Abstract
Despite impressive improvement in long-term survival, adults with congenital heart disease (CHD) remain exposed to a significant cardiovascular morbidity over lifetime. Thromboembolic events (TE) are a major issue. Specific anatomic groups have been shown a particular high risk of TE, including cyanotic heart disease and Fontan circulation. Many intercurrent clinical factors add a substantial risk such as intracardiac medical devices, atrial arrhythmia, endocarditis, or pregnancy. Nevertheless, what is unknown exceeds what is known, especially regarding the management of this heterogenous patient population. Anticoagulation decision should always be individualized weighing balanced with the alternative risk of hemorrhagic complications. In this review, we aim to synthetize existing literature on TE in adults with CHD, discuss management issues, highlight gaps in knowledge, and intend to suggest high priority research.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: overview of the issue
The steadily growing population of patients with congenital heart disease (CHD) represents more than 1 million people in Europe [1, 2]. While long-term prognosis of most cardiac defects has been improved by recent advances in pediatric and adult cardiology, these patients remain exposed to a significant cardiovascular morbidity over lifetime [3,4,5,6].
Along with arrhythmic events and heart failure, thromboembolic (TE) complications are a central issue, accounting for approximately 4% of the overall CHD mortality [3,4,5,6,7,8,9]. In an analysis of 23,153 adult patients with CHD from European and Canadian centers, the annual incidence of cerebrovascular accidents was 0.05%, representing more than ten times the rate expected in control populations of comparable age [6]. Ischemic strokes (or transient ischemic attacks) accounted for the majority of cases (1.5% hemorrhagic causes) and were associated with persistent neurological deficits in 25% [6]. An even higher incidence was reported in the Euro Heart Survey, in which a stroke was recorded in 4% among 4000 patients followed over a 5-year period [3]. The cumulative risk appears considerably higher than in the general population, especially at a younger age, with 1 in 11 men and 1 in 15 women experiencing a stroke between ages 18 and 64 years [4]. Venous thrombosis and pulmonary embolism complications are less described in CHD patients. Reported incidence widely varies from 3 to 40% according to cardiac defect and study design. However, specific data about venous thrombosis in CHD are scarce.
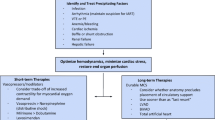
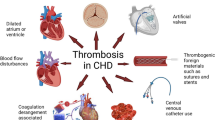
The risk for developing TE complications depends on the conjunction of many interlinked factors and conditions (central illustration). It varies also widely according to the nature and severity of the underlying cardiac defect (Fig. 1). However, given the specificities and heterogeneity of patients with CHD, risk stratification and management of TE remains challenging in this population, and several questions remain unanswered. In this review, we synthetize existing literature on TE complications in adults with CHD, address management issues, and discuss knowledge gaps and areas for future research. Main CHD and specific clinical scenario associated with TE complications are detailed.
Annualized incidences of thromboembolism complications in adults with congenital heart diseases (CHD), in mild, moderate, and complex CHD, in ACHD with valvular heart diseases (VHD), in cyanotic CHD and in patients with Fontan circulation. Annualized incidences are provided in CHD subgroups with regards to the predisposing factors (atrial arrhythmias and pregnancy). ACHD adult with congenital heart disease
Underlying conditions and intercurrent factors linked to TE risk
TE risk related to the anatomical complexity of the disease and the operation performed to correct the disease
Univentricular heart and Fontan circulation
In patients with functionally univentricular heart, the Fontan surgery, connecting the systemic venous return to the pulmonary arteries, has been developed to bypass the inadequate or absent sub-pulmonary ventricle. TE is a common complication after Fontan surgery and is one of the main causes of long-term mortality [10]. However, unknowns about this complication exceed the known. Long-term TE incidence was estimated between 3.3 and 7.4 per 100 patient-years [11,12,13,14] with different rates of systemic venous (1–5.6%) and arterial emboli (1.4–2.2%) [14,15,16]. However, the incidence depends on study design, methods of diagnosis, and follow-up time. Moreover, the possibility of clinically silent thrombi in the Fontan circulation and silent pulmonary embolism is significant [17]; as a consequence, the true incidence of TE events is still undetermined.
The underlying pathophysiology of TE in this setting is complex and incompletely understood, but involves all three factors of Virchow’s triad: hypercoagulability, abnormal hemodynamics, and endothelial injury/dysfunction. Coagulation factor abnormalities predisposing to TE have been described in various constellations after the Fontan procedure, suggesting an unpredictable risk of imbalance in procoagulant and anticoagulant factors [18, 19]. Decreased levels of protein C, protein S, antithrombin III, and coagulation factors have been reported [20,21,22]. An impaired fibrinolytic potential, with increased thrombin activatable fibrinolysis inhibitor, and increased platelet reactivity in patients who experienced TE have also been described in Fontan adults [20, 21]. Finally, impaired endothelial function has been demonstrated [21, 23, 24]. All these mechanisms are illustrated in Fig. 2a. Chronic systemic venous hypertension in Fontan patients, hemodynamic changes, and temporary liver dysfunction have been proposed as a plausible hypotheses for abnormal liver protein synthesis and hypercoagulability [19, 25, 26]. Protein losing enteropathy that occurs in 8% of patients after Fontan surgery [27] also results in a loss of serum proteins into the gastrointestinal tract with subsequent impairment of the clotting cascade [28].
a Clotting cascade in Fontan patient. Fontan patients are characterized by enhanced platelet activation and endothelial injury, reduced free protein S and C levels, reduced plasma levels of coagulation factors and plasminogen, and increased von Willebrand factor (vWF). These complex hemostatic disturbances can favor bleeding as much as promote thrombosis. b Clotting cascade in cyanotic adults with congenital heart diseases. These patients have both an increased risk of thrombosis and bleeding resulting from multiple pathways
The combined effects of the abnormalities of coagulation, platelet activation, and endothelial dysfunction on thrombus formation are imperfectly established, and correlation to TE has not been clearly shown. Therefore, the therapeutic target to prevent a TE complication is still undetermined. Patients with an atrio-pulmonary connection are known to be at higher risk for TE [29, 30], and in patients with an atrial arrhythmia, TE risk increases significantly up to 6.5% per 100 patient-years [14]. However, the identification of Fontan patients at high risk of TE remains challenging.
Cyanotic congenital heart diseases and pulmonary hypertension
Patients with cyanotic congenital heart disease have a high prevalence of thrombosis and an increasing risk of bleeding. The hypoxaemia results from CHD with right to left shunt. Hypoxaemia is observed in patients with an intracardiac defect and pulmonary stenosis or pulmonary hypertension (e.g., Eisenmenger syndrome).
Pulmonary hypertension is associated with an increased risk of thrombosis both in patients with and without congenital heart disease. Patients with Eisenmenger syndrome are at the higher risk, combining pulmonary vascular lesions resulting from pulmonary arterial hypertension and hypoxemia.
Cerebral and pulmonary thromboses are the most common described locations. Its prevalence arises 20–40% in tomodensitometry studies [31,32,33]. Patients with complex cyanotic CHD without pulmonary hypertension such as univentricular heart seem to have a higher risk of cerebral thrombosis [3, 6], while patients with Eisenmenger syndrome have a higher risk of pulmonary thrombosis. Incidence of thrombotic events in patients with Eisenmenger syndrome was measured at 0.012% per year in a retrospective study [34].
Multiple pathophysiology pathways leading to prothrombotic condition have been suggested in these latter patients (Fig. 2b) [32]. Pulmonary artery endothelial dysfunction resulting from shear stress and local vascular injury is associated with chronic intravascular coagulation [35]. Slow blood flow in the pulmonary arteries promotes red cell aggregation [32, 33] and in situ thrombosis. Erythrocytosis secondary to hypoxaemia and hemostatic abnormalities may contribute to thrombogenesis [33, 34]. Iron deficiency due to repeated venesection results in spherocytosis and hyperviscosity. However, hypercoagulability status remains controversial [32, 36, 37]. Recent data suggest that hypercoagulability in Eisenmenger syndrome is platelet dependent [38]. Increased mean platelet volume is a risk marker of thrombosis in these patients [34]. Larger platelets appear to be more active and more thrombogenic, with an increased aggregating capacity compared with normal size platelets [34]. Peak thrombi generation is increased in Eisenmenger syndrome platelet-rich plasma compared to controls [38].
Thrombi are reported more common in women and in those with lower oxygen saturation [33]. Thrombosis risk observed in patients with cyanotic CHD with or without pulmonary hypertension is also linked to a higher incidence of predisposing factors such as atrial arrhythmias, heart failure [32], pregnancy, and infective endocarditis [39].
On the opposite, hypocoagulability status is associated with impaired fibrinogen function, higher haematocrit, and platelet activity [36,37,38] (Fig. 2b). Platelet activity seems to paradoxically modulate the activity of endogenous anticoagulant Protein C-related pathway in a manner that could promote bleeding [38]. Pulmonary infarction secondary to arterial thrombosis may contribute to the risk of hemoptysis in patients with Eisenmenger syndrome [40].
Incidence of Eisenmenger syndrome is decreasing over time given improvement in care in developing countries [41]. Accurate estimation of incidence and hazard ratio of risk markers in subgroups of this population is unfortunately unlikely to be precisely known, but a better understanding of physio-pathological pathways leading to thrombotic and bleeding risk is still achievable and should be encouraged as illustrated by recent studies investigating the role of platelets [34, 38].
TE risk related to valve and devices implanted as part of treatment
Valve-related procedures have become the most frequent indication for intervention in CHD patients. [42, 43]. This trend concerns mainly patients with tetralogy of Fallot, for whom the indication for pulmonary valve replacement has more than tripled over the past decade [44]. Incidence of valvular thrombosis for mechanical valves in the pulmonary position is higher (1.7% per patient-year) than in aortic and mitral position (around 0.3% per patient-year) [45,46,47]. Consequently, biological pulmonary valve is widely preferred, especially with the emergence of percutaneous pulmonary valve replacement techniques [48]. However, subclinical thrombus formation at the base of bioprosthetic valve cusps and on leaflets of a percutaneously implanted pulmonary valve has been frequently reported [49, 50]. To date, the clinical impact of thrombi in pulmonary bioprosthesis location remains uncertain. Subclinical leaflet thrombosis on aortic valves have been associated with increased rates of transient ischemic attack or strokes [51] and reoperations [52].
Along with various types of valves, different types of prosthetic material including surgical baffle, stent, pacing, or defibrillation lead, and shunt closure device are frequently used in patients with CHD, favoring thrombosis formation. In a large meta-analysis, device-related thrombosis was reported in 1.2% of cases after percutaneous atrial septal defect closure and mainly related to an old-fashioned device (0% with Amplatzer occluder versus 7% with CardioSEAL device) [53]. The pooled estimate rate of cerebrovascular events was 1.1% [53] and strongly related to the presence of atrial arrhythmia [54, 55]. A more than twofold increase in the risk of systemic TE has also been reported in patients with transvenous leads and intracardiac shunts [56]. The link between atrial arrhythmia and atrial device has to be determined especially after a long period of follow-up.
TE risk related to arrhythmias related to the disease
The prevalence of atrial arrhythmias is estimated around 15% in adult patients with CHD, and projections indicate that 50% of 20-year-old subjects will experience atrial tachyarrhythmia during their lifespan [57]. The burden of arrhythmias continues to grow with the aging of the CHD population, and patterns tend to change with an increase of atrial fibrillation and permanent forms of atrial arrhythmias [58].
Atrial arrhythmias are associated with a 60–190% increase in the risk of stroke in CHD patients compared with CHD patients without atrial arrhythmias [57, 59,60,61]. Annual TE event rates of 0.00%, 0.93%, and 1.95% per year in patients with atrial arrhythmias and simple, moderate, and severe CHD forms have been reported in a multicenter study with antithrombotic therapy let at the physician’s discretion [62]. The mean annual TE rate was as high as 1.14% and this is comparable to rates observed in studies including non-CHD patients with non-valvular atrial fibrillation despite their younger age and fewer traditional risk factors. A recent prospective study found a similar annual rate (1.0%) of TE complications in a population of adult patients with CHD receiving NOACs mainly for atrial arrhythmias [63].
A comprehensive appraisal of the mechanistic basis of TE complications in patients with atrial arrhythmia is beyond the scope of this review, but involves a complex interplay between systemic factors, contractile dysfunction and stasis during arrhythmia episodes, and progressive structural and electrical remodeling of the atrium [64]. Surgical scarring and chronic hemodynamic or hypoxic stress in patients with CHD result in the development of an atrial substrate prone to TE complications with important atrial dilatation and remodeling.
Several questions remain unanswered. First, most studies have lacked the granularity to distinguish between types of atrial arrhythmias in assessing associations with adverse events. Only one multicenter study specifically addressed the TE risk associated with atrial fibrillation compared with other forms of atrial arrhythmias (intra-atrial reentrant tachycardia or focal atrial tachycardia) and did not observe a significant difference [62]. Second, no specific score to estimate the TE risk associated with atrial arrhythmias has been developed in CHD, and CHADS2 or CHA2DS2-VASc scores have shown mixed results in this population. While CHADS2 or CHA2DS2-VASc scores were similar in patients with and without events in different studies [62, 65], the CHA2DS2-VASc score at a cut-off of ≥ 2 was associated with an annual rate of 3% vs. 0.7% (HR 3.7; 95% CI 1.2–11.5) in another retrospective study [66]. Rather than traditional atherosclerotic risk factors, complexity of CHD appears to be the main risk factor in patients with CHD [62]. Consequently, future score models should be developed integrating CHD complexity. Third, considering the important improvement of outcomes of interventional approaches and given the young age of most patients, another important issue is the possibility to stop anticoagulation after a successful ablation. However, recurrences remain common, and there are for now no specific guidelines on this topic. Individualized decisions should integrate the complete history of arrhythmic events, underlying CHD complexity, and associated TE risk factors. Electrophysiological findings during an ablation procedure, including arrhythmia mechanisms and circuits, extent of electrical remodeling, and inducibility of other arrhythmias, may also be useful.
TE risk related to other complications, notably endocarditis
Infective endocarditis is a common complication especially with large vegetations [67]. In different CHD populations, TE has been documented in up to 40% of patients, with a majority of cerebrovascular events [68, 69]. Both emboli [69] and large vegetations over 20 mm [70] have been associated with overall mortality in adults with CHD. Furthermore, with the increased number of patients with an intravascular device or valvular prosthesis, the potential risk of infective endocarditis and so of septic emboli has been expanded.
Incidence of TE after cardiac surgery is around 1% in adults with CHD [71]. In a European multicenter study, 19 (0.9%) patients out of 2012 had persistent neurological deficit at discharge after congenital cardiac surgery [72]. After percutaneous procedures in patients with CHD, the risk appears very low with a rate of 0.04% reported in a large national registry in the United States [73].
Heart failure is also strongly associated with stroke in CHD, especially among younger patients: the risk of TE has been reported sixfold higher in patients aged 18–49 years [4]. Blood stasis involved in thrombus formation, and neuroendocrine and hemorheological abnormalities are possible underlying mechanisms.
Indication for antithrombotic therapy in CHD
Most guidelines for preventive antithrombotic therapy in CHD are backed only by level C evidence (Table 1) [74,75,76,77,78,79,80,81]. Indeed, randomized clinical trials to guide management decisions are almost non-existent. Consequently, most recommendations are extrapolated from guidelines in acquired cardiac diseases.
Risk stratification and optimal strategy to prevent TE in Fontan patients are under debate, and practices vary considerably among institutions. A lower incidence of TE was suggested with prophylaxis (either aspirin or warfarin) in a meta-analysis [30]. These results have been confirmed in a recent study using a propensity score matching analysis based on a large population of patients with an extracardiac Fontan [82]. However, failure rates were still relatively high (∼9%) with either agent. This may be partly explained by a suboptimal anticoagulation/antiplatelet therapy. Indeed, some studies reported an increased risk of thrombosis in patients under vitamin K antagonists (VKA) who had subtherapeutic INRs, in patients who spent less time in the therapeutic range and in patients who discontinued the anticoagulation [14, 82, 83]. Except cases with atrial arrhythmia or previous history of TE, the benefit to warfarin over aspirin for preventing TE in patients, although theoretically maybe obvious, has not been clearly demonstrated. On the other hand, bleeding complications have been reported in 3–7% of patients with a higher rate in patients treated with VKA [14, 82]. Therefore, the decision to treat a Fontan patient with targeting platelet function or with oral anticoagulation for prevention of TE needs an individual approach.
In patients with idiopathic pulmonary arterial hypertension, antithrombotic therapy may be considered with a low level of evidence (grade IIb, C) [75]. There are no data on this issue in patients with CHD and pulmonary hypertension and definite recommendations favor an individual approach [75, 77]. Oral anticoagulation may be considered in patients without significant haemoptysis in the presence of systemic or peripheral thromboembolism, or pulmonary embolism/thrombosis, particularly when concomitant TE risk factors exist such as atrial arrhythmias, heart failure, or artificial valves/conduits [32, 75, 79]. However, in a 2018 consensus statement from Germany, it is reminded that, currently, there are no data indicating that oral anticoagulation has a positive effect on morbidity and mortality in patients with Eisenmenger syndrome (ES) [79, 84]. Moreover, technical challenges to determine coagulation parameters in this particular population may be another issue to follow patients under antithrombotic therapy [79]. In patients with cyanotic CHD without pulmonary hypertension, predisposing condition to both thrombosis and bleeding limit the indication of antithrombotic therapy [77]. Therapy may be restricted to patients with associated risk factors in a case-by-case analysis.
In patients with intracardiac leads and residual shunt or at high risk of paradoxical emboli, surgical or percutaneous closure is crucial to prevent systemic emboli [56, 74]. After stent implantation, surgical of transpulmonary pulmonary valve replacement, or closure device, antiplatelet agents are usually empirically prescribed for 3–6 months [85]. Strikingly, medical therapy after pulmonic bioprosthesis replacement is not discussed in any guidelines (neither valvular nor congenital) [74, 77, 86, 87]. Empirically aspirin is prescribed 3–6 months following the replacement. A few centers recommend long-life aspirin to prevent infective endocarditis [88]. An expert consensus has to be achieved.
In the absence of specific data for TE prevention in atrial arrhythmia in CHD, latest guidelines endorsed by international heart rhythm societies suggest to use CHA2DS2-VASc score to guide anticoagulation therapy in simple CHD [80, 81] (Table 1). In patients with moderate and complex CHD and atrial arrhythmias, anticoagulation is recommended. However, these recommendations date from 2014, when data on NOAC therapy were only scarce. While evidence is emerging on safety and efficacy of NOACs in this population [63, 89], VKA remains the anticoagulant agent of choice in complex CHD (in particular in Fontan patients). However, an individual approach again is needed to determine to most appropriate anticoagulation therapy.
As pregnancy increases TE risk, international guidelines [76] recommend considering therapeutic anticoagulation in pregnant women with a Fontan circulation (IIaC), PAH, or Eisenmenger syndrome (balanced with the risk of bleeding). For the other cardiac conditions which can be met during pregnancy (i.e., atrial arrhythmia, heart failure, or valvular heart disease), indication of anticoagulation treatment and regimen follows general guidelines from the European Society of Cardiology [76] and the AHA/American College of Cardiology [90].
Non-vitamin K antagonist oral anticoagulants
Non-vitamin K antagonist oral anticoagulants (NOACs) have emerged as an alternative to vitamin K antagonists (VKA) [91]. In non-valvular atrial fibrillation, guidelines suggest to use NOACs as first-line therapy in eligible patients, considering at least non-inferior efficacy in preventing thromboembolism and more favorable safety profile [92]. The convenience of NOACs use, with no need for monitoring or frequent dose adjustments, appears particularly attractive in the young and active CHD population. In CHD patients with atrial arrhythmias, in the absence of robust data, an expert consensus statement (2014) discouraged the use of NOACs in moderate and complex forms, especially those with Fontan palliation [80]. However, NOAC use in adult with CHD has been recently promoted by the last ACC/AHA recommendations [77], and a comprehensive overview on NOAC use in adult patients with CHD has been recently published by a consortium of CHD experts [93]. Moreover, data from a large ongoing prospective registry, NOTE registry, have recently confirmed the short-term safety and efficacy of NOAC in 530 adult patients with CHD [63]. The most common indication was atrial arrhythmia. Outcomes on NOAC safety and efficacy in 74 Fontan patients from this registry were also promising [89].
Research priorities on thromboembolism complications in adult with CHD
Multiple unknowns are raised by this review. We listed the main gaps of knowledge in Table 2 with the corresponding research priorities. Two major priorities emerged: 1/ to determine the risk of TE complications in specific subgroups and thus stratify the risk of CHD, and 2/ to define the optimal preventive strategy. This kind of research requires large-scale studies implying international collaborations. Nevertheless, in such a heterogeneous ACHD population, we would need thousands of patients per defect. And we should include those patients within a limited period of time (< 5 years or so), to avoid trouble in evaluating patients from different era or treatment strategies. However, for outcome studies, we would need a long follow-up period, since the event rate is low. While large randomized-controlled trials are in most cases unrealistic in attempting to answers these questions, international collaborative multicenter cohort studies are feasible and should be encouraged. Alternatively, using artificial intelligence with machine learning algorithms trained on large datasets appears as a promising option in the ACHD population [94] (Fig. 1).
Pregnancy
Both CHD and pregnancy are prothrombotic conditions [95, 96]. Venous thromboembolism in pregnancy represents an important cause of maternal morbidity and mortality in developed countries, with an incidence of 0.5–2.2 per 1000 pregnancies [97]. In comparison, TE risk in CHD women is significantly increased during pregnancy. Among 3295 pregnant women with CHD included in the ROPAC registry over 10 years, the incidence of TE events was 1% [98]. This is partly explained by the complexity of the underlying cardiac defect. Women with a mechanical valve had the highest rate of TE complications with an incidence of valve thrombosis during pregnancy of 7% in ROPAC registry, which was lethal in 18% [98]. TE complications have also been reported in 4% of pregnant women with Fontan surgery [99], and pulmonary embolism was found as a main cause of death in Eisenmenger patients during postpartum [100]. However, none TE was reported in a contemporary observational study on 71 pregnancies in patients with cyanotic CHD [39]. Hemodynamic changes related to pregnancy can promote atrial arrhythmia which occurred in 2% of women from ROPAC [98]. This event was associated with an increase in maternal deaths partly attributable to thromboembolic complications [101]. Unfortunately, there is a paucity of high-quality evidence on the prevention and treatment of pregnancy-related VTE specifically in CHD patients, and the current recommendations are based on observational studies or evidence gathered from studies in the non-pregnant population. In the absence of randomized control trials, guidelines recommend to consider VKAs continuation in women with a mechanical valve when the VKA dose is low, with VKAs being the most effective regimen to prevent valve thrombosis during pregnancy, otherwise discontinue and replace VKAs with adjusted-dose UFH or LMWH. They recommend to consider LMWH prophylactic anticoagulation in high TE risk CHD such as patients with Fontan circulation or pulmonary hypertension and to apply the same rules in patients who experienced atrial arrhythmia as in non-pregnant patients [76].
Conclusion
TE are an important source of morbidity and mortality in CHD. Multiple and sometimes cumulative underlying conditions and interlaying factors, including arrhythmia, persistent shunt, intracardiac device, prosthetic valve, endocarditis, medical interventions, impaired hemostasis, or hypercoagulable states, are behind the risk. The highest-risk CHD such as Fontan circulation and cyanotic patients require peculiar attention. However, little is known about the optimal management and preventive strategies of TE in the CHD population, and a global risk score such as the CHADSVASC but including specificities of CHD complexity should be developed. Multicenter and prospective studies are imperatively needed to shed light on these complex issues.
Abbreviations
- CHD:
-
Congenital heart disease
- NOAC:
-
Non-vitamin K antagonist oral anticoagulants
- TE:
-
Thromboembolic event
- VHD:
-
Valvular heart disease
- VKA:
-
Vitamin K antagonists
References
Baumgartner H (2014) Geriatric congenital heart disease: a new challenge in the care of adults with congenital heart disease? Eur Heart J 35:683–685
Moons P, Bovijn L, Budts W, Belmans A, Gewillig M (2010) Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 122:2264–2272
Engelfriet P, Boersma E, Oechslin E et al (2005) The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The Euro Heart Survey on adult congenital heart disease. Eur H J 26:2325–2333
Lanz J, Brophy JM, Therrien J, Kaouache M, Guo L, Marelli AJ (2015) Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation 132:2385–2394
Ammash N, Warnes CA (1996) Cerebrovascular events in adult patients with cyanotic congenital heart disease. J Am Coll Cardiol 28:768–772
Hoffmann A, Chockalingam P, Balint OH et al (2010) Cerebrovascular accidents in adult patients with congenital heart disease. Heart 96:1223–1226
Nieminen HP, Jokinen EV, Sairanen HI (2007) Causes of late deaths after pediatric cardiac surgery: a population-based study. J Am Coll Cardiol 50:1263–1271
Raissadati A, Nieminen H, Haukka J, Sairanen H, Jokinen E (2016) Late causes of death after pediatric cardiac surgery: a 60-year population-based study. J Am Coll Cardiol 68:487–498
Karsenty C, Zhao A, Marijon E, Ladouceur M (2018) Risk of thromboembolic complications in adult congenital heart disease: a literature review. Archiv Cardiovasc Dis 111:613–620
Alsaied T, Bokma JP, Engel ME et al (2017) Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart 103:104–110
Cheung YF, Chay GW, Chiu CS, Cheng LC (2005) Long-term anticoagulation therapy and thromboembolic complications after the Fontan procedure. Int J Cardiol 102:509–513
Seipelt RG, Franke A, Vazquez-Jimenez JF et al (2002) Thromboembolic complications after Fontan procedures: comparison of different therapeutic approaches. Ann Thorac Surgery 74:556–562
Idorn L, Jensen AS, Juul K et al (2013) Thromboembolic complications in Fontan patients: population-based prevalence and exploration of the etiology. Pediatr Cardiol 34:262–272
Egbe AC, Connolly HM, McLeod CJ et al (2016) Thrombotic and embolic complications associated with atrial arrhythmia after fontan operation: role of prophylactic therapy. J Am Coll Cardiol 68:1312–1319
d'Udekem Y, Iyengar AJ, Galati JC et al (2014) Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 130:S32–S38
Kaulitz R, Ziemer G, Rauch R et al (2005) Prophylaxis of thromboembolic complications after the Fontan operation (total cavopulmonary anastomosis). J Thorac Cardiovasc Surg 129:569–575
Grewal J, Al Hussein M, Feldstein J et al (2013) Evaluation of silent thrombus after the Fontan operation. Congenit Heart Dis 8:40–47
Tomita H, Yamada O, Ohuchi H et al (2001) Coagulation profile, hepatic function, and hemodynamics following Fontan-type operations. Cardiol Young 11:62–66
Odegard KC, McGowan FX Jr, Zurakowski D et al (2003) Procoagulant and anticoagulant factor abnormalities following the Fontan procedure: increased factor VIII may predispose to thrombosis. J Thorac Cardiovasc Surg 125:1260–1267
Ravn HB, Hjortdal VE, Stenbog EV et al (2001) Increased platelet reactivity and significant changes in coagulation markers after cavopulmonary connection. Heart 85:61–65
Tomkiewicz-Pajak L, Hoffman P, Trojnarska O, Lipczynska M, Podolec P, Undas A (2014) Abnormalities in blood coagulation, fibrinolysis, and platelet activation in adult patients after the Fontan procedure. J Thorac Cardiovas Surg 147:1284–1290
Cromme-Dijkhuis AH, Henkens CM, Bijleveld CM, Hillege HL, Bom VJ, van der Meer J (1990) Coagulation factor abnormalities as possible thrombotic risk factors after Fontan operations. Lancet 336:1087–1090
Mahle WT, Todd K, Fyfe DA (2003) Endothelial function following the Fontan operation. Am J Cardiol 91:1286–1288
Binotto MA, Maeda NY, Lopes AA (2008) Altered endothelial function following the Fontan procedure. Cardiol Young 18:70–74
Chaloupecky V, Svobodova I, Hadacova I et al (2005) Coagulation profile and liver function in 102 patients after total cavopulmonary connection at mid term follow up. Heart 91:73–79
Procelewska M, Kolcz J, Januszewska K, Mroczek T, Malec E (2007) Coagulation abnormalities and liver function after hemi-Fontan and Fontan procedures—the importance of hemodynamics in the early postoperative period. Eur J Cardio-thorac Surg Off J Eur Assoc Cardio-thorac Surg 31:866–872
Atz AM, Zak V, Mahony L et al (2017) Longitudinal outcomes of patients with single ventricle after the fontan procedure. J Am Coll Cardiol 69:2735–2744
Rychik J (2007) Protein-losing enteropathy after Fontan operation. Congenit Heart Dis 2:288–300
Egbe AC, Connolly HM, Niaz T et al (2017) Prevalence and outcome of thrombotic and embolic complications in adults after Fontan operation. Am Heart J 183:10–17
Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR (2015) Strategies for thromboprophylaxis in Fontan circulation: a meta-analysis. Heart 101:1731–1737
Jensen AS, Idorn L, Thomsen C et al (2015) Prevalence of cerebral and pulmonary thrombosis in patients with cyanotic congenital heart disease. Heart 101:1540–1546
Broberg CS, Ujita M, Prasad S et al (2007) Pulmonary arterial thrombosis in eisenmenger syndrome is associated with biventricular dysfunction and decreased pulmonary flow velocity. J Am Coll Cardiol 50:634–642
Silversides CK, Granton JT, Konen E, Hart MA, Webb GD, Therrien J (2003) Pulmonary thrombosis in adults with Eisenmenger syndrome. J Am Coll Cardiol 42:1982–1987
Martin-Garcia AC, Arachchillage DR, Kempny A et al (2018) Platelet count and mean platelet volume predict outcome in adults with Eisenmenger syndrome. Heart 104:45–50
Lopes AA, Caramuru LH, Maeda NY (2002) Endothelial dysfunction associated with chronic intravascular coagulation in secondary pulmonary hypertension. Clin Appl Thrombosis/hemostas Off J Int Acad Clin Appl Thrombosis/Hemostas 8:353–358
Jensen AS, Johansson PI, Bochsen L et al (2013) Fibrinogen function is impaired in whole blood from patients with cyanotic congenital heart disease. Int J Cardiol 167:2210–2214
Jensen AS, Johansson PI, Idorn L et al (2013) The haematocrit–an important factor causing impaired haemostasis in patients with cyanotic congenital heart disease. Int J Cardiol 167:1317–1321
Kevane B, Allen S, Walsh K et al (2018) Dual endothelin-1 receptor antagonism attenuates platelet-mediated derangements of blood coagulation in Eisenmenger syndrome. J Thrombos Haemostas 16:1572
Ladouceur M, Benoit L, Basquin A et al (2017) How pregnancy impacts adult cyanotic congenital heart disease: a multicenter observational study. Circulation 135:2444–2447
Wood P (1958) The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. BMJ 2:755–762
Hjortshoj CS, Jensen AS, Sorensen K et al (2017) Epidemiological changes in eisenmenger syndrome in the nordic region in 1977–2012. Heart 103:1353–1358
Ionescu-Ittu R, Mackie AS, Abrahamowicz M et al (2010) Valvular operations in patients with congenital heart disease: increasing rates from 1988 to 2005. Ann Thorac Surg 90:1563–1569
Horer J, Belli E, Roussin R et al (2018) Evaluation of the adult congenital heart surgery mortality score at two european centers. Ann Thorac Surg 105:1441–1446
O'Byrne ML, Glatz AC, Mercer-Rosa L et al (2015) Trends in pulmonary valve replacement in children and adults with tetralogy of fallot. Am J Cardiol 115:118–124
Pragt H, van Melle JP, Javadikasgari H et al (2017) Mechanical valves in the pulmonary position: an international retrospective analysis. J Thorac Cardiovasc Surg 154(1371–1378):e1
Toole JM, Stroud MR, Kratz JM et al (2010) Twenty-five year experience with the St. Jude medical mechanical valve prosthesis. Ann Thorac Surg 89:1402–1409
Nishida T, Sonoda H, Oishi Y et al (2014) Single-institution, 22-year follow-up of 786 CarboMedics mechanical valves used for both primary surgery and reoperation. J Thorac Cardiovasc Surg 147:1493–1498
Caughron H, Kim D, Kamioka N et al (2018) Repeat pulmonary valve replacement: similar intermediate-term outcomes with surgical and transcatheter procedures. JACC Cardiovasc Intervent 11:2495–2503
Jewgenow P, Schneider H, Bokenkamp R et al (2019) Subclinical thrombus formation in bioprosthetic pulmonary valve conduits. Int J Cardiol 281:113–118
Hascoet S, Dalla Pozza R, Bentham J et al (2019) Early outcomes of percutaneous pulmonary valve implantation using the Edwards SAPIEN 3 transcatheter heart valve system. EuroIntervent J EuroPCR Collaborat Working Group on Intervent Cardiol Eur Soc Cardiol 14:1378–1385
Chakravarty T, Sondergaard L, Friedman J et al (2017) Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 389:2383–2392
Egbe AC, Pislaru SV, Pellikka PA et al (2015) Bioprosthetic valve thrombosis versus structural failure: clinical and echocardiographic predictors. J Am Coll Cardiol 66:2285–2294
Abaci A, Unlu S, Alsancak Y, Kaya U, Sezenoz B (2013) Short and long term complications of device closure of atrial septal defect and patent foramen ovale: meta-analysis of 28,142 patients from 203 studies. Catheter Cardiovasc Intervent Off J Soc Cardiac Angiogr Intervent 82:1123–1138
Nyboe C, Olsen MS, Nielsen-Kudsk JE, Hjortdal VE (2015) Atrial fibrillation and stroke in adult patients with atrial septal defect and the long-term effect of closure. Heart 101:706–711
Jalal Z, Hascoet S, Gronier C et al (2018) Long-term outcomes after percutaneous closure of ostium secundum atrial septal defect in the young: a nationwide cohort study. JACC Cardiovasc Intervent 11:795–804
Khairy P, Landzberg MJ, Gatzoulis MA et al (2006) Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: a multicenter study. Circulation 113:2391–2397
Bouchardy J, Therrien J, Pilote L et al (2009) Atrial arrhythmias in adults with congenital heart disease. Circulation 120:1679–1686
Labombarda F, Hamilton R, Shohoudi A et al (2017) Increasing prevalence of atrial fibrillation and permanent atrial arrhythmias in congenital heart disease. J Am Coll Cardiol 70:857–865
Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Hansson PO, Dellborg M (2016) Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc 5:2
Mandalenakis Z, Rosengren A, Lappas G et al (2018) Atrial fibrillation burden in young patients with congenital heart disease. Circulation 137:928–937
Lin YS, Liu PH, Wu LS, Chen YM, Chang CJ, Chu PH (2014) Major adverse cardiovascular events in adult congenital heart disease: a population-based follow-up study from Taiwan. BMC Cardiovasc Disord 14:38
Khairy P, Aboulhosn J, Broberg CS et al (2016) Thromboprophylaxis for atrial arrhythmias in congenital heart disease: a multicenter study. Int J Cardiol 223:729–735
Yang H, Bouma BJ, Dimopoulos K et al (2020) Non-vitamin K antagonist oral anticoagulants (NOACs) for thromboembolic prevention, are they safe in congenital heart disease? Results of a worldwide study. Int J Cardiol 299:123–130
Kamel H, Okin PM, Elkind MS, Iadecola C (2016) Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke 47:895–900
Masuda K, Ishizu T, Niwa K et al (2017) Increased risk of thromboembolic events in adult congenital heart disease patients with atrial tachyarrhythmias. Int J Cardiol 234:69–75
Heidendael JF, Bokma JP, de Groot JR, Koolbergen DR, Mulder BJ, Bouma BJ (2015) Weighing the risks: thrombotic and bleeding events in adults with atrial arrhythmias and congenital heart disease. Int J Cardiol 186:315–320
Thuny F, Di Salvo G, Belliard O et al (2005) Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 112:69–75
Thom K, Hanslik A, Russell JL et al (2018) Incidence of infective endocarditis and its thromboembolic complications in a pediatric population over 30years. Int J Cardiol 252:74–79
Moore B, Cao J, Kotchetkova I, Celermajer DS (2017) Incidence, predictors and outcomes of infective endocarditis in a contemporary adult congenital heart disease population. Int J Cardiol 249:161–165
Yoshinaga M, Niwa K, Niwa A et al (2008) Risk factors for in-hospital mortality during infective endocarditis in patients with congenital heart disease. Am J Cardiol 101:114–118
Karangelis D, Mazine A, Narsupalli S, Mendis S, Veldtman G, Nikolaidis N (2018) Morbidity after cardiac surgery in patients with adult congenital heart disease in comparison with acquired disease. Heart Lung Circ 27:739–744
Vida VL, Berggren H, Brawn WJ et al (2007) Risk of surgery for congenital heart disease in the adult: a multicentered European study. Ann Thorac Surg 83:161–168
Stefanescu Schmidt AC, Armstrong A, Kennedy KF, Nykanen D, Aboulhosn J, Bhatt AB (2017) Prediction of adverse events after catheter-based procedures in adolescents and adults with congenital heart disease in the IMPACT registry. Eur Heart J 38:2070–2077
Baumgartner H, Bonhoeffer P, De Groot NM et al (2010) ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 31:2915–2957
Galie N, Humbert M, Vachiery JL et al (2015) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS): endorsed by: association for european paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Respir J 46:903–975
Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J et al (2018) 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 39:3165–3241
Stout KK, Daniels CJ, Aboulhosn JA et al (2019) 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol 73:1494–1563
Rychik J, Atz AM, Celermajer DS et al (2019) Evaluation and management of the child and adult with fontan circulation: a scientific statement from the american heart association. Circulation 140:e234
Kaemmerer H, Apitz C, Brockmeier K et al (2018) Pulmonary hypertension in adults with congenital heart disease: updated recommendations from the cologne consensus conference 2018. Int J Cardiol 272S:79–88
Khairy P, Van Hare GF, Balaji S et al (2014) PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Can J Cardiol 30:e1–e63
Hernandez-Madrid A, Paul T, Abrams D et al (2018) Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace Eur Pacing Arrhythm Cardiac Electrophysiol J Working Group Cardiac Pacing Arrhythm Cardiac Cell Electrophysiol Eur Soc Cardiol 20:1719–1753
Iyengar AJ, Winlaw DS, Galati JC et al (2016) No difference between aspirin and warfarin after extracardiac Fontan in a propensity score analysis of 475 patients. Eur J Cardio-thorac surg Off J Eur Assoc Cardio-thorac Surg 50:980–987
Monagle P, Cochrane A, McCrindle B, Benson L, Williams W, Andrew M (1998) Thromboembolic complications after fontan procedures–the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg 115:493–498
Rosenkranz S, Lang IM, Blindt R et al (2018) Pulmonary hypertension associated with left heart disease: updated recommendations of the cologne consensus conference 2018. Int J Cardiol 272S:53–62
Feltes TF, Bacha E, Beekman RH 3rd et al (2011) Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 123:2607–2652
Baumgartner H, Falk V, Bax JJ et al (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38:2739–2791
Nishimura RA, Otto CM, Bonow RO et al (2017) 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol 70:252–289
Hascoet S, Acar P, Boudjemline Y (2014) Transcatheter pulmonary valvulation: current indications and available devices. Archiv Cardiovasc Dis 107:625–634
Yang H, Veldtman GR, Bouma BJ et al (2019) Non-vitamin K antagonist oral anticoagulants in adults with a Fontan circulation: are they safe. Open heart 6:e000985
Nishimura RA, Otto CM, Bonow RO et al (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on practice guidelines. Circulation 129:e521–643
Marzec LN, Wang J, Shah ND et al (2017) Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 69:2475–2484
January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology 2019.
Mongeon FP, Macle L, Beauchesne LM et al (2019) Non-vitamin K antagonist oral anticoagulants in adult congenital heart disease. Can J Cardiol 35:1686
Diller GP, Kempny A, Babu-Narayan SV et al (2019) Machine learning algorithms estimating prognosis and guiding therapy in adult congenital heart disease: data from a single tertiary centre including 10 019 patients. Eur Heart J 40:1069–1077
Greer IA (1999) Thrombosis in pregnancy: maternal and fetal issues. Lancet 353:1258–1265
Toglia MR, Weg JG (1996) Venous thromboembolism during pregnancy. N Eng J Med 335:108–114
Liu S, Rouleau J, Joseph KS et al (2009) Epidemiology of pregnancy-associated venous thromboembolism: a population-based study in Canada. J Obstet Gynaecol Can 31:611–620
Roos-Hesselink J, Baris L, Johnson M et al (2019) Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J 4:3848
Garcia Ropero A, Baskar S, Roos Hesselink JW et al (2018) Pregnancy in women with a fontan circulation: a systematic review of the literature. Circ Cardiovasc Qual Outcomes 11:e004575
Bedard E, Dimopoulos K, Gatzoulis MA (2009) Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 30:256–265
Salam AM, Ertekin E, van Hagen IM et al (2015) Atrial fibrillation or flutter during pregnancy in patients with structural heart disease: data from the ROPAC (Registry on pregnancy and cardiac disease). JACC Clin Electrophysiol 1:284–292
Acknowledgements
Dr Ségolène Claeyssens, Pr Philippe Acar
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karsenty, C., Waldmann, V., Mulder, B. et al. Thromboembolic complications in adult congenital heart disease: the knowns and the unknowns. Clin Res Cardiol 110, 1380–1391 (2021). https://doi.org/10.1007/s00392-020-01746-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-020-01746-2