortality [1, 2], the development of end-organ damage [3, 4] and cardiovascular events [1, 2]. The gold-standard for assessing arterial stiffness is invasively obtained aortic pulse wave velocity (PWV) [1]. Typically this involves insertion of catheters into the aortic root [5] which is not indicated/desirable in the majority of patients. A number of non-invasive techniques have been proposed to examine either the physiological [6,7,8,9,10,11,12] or anatomical [13,14,15,16,17,18,19] aspects of aortic stiffness but accuracy, reproducibility, operator dependence or other reasons preclude their routine clinical use [20].
Computer tomography (CT) is widely used in the assessment of cardiovascular disease. However, the use of CT to assess aortic stiffness through PWV has not yet been reported. Unlike other modalities, CT can assess both physiological and anatomical components of aortic stiffness: through cross-sectional distensibility of the aorta identified during ECG-synchronised contrast-enhanced CT scanning and through the quantification of aortic calcification.
This study contained two parts. First with cohort 1, we set out to correlate these two features with invasively measured PWV and to use them to construct a CT Aortic Stiffness Index (CTASI). Second, as we have previously shown that invasively measured PWV predicts 1 year mortality in patients undergoing TAVI [5], we sought to further extend these findings using CTASI similarly in a separate cohort of post-TAVI patients (cohort 2).
Methods
Patient flow through the study protocol is displayed in Fig. 1. To construct CTASI, the necessary invasive data were consecutively gathered from patients who had TAVI in our centre by those operators who routinely measure it during the procedure. 106 such patients underwent TAVI from January until September 2017. 25 patients were excluded because of inadequate CT data and one patient because of poor-quality invasive data. The remaining 80 patients were included in this analysis.
For the prognostic validation cohort, 423 patients who underwent TAVI between August 2015 and December 2016 were identified. Patients who died within 30 days after the TAVI were excluded (n = 8). We also excluded 177 patients in whom the CT data were inadequate (n = 35) or unavailable (n = 75), or in whom blood pressure was not recorded simultaneously with the CT (n = 67). The remaining 238 patients made up this cohort.
Invasive PWV
Invasive PWV was calculated according to our previously published methodology [5]. Briefly, prior to TAVI simultaneous pressure signals were obtained from a fluid-filled catheter at the aortic root and the 6F sheath in the common femoral artery. A bespoke foot-to-foot algorithm written in Matlab (Version R2015a, the MathWorks, Natick, MA, USA) was used to calculate pulse wave time. If in sinus rhythm, data were ensembled from a series of cardiac cycles (typically 10–15) through automated recognition of the peak of the QRS complex. If in AF, to avoid errors with ensemble averaging, a single cardiac cycle was assessed; pulse wave time readings from a minimum of five cardiac cycles were then averaged to provide the mean pulse wave time. An automated step was used to identify the peak of the second derivate to select the point of the pressure upstroke in an objective fashion. Pulse wave distance was calculated using a semi-automated vessel identification step which was then measured from the contrast-enhanced CT using a commercial package (Syngo.via, Siemens Healthineers, Erlangen, Germany). PWV was then calculated:
CT scanning
All patients underwent a CT scan for TAVI planning using a standardised protocol with a third-generation dual source CT scanner (Somatom FORCE, Siemens Healthineers, Germany). No premedication for heart rate control (which is unnecessary in TAVI-planning CT) or vasodilatation (which is contraindicated in severe aortic stenosis) was added for CT acquisition. CT images of the thoracic aorta were obtained using a retrospective electrocardiographically gated protocol with automatic exposure control x-ray tube current modulation, providing datasets at 10% increments of the R–R interval. CT examination of the ilio-femoral arteries were performed using a high-pitch (TurboFlash) helical scan. A dose optimisation algorithm (CARE-Dose4D) automatically adjusted the x-ray tube voltage (range 70–140 kV in 10 kV increments) and the x-ray tube current (mAs) based on the patient size. The images were obtained using 85 ml of contrast agent (Omnipaque-350; 50 ml injected at 5 ml/s followed by 35 ml injected at 3.5 ml/s, followed by 40 ml of saline injected at 3.5 ml/s). The dataset was imported to a post-processing workstation with a commercial package (Syngo.via, Siemens Healthineers, Erlangen, Germany) for further analysis.
Aortic calcification
Total aortic calcium was quantified by Agatston’s methodology [21], with a threshold for calcium detection set at 850 Hounsfield Units as previously suggested for contrast scans [22]. Calcification was identified at this threshold in an automated fashion and segmented semi-automatically using a commercial package (Syngo.CT CAScoring, Syngo.via, Siemens Healthineers, Erlangen, Germany) from aortic root to the right or left common femoral artery at the femoral head. Aortic leaflet and LVOT calcifications were excluded. Data were obtained from the same iliac and femoral vessels as the invasive data (Fig. 2).
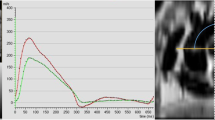
Example of aortic area (a) and aortic calcification (b) measurements obtained from contrast CT. a ECG-sequenced cross-sections of the ascending aorta are taken at 10% intervals throughout the cardiac cycle and aortic area calculated using automated software. The largest and smallest aortic areas are identified (highlighted with a red box in this example) and processed. b Using automated commercial software, the degree of aortic calcification can be calculated by selecting each body of calcium from aortic root to femoral head
Aortic distensibility
Images were analysed using Syngo.CT Vascular Analysis (Syngo.Via, Siemens Healthineers, Erlangen, Germany). A section of the ascending aorta that was en-face to the imaging plane was identified manually and images at 10% increments of the cardiac cycle were analysed. Data were exported in DICOM format and analysed using Radiant DICOM viewer (Medixant, Poznań, Poland). Aortic area was measured in an automated fashion using a bespoke Matlab programme (Version R2015a, the MathWorks, Natick, MA, USA) and the maximum (Amax) and minimum (Amin) areas identified (Fig. 2).
Brachial artery blood pressure was measured non-invasively using an automated sphygmomanometer at the same time as the CT scan with the patient supine prior to scanning.
Distensibility and distensibility-PWV were calculated according to established principles [23] where:
and:
where ρ is the density of blood, assumed to be 1055 kg/m3.
Patient follow-up
All patient data were collected and entered prospectively on a local online database from the point of referral to follow-up. Mortality data were obtained from the NHS Spine (a central framework including most NHS organisations across the UK).
Statistics
Sample size was determined using Freedman’s method [24] and hazard ratios estimated from previous invasive data. Assuming an uneven distribution between groups (with a ratio of 0.66) as before, we determined a minimum of 239 patients were required with an expectation of 35 events to give an 80% power to reject a hazard ratio of at least 0.30 at the 0.05 significance (two-tail).
Distribution of data was assessed using a Shapiro–Wilk test. If normally distributed, continuous data are displayed as mean ± standard deviation; if non-normally distributed as median (95% confidence interval [CI]). Unpaired data were compared with Student’s t test or Mann–Whitney U test as appropriate. Correlation between non-normally distributed data was assessed with Spearman’s rank order correlation.
Potential variables that may influence 1-year survival were identified from previous registry-based studies [25,26,27]. Linear and logistic regression analysis were performed to assess univariate relationships between continuous and discrete variables, respectively. A multivariate regression model was constructed for all variables with a p value of < 0.1. Aortic calcification, aortic distensibility and the linear regression derived CTASI were compared for diagnostic efficiency against an invasively obtained cut-off of 10.0 m/s [28] using receiver-operator characteristic (ROC) curve analysis.
Because of the debate over the appropriate pulse wave velocity cut-point [20, 28] and the variability of PWV according to age [29], the optimum PWV for this patient group was determined according to the highest Youden index from a series of ROC calculations against 1 year mortality using the function “cutpt” in STATA. This value was used to categorise individuals into high- and low-risk groups. Survival analysis was assessed using a Kaplan–Meier analysis.
Data analysis was performed using STATA 13.1 for Windows (STATA software, College Station, TX, USA). A p value of < 0.05 was considered statistically significant.
Results
Baseline demographics for both cohorts are shown in Table 1.
Cohort1: construction of CTASI
Invasive and non-invasive data in these 80 patients are displayed in Table 1. Median invasive-PWV was 9.71 m/s (95% CI 8.9–10.4) and distensibility-PWV 11.7 (95% CI 11.0–12.9) m/s. Aortic calcification was markedly skewed and was logarithmically transformed. A reasonable correlation was noted between invasive-PWV and distensibility-PWV (rho = 0.35, p < 0.01) and log-transformed aortic calcification (rho = 0.36, p < 0.01).
Linear regression analysis showed a relationship between invasive-PWV and both log-transformed aortic calcification (β = 1.29, 95% CI 0.31–2.29, p = 0.01) and distensibility-PWV (β = 0.19, 95% CI 0.04–0.33, p = 0.01). Using this regression analysis, CTASI was constructed:
CT Aortic Stiffness Index(CTASI,m/s) = 3.40 + (0.19 × Distensibility PWV) + (1.29 × log10[aortic calcification])
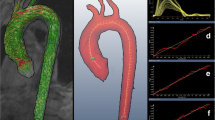
Median CTASI was 10.0 m/s (9.7–10.4). CTASI correlated with invasive pulse wave velocity (r = 0.47, p < 0.01) with a higher rank correlation coefficient than the individual CT-derived measures (Fig. 3).
a Invasively measured PWV correlated with distensibility-PWV (left), log-transformed aortic calcification (middle) and CT aortic stiffness index (CTASI, right). b Diagnostic characteristics of distensibility-PWV, log-transformed aortic calcification and CTASI. A significant correlation was noted with both CT-derived measures (distensibility-PWV and log-transformed aortic calcification); however, a stronger correlation was apparent with CTASI. Furthermore, when an invasive-PWV value of 10 m/s was taken as the ‘gold standard’ value a good area under the curve value of 0.72 was found, significantly higher than those according to distensibility-PWV or aortic calcification alone
Univariate analysis revealed a relationship between CTASI and systolic BP (β = 0.01, 95% CI 0.00–0.01, p = 0.04), pulse pressure (β = 0.01, 95% CI 0.00–0.02, p < 0.01), body mass index (β = −0.02, 95% CI: −0.04 to 0.00, p = 0.01) and age (β = 0.05, 95% CI 0.04–0.08, p < 0.01) of which only body mass index (β = −0.02, 95% CI −0.04 to 0.00, p = 0.04) and age (β = 0.05, 95% CI 0.03–0.07, p < 0.01) were preserved on multivariate analysis (Table 2).
ROC curves were created using an invasive PWV threshold of 10 m/s and demonstrated CTASI to have good accuracy as a diagnostic test, better than distensibility-PWV and aortic calcification alone (CTASI Area Under Curve (AOC) 0.72 [95% CI 0.61–0.84], distensibility-PWV AOC 0.69 [95% CI 0.57–0.80], log aortic calcification AOC 0.65 [95% CI 0.53–0.77]; χ2 = 0.82, p = 0.02, Fig. 3).
Cohort 2: prognostic validation of CTASI following TAVI
Median follow-up of cohort 2 was 771 days (95% CI 751.4–790.5) during which time there were 61 deaths with a 1-year mortality of 12.6%. Using a series of ROC curves a CTASI value of 10.5 m/s was identified as most informative for predicting 1-year mortality (Youden index = 0.23, sensitivity = 0.57, specificity = 0.66, AOC = 0.62) and this value was used to stratify patients into low- and high-risk groups (patient characteristic data shown in Table 3). Using this cut-off, univariate analysis revealed significance for CTASI only in predicting 1-year mortality (OR 2.58, 95% CI 1.18–5.61, p = 0.02, Table 4). Kaplan–Meier survival analysis was also significant (log-rank p = 0.03, Fig. 4).
Kaplan–Meier survival analysis following successful TAVI according to CT aortic stiffness index (CTASI). Using CTASI long-term outcome of patients undergoing TAVI can be predicted. CTASI cut-off was 10.5 m/s. Patients who died within 30-days after TAVI were excluded to avoid confounding from procedural mortality
Using similarly generated cut-points for calcification and distensibility-PWV individually showed both to be predictive of 1-year mortality (aortic calcification cut-point 3.69: OR 2.14, 95% CI 1.06–4.35, p = 0.04; distensibility PWV cut-point 12.19 m/s: OR 2.55, 95% CI 1.20–5.41, p = 0.02) but not significant for survival analysis (aortic calcification log-rank p = 0.1, distensibility PWV log-rank p = 0.06).
Discussion
Pulse Wave Velocity is a powerful tool for identifying cardiovascular risk and can be used to guide therapeutic preventative strategies. Its use has been limited by difficulties in obtaining accurate non-invasive measures. In this study, we have derived a novel CT aortic stiffness index (CTASI) from two features that reflect pulse wave velocity (aortic distensibility and calcification); this index shows a good correlation with invasive PWV. In a second patient cohort, we have shown that CTASI can risk-stratify patients with regards to 1-year mortality.
Direct vascular assessment by CTASI
Conventional cardiovascular risk factors, while useful and modifiable, do not give a direct measure of cardiovascular health and cannot therefore consistently predict the development of overt disease [30] nor identify preclinical states [31]. There are several alternative modalities that can offer a more direct assessment of the vascular system by measuring either physiological or anatomical characteristics.
Physiological assessment tools include endothelial function testing using techniques such as flow mediated dilatation or peripheral artery tonometry both of which are prognostic markers of cardiovascular events and mortality [6]. However, these are relatively operator dependent [7], have modest levels of reproducibility [8] and their results have not been consistent in all studies [7]. MRI-calculated PWV velocity has been shown to predict cardiovascular events in some (but not all) age-groups [9]. Similarly, aortic distensibility can also be measured using MRI [12] where it correlates with mortality and cardiovascular events [11]. Alternatively, a number of specific physiology-based non-invasive tools have been devised to assess PWV [10] but at present none are accurate enough for clinical recommendation [20].
Carotid Intima-Medial Thickness is an anatomical assessment measured by ultrasound and is predictive of future cardiovascular events [13] although its additive effect over other conventional risk factors is small [14]. Coronary artery calcium scoring through CT scanning appears to provide greater insight into cardiovascular event risk [15] although is more expensive and laborious. Additionally, aortic calcification in the descending aorta measured by CT has been shown to correlate with non-invasive PWV [16] and accordingly can predict cardiac and all-cause mortality [17, 18], particularly if more rapidly progressive [19].
Given the ability of either physiological and anatomical characteristics to measure vascular stiffness, we identified the potential for CT to assess both of these domains. Whilst CT carries a good spatial resolution, its contrast and temporal resolution is lower than MRI but despite this, we found relative clarity in identifying the dynamic changes in ascending aorta diameter during the cardiac cycle in the majority (90%) of patients. Even without the integration of calcification, we found a reasonable correlation with invasively obtained PWV. Calcification was assessed using a modified Agatston methodology [22] thus allowing the protocol to be completed in a single scan.
Our resultant measure of vascular health (CTASI) therefore directly quantifies aortic stiffness, one of the earliest subclinical changes in the progression of cardiovascular disease [32]. By incorporating both anatomical (aortic calcification) and physiological (aortic distensibility) characteristics, we have created an index that correlates well with invasive-PWV and is able to predict an abnormal invasvie-PWV [28] more impressively than either characteristic alone. Furthermore, CTASI was appropriately related to patient factors (BMI and age) [5, 33].
CTASI and prognosis
Aortic stiffness is superior to brachial systolic or pulse pressure in its assessment of cardiovascular health [34]. Invasively obtained PWV is the gold-standard measure of aortic stiffness [1] and is a good predictor of subclinical [4] and clinical [3] end-organ damage, as well as cardiovascular mortality independent of classical cardiovascular risk factors [2]. Recent work has shown that it predicts mortality in patients undergoing TAVI [5].
Thus, to demonstrate the clinical importance of CTASI, we wanted to replicate these findings. In a second cohort of patients undergoing only CTASI calculation, we have confirmed this. Appropriately, the estimated cut-off value of 10.5 m/s is very similar to our previous invasive findings [5] and consistent with current guidelines [28]. Whilst both of the individual measures of calcification and distensibility have some prognostic abilities in this regard neither was as strong as CTASI. Furthermore, in our moderately sized cohort, CTASI was more informative than other known or potential prognostic markers.
Therefore, CTASI has the potential for prospective use to risk stratify and guide therapy [35,36,37] in all patients undergoing contrast CT, or could be used as a tool to assess cardiovascular health in its own right. A particular group of patients for which this will be applicable is those undergoing CT coronary assessment where a broader assessment of vascular health, in addition to the recognition of coronary disease, can now be assessed and treated.
Limitations
In clinical practice, a significant number of cardiac contrast CT scans are limited to the thorax and will not include more distal vasculature. However, they will therefore include the ascending aorta, arch and descending aorta and calcification from this section could be used to calculate CTASI. Further work to clarify the relationship between this quantification of calcification and the measure of calcification we have used here will be required to confirm the weighting for this more focussed assessment. Encouragingly, the aortic arch seems the most informative in terms of aortic calcification and risk [18].
For the purposes of our follow-up cohort, we were forced to exclude 177 patients because of inadequate or unavailable CT data, or because blood pressure was not recorded simultaneously. However, there is nothing to suggest these patients differed in any way from our final cohort with similar Logistic Euroscore 1 values (15.4 [95% CI 14.2–17.6] vs 17.2 [95% CI 15.6–19.9], p = 0.32).
The rate of exclusion with regards to adequate CT data occurred in 10% of patients in this study. Whilst this partly reflects the limitations of CT with some implications for further work, it is recognised that the TAVI cohorts present some challenges in scanning where beta-blockade is not used and the patient’s ability to adequately breath-hold is often impaired. Therefore, we would anticipate a lower exclusion rate in the general population.
In comparison to MRI, the temporal resolution of CT is lower. This may have implications on accuracy when the two modalities are compared but we anticipate this being minor. Unlike MRI, we were able to include our second assessment of stiffness (that of calcification) that ultimately improved the accuracy of our measurement. We also acknowledge that retrospective ECG-synchronisation carries a higher radiation dose and further work may be required to establish whether prospective ECG- synchronisation is as accurate for CTASI.
We opted to exclude patients from follow-up if they died within the first 30 days after TAVI on the assumption that their cause of death may have stemmed from the procedure rather than their cardiovascular health. When they were included in the analysis, the predictive value of CTASI was preserved (Kaplan–Meier log-rank p = 0.01) demonstrating that this action did not cloud our analysis.
The concept of quantifying aortic calcification by the Agatston method with a modified Hounsfield threshold has been used by several groups previously [16, 17, 19]. We chose to use a cut-off value that has been demonstrated to be most insightful when contrast is administered [22]. However, further work may be required comparing contrast and non-contrast scans to ensure maximal accuracy has been achieved.
Conclusion
By assessing the distensibility of the ascending aorta and incorporating the degree of aortic calcification, we have created the CT aortic stiffness index (CTASI). We have shown the clinical relevance of CTASI in that it is able to predict mortality in patients following a TAVI. This measure could now be obtained in any patient undergoing a contrast CT scan to provide a direct assessment of vascular health, identify preclinical disease states and potentially guide early intervention strategies. Further evaluation of CTASI in other cardiovascular risk groups is warranted.
Availability of data and material
Datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Code availability
Code is custom built by one of the authors (CJB).
References
Laurent S, Cockcroft J, Van Bortel L et al (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27:2588–2605. https://doi.org/10.1093/eurheartj/ehl254
Ben-Shlomo Y, Spears M, Boustred C et al (2014) Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63:636–646. https://doi.org/10.1016/j.jacc.2013.09.063
Townsend RR, Anderson AH, Chirinos JA et al (2018) Association of pulse wave velocity with chronic kidney disease progression and mortality: findings from the CRIC study (chronic renal insufficiency cohort). Hypertens 71:1101–1107. https://doi.org/10.1161/HYPERTENSIONAHA.117.10648 (Dallas, Tex 1979)
Coutinho T, Turner ST, Kullo IJ (2011) Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc Imaging 4:754–761. https://doi.org/10.1016/j.jcmg.2011.04.011
Broyd CJ, Patel K, Pugliese F et al (2019) Pulse wave velocity can be accurately measured during transcatheter aortic valve implantation and used for post-procedure risk stratification. J Hypertens. https://doi.org/10.1097/HJH.0000000000002141
Xu Y, Arora RC, Hiebert BM et al (2014) Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Hear J - Cardiovasc Imaging 15:736–746. https://doi.org/10.1093/ehjci/jet256
Yeboah J, McClelland RL, Polonsky TS et al (2012) Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 308:788–795. https://doi.org/10.1001/jama.2012.9624
Brant LCC, Barreto SM, Passos VMA, Ribeiro ALP (2013) Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens 31:1984–1990. https://doi.org/10.1097/HJH.0b013e328362d913
Ohyama Y, Ambale-Venkatesh B, Noda C et al (2017) Aortic arch pulse wave velocity assessed by magnetic resonance imaging as a predictor of incident cardiovascular events. Hypertension 70:524–530. https://doi.org/10.1161/HYPERTENSIONAHA.116.08749
Milan A, Zocaro G, Leone D et al (2019) Current assessment of pulse wave velocity: comprehensive review of validation studies. J Hypertens. https://doi.org/10.1097/HJH.0000000000002081
Redheuil A, Wu CO, Kachenoura N et al (2014) Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: the MESA study. J Am Coll Cardiol 64:2619–2629. https://doi.org/10.1016/j.jacc.2014.09.060
Stoiber L, Ghorbani N, Kelm M et al (2020) Validation of simple measures of aortic distensibility based on standard 4-chamber cine CMR: a new approach for clinical studies. Clin Res Cardiol 109:454–464. https://doi.org/10.1007/s00392-019-01525-8
O’Leary DH, Polak JF, Kronmal RA et al (1999) Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 340:14–22. https://doi.org/10.1056/NEJM199901073400103
Zhang Y, Guallar E, Qiao Y, Wasserman BA (2014) Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arterioscler Thromb Vasc Biol 34:1341–1345. https://doi.org/10.1161/ATVBAHA.113.302075
Greenland P, LaBree L, Azen SP et al (2004) Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 291:210–215. https://doi.org/10.1001/jama.291.2.210
Temmar M, Liabeuf S, Renard C et al (2010) Pulse wave velocity and vascular calcification at different stages of chronic kidney disease. J Hypertens 28:163–169. https://doi.org/10.1097/HJH.0b013e328331b81e
Alon E, Alexander T, Nira K-M et al (2008) Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients. Circulation 118:1328–1334. https://doi.org/10.1161/CIRCULATIONAHA.107.712141
Harbaoui B, Montoy M, Charles P et al (2016) Aorta calcification burden: Towards an integrative predictor of cardiac outcome after transcatheter aortic valve implantation. Atherosclerosis 246:161–168. https://doi.org/10.1016/j.atherosclerosis.2016.01.013
Kalsch H, Mahabadi AA, Moebus S et al (2018) Association of progressive thoracic aortic calcification with future cardiovascular events and all-cause mortality: ability to improve risk prediction? Eur Heart J Cardiovasc Imaging, Results of the Heinz Nixdorf Recall (HNR) study. https://doi.org/10.1093/ehjci/jey173
Williams B, Mancia G, Spiering W et al (2018) 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. https://doi.org/10.1093/eurheartj/ehy339
Agatston AS, Janowitz WR, Hildner FJ et al (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–832. https://doi.org/10.1016/0735-1097(90)90282-T
Jilaihawi H, Makkar RR, Kashif M et al (2014) A revised methodology for aortic-valvar complex calcium quantification for transcatheter aortic valve implantation. Eur Hear J - Cardiovasc Imaging 15:1324–1332. https://doi.org/10.1093/ehjci/jeu162
Bramwell JC, Hill AV (1922) The velocity of the pulse wave in man. Proc R Soc B Biol Sci 93:298–306. https://doi.org/10.1098/rspb.1922.0022
Freedman LS (1982) Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1:121–129. https://doi.org/10.1002/sim.4780010204
Holmes DR, Brennan JM, Rumsfeld JS et al (2015) Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA 313:1019. https://doi.org/10.1001/jama.2015.1474
Zahn R, Gerckens U, Linke A et al (2013) Predictors of one-year mortality after transcatheter aortic valve implantation for severe symptomatic aortic stenosis. Am J Cardiol 112:272–279. https://doi.org/10.1016/j.amjcard.2013.03.024
Wendler O, Schymik G, Treede H et al (2017) SOURCE 3: 1-year outcomes post-transcatheter aortic valve implantation using the latest generation of the balloon-expandable transcatheter heart valve. Eur Heart J 38:2717–2726. https://doi.org/10.1093/eurheartj/ehx294
Mancia G, De Backer G, Dominiczak A et al (2007) 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 25:1105–1187. https://doi.org/10.1097/HJH.0b013e3281fc975a
Collaboration TRV, for AS, (2010) Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values.’ Eur Heart J 31:2338–2350
Wang TJ (2011) Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation 123:551–565. https://doi.org/10.1161/CIRCULATIONAHA.109.912568
Devereux RB, Alderman MH (1993) Role of preclinical cardiovascular disease in the evolution from risk factor exposure to development of morbid events. Circulation 88:1444–1455. https://doi.org/10.1161/01.cir.88.4.1444
Redheuil A, Yu W-C, Wu CO et al (2010) Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertens 55:319–326. https://doi.org/10.1161/HYPERTENSIONAHA.109.141275 (Dallas, Tex 1979)
Huisman HW, Schutte R, Venter HL, van Rooyen JM (2015) Low BMI is inversely associated with arterial stiffness in Africans. Br J Nutr 113:1621–1627. https://doi.org/10.1017/S0007114515000975
Mitchell GF, Hwang S-J, Vasan RS et al (2010) Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121:505–511. https://doi.org/10.1161/CIRCULATIONAHA.109.886655
Shahin Y, Khan JA, Chetter I (2012) Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: a meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis 221:18–33. https://doi.org/10.1016/j.atherosclerosis.2011.12.005
D’elia L, La Fata E, Iannuzzi A, Rubba PO, (2018) Effect of statin therapy on pulse wave velocity: a meta-analysis of randomized controlled trials. Clin Exp Hypertens 40:601–608. https://doi.org/10.1080/10641963.2017.1411498
Metsamarttila E, Rodilla E, Jokelainen J et al (2018) Effect of physical activity on pulse wave velocity in elderly subjects with normal glucose, prediabetes or Type 2 Diabetes. Sci Rep 8:8045. https://doi.org/10.1038/s41598-018-25755-4
Funding
FP—this work form part of the translational portfolio of the Cardiovascular Biomedical Research Centre at Barts which is supported and funded by the NIHR. KP is supported by an unrestricted educational grant from Edwards Lifesciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest/competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent to participate
Data were collected as part of the routine clinical care for each patient. No individual is identifiable from the manuscript.
Rights and permissions
About this article
Cite this article
Broyd, C.J., Pugliese, F., Patel, K. et al. Development of a novel CT-derived measure of cardiovascular health: the CT aortic stiffness index (CTASI). Clin Res Cardiol 110, 1781–1791 (2021). https://doi.org/10.1007/s00392-021-01861-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01861-8