Abstract
Background
Depression is common in patients after acute coronary syndromes (ACS) and with stable coronary artery disease (CAD) and has been associated with increased mortality and morbidity. However, it is unclear whether selective serotonin receptor inhibitors (SSRIs) reduce mortality or cardiac events in patients with CAD and depression.
Objective
We conducted a systematic review and meta-analysis to assess the effects of SSRIs on cardiovascular events in depressed CAD patients.
Methods
The CENTRAL, MEDLINE, and PsycINFO databases were searched (April 2020) for randomized controlled trials (RCTs) and extended follow-up analyses of RCTs that compared SSRIs with placebo or no intervention in patients with CAD and depression. The primary outcomes were all-cause mortality, cardiovascular mortality, and myocardial infarction incidence. The results were calculated through random-effect meta-analyses and reported in terms of risk ratio (RR) with 95% confidence intervals (CI).
Results
We retrieved 8 RCTs (2 of which with extended follow-up analyses), comprising a total of 1148 patients. 7 studies only included post-ACS patients. SSRIs were associated with a significantly lower risk of myocardial infarction in patients with CAD and depression (RR 0.54, 95% CI 0.34–0.86), and in post-ACS patients with depression (RR 0.56, 95% CI 0.35–0.90). We found no statistically significant difference in all-cause mortality, cardiovascular mortality, hospitalizations, angina, congestive heart failure, or stroke incidence.
Conclusion
The use of SSRIs in post-ACS patients with depression was associated with a 44% relative risk reduction of myocardial infarction. No difference in mortality was found. Given that the quality of the evidence was low, further research is warranted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Depression and depressive symptoms are relatively common in patients after acute coronary syndromes (ACS) [1,2,3] and have been associated with poorer cardiovascular prognosis, with increased mortality and morbidity [4,5,6,7,8], increasing mortality even when the symptoms of depression are minimal [9]. Depression not only affects the prognosis of patients with ACS, but also of patients with stable coronary disease [10]. Some studies have pointed to a 31–45% prevalence of clinically significant depressive symptoms in patients with coronary artery disease (CAD) and 15–20% of post-ACS patients meet the criteria for major depressive disorder [11]. It is estimated that depressed postmyocardial infarction (MI) patients have a 2–2.5-fold higher mortality rate and also a higher rate of cardiac events [11,12,13]. Some post hoc analyses of randomized controlled trials (RCTs) [14, 15] have also shown that the severity of depression or non-improvement of depression in post-ACS patients is associated with a significantly higher mortality rate and a recent observational study found that patients with untreated depression had a 70–90% higher risk of dying 1 year after their MI than patients without depression or with treated depression [16], although no cause of death specific correlation was carried out (cardiovascular mortality, suicide, or other causes).
SSRIs are the first-line treatment for pharmacological management of major depression [17], especially in CAD patients, because they do not have the cardiac adverse effects of tricyclic antidepressants [18, 19]. The efficacy of SSRI in reducing depressive symptoms in post-ACS patients with depression has long been established [20, 21]. However, whether SSRIs decrease the risk of cardiovascular events in those patients is still a matter of research. Several RCTs have not individually shown cardiovascular benefit of SSRI use in the post-ACS setting [21, 22], possibly due to their short follow-up period [14]. However, a recent 8.1-year extended follow-up analysis of an RCT [14] showed a 31% relative reduction of major cardiac events with the use of escitalopram in post-ACS patients with depression (HR 0.69; 0.49–0.96).
Given the conflicting results of those studies and the absence of a recent systematic review on the issue, we decided to conduct a systematic review of RCTs and extended follow-up analyses of RCTs (FUs) on the effects of SSRIs (assuming class effect) versus placebo or no intervention on mortality and cardiovascular events in patients with CAD and depression.
Methods
This systematic review follows the reporting principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [23].
Information sources and search strategy
We conducted an online electronic search of Cochrane Central Register of Controlled Trials (CENTRAL), Medline, and PsycINFO in April 2020 using free words and MeSH terms. The complete search strategy is available in the supplemental material. Reference lists of articles and reviews were comprehensively searched to select additional eligible studies. No language restrictions were applied.
Eligibility criteria
We included randomized controlled trials (RCTs) and extended follow-up analyses of randomized controlled trials (FUs) that compared the impact of SSRIs versus placebo or no intervention. The definition of SSRIs used can be seen in the supplemental material. The target population was stable coronary disease or post-acute coronary syndrome patients with depression. The primary outcomes of our systemic review were all-cause mortality, cardiovascular mortality, and myocardial infarction (MI) incidence. Secondary outcomes included percutaneous coronary intervention (PCI), hospitalization for cardiovascular causes, angina, heart failure, and stroke. No follow-up length, duration of treatment, or language limits were set.
Additional interventions given to all patients in both arms were allowed provided the only difference between the arms was SSRI use.
Study selection, data collection, and treatment
Two reviewers (NF and LP or DC) independently screened the title and abstract of the articles returned by the search to determine the appropriateness for inclusion in the full-text phase. Two reviewers (NF and DC) then assessed the full texts to determine the eligibility for inclusion in the systematic review. Any doubts and disagreements were discussed and solved by consensus. The reasons for exclusion were recorded at the full-text screening stage.
All studies that explicitly reported any of the relevant outcomes were included for the qualitative analysis. Study characteristics and results were independently extracted into a standardized spreadsheet, which included the main characteristics of the sample, intervention, comparator, and outcome.
All data treatment decisions and assumptions were made by consensus and prior to the statistical analyses.
Risk of bias assessment
The risk of bias was independently assessed by two authors (NF and DC) using the Cochrane revised tool for Risk of Bias in randomized trials (Rob 2.0 tool) [24] and was classified as “low risk”, “some concerns”, or “high risk” at the study level. Some studies were downgraded for reasons not considered in the RoB2 tool.
Statistical analysis and synthesis of results
We used OpenMetaAnalyst [25] for statistical analysis and to derive forest plots. For the primary quantitative analyses for each outcome, only studies with non-null event rates were included; studies with null event rates were included in some of the exploratory analyses. In the case of the same study being reported as an RCT and as an FU, only one was included in each analysis (in the primary analyses for each outcome, the data used was the one with the longest follow-up time).
We reported pooled dichotomous data using risk ratios (RRs), reporting 95% CIs, and corresponding p values. We used the DerSimonian–Laird random-effects analysis method, with a correction factor of 0.5 if the number of events/patients with events was 0. Heterogeneity was assessed using I2 and was considered to be substantial above 50%.
Additional analyses
The following pre-planned exploratory analyses were carried out: sensitivity (leave one out) analyses for the outcomes whose primary meta-analysis showed statistical significance; analyses including studies with null events; subgroup analyses stratified by study design (RCTs or FUs); risk of bias (excluding the “high risk” of bias studies); population type (post-ACS only); and excluding the studies in which both arms were given the same extra intervention.
Quality of evidence
We used the Grading of Recommendations, Assessment and Evaluation (GRADE) framework to report the overall quality of evidence for each outcome. The certainty in the evidence for each outcome was graded as “high”, “moderate”, “low”, or “very low” [26, 27].
Results
Included studies
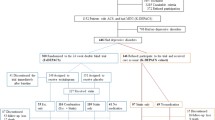
The search returned 689 records (675 records after removing duplicates), 461 of which were excluded after title and abstract screening, and 204 excluded in the full-text phase. 8 RCTs [28,29,30,31,32,33,34,35] as well as 2 FUs [14, 15] of RCTs already included [30, 34] were included for qualitative analysis, 6 RCTs [28, 30, 31, 33,34,35] and 2 FUs [14, 15] of which were included in the primary quantitative analyses (Fig. 1). 1 of the RCTs (Lespérance et al.) [33] was subdivided into 2 substudy analyses. We retrieved an unpublished study (Kennedy) [32]. The specific reasons for the exclusion of the articles in the full-text phase are reported in the supplementary material. The assumptions made and the data treatment are also available in the supplemental material.
PRISMA flow chart of the identification and selection of relevant studies. Based on [23]. (Asterisk) 8 RCTs and 2 FUs of RCTs already included. (Double asterisks) 6 RCTs and 2 FUs of RCTs already included
The main characteristics and the relevant results extracted from the included studies can be seen in Table 1 and in the supplemental material. The sample sizes ranged from 17 and 369 patients, with a total of 1791 patients being included in our review. The average age ranged from 55.8 to 61 years old, and around 65% were males. Study publication dates for RCTs ranged from 2000 to 2016, and 2009 and 2018 for the follow-up analyses. 7 studies included only depressed post-ACS patients and 1 study included depressed patients with stable CAD. 3 RCTs studied sertraline, 2 studied escitalopram, 1 studied citalopram, 1 studied fluoxetine, and 1 studied both paroxetine and fluoxetine.
83% of patients came from the 3 largest studies (SADHART [15, 30], CREATE [33], and EsDEPACS [14, 34]). Both SADHART and EsDEPACS studied post-ACS patients (79.7% and 61.3% being MI, respectively) with 100% (inclusion criteria) and 55.8% of patients having major depressive disorder (MDD). The CREATE trial included patients with stable CAD (64.8% had a history of previous MI; median time since the latest cardiac event was 18.9 months) and MDD. The main baseline characteristics of the studies included can be seen in the supplemental material.
Risk of bias
The analyses of the risk of bias can be seen in Table 1 and are further detailed in the supplemental material. We classified 2 RCTs to be at a high risk of bias: Mohapatra et al. [31] due to randomization process concerns and possible deviations from intended interventions and Kennedy [32] due to insufficient recruitment and early termination. 5 RCTs [28,29,30, 34, 35] were judged to be at some concern of bias: Glassman [30] and Kim et al. [34] for missing data (non-insignificant losses to follow-up/discontinuations), Strik et al. [28] and Tian et al. [35] for randomization process concerns (allocation concealment and/or blinding concerns) and McFarlane et al. [29] for per-protocol analysis. Lespérance et al. [33] was considered to be at low risk of bias. Both FUs [14, 15] were considered to be at low risk of bias in all RoB2 categories, but were then downgraded to “some concerns”, due to the observational nature of the extended follow-up period and the possibility of different treatment choices during that period.
Primary outcomes: all-cause mortality, cardiovascular mortality, and myocardial infarction
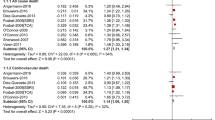
5 RCTs [28,29,30,31,32] and 2 FU [14, 15] explicitly reported mortality. 3 of the RCTs [28, 29, 32] reported no deaths and, as a result, were excluded from the primary quantitative meta-analysis. The primary analysis included 1 RCTs (n = 17) and 2 FU (n = 659) (one RCT [30] was the same as one FU [15]). Pooled results showed no statistically significant difference in all-cause mortality between the SSRI and placebo groups (RR 0.91, 95% CI 0.68–1.21; p = 0.50; I2 = 0%; Fig. 2a). The exploratory analyses carried out showed similar results (supplemental material).
Forest plot of the meta-analyses evaluating the effect of SSRIs compared to control on a all-cause mortality, and b myocardial infarction risk in patients with coronary artery disease and depression. The majority of the events in the FUs occurred during the follow-up phase (number of events during the follow-up phase/number of total events in that group: GlassmanFU mortality: 36/38 and 32/37; KimFU mortality: all deaths occurred during the follow-up phase; KimFU myocardial infarction: 12/13 and 23/23 for the treatment and control groups, respectively)
Regarding the cardiovascular mortality, only one study [14] reported cardiovascular mortality (RR 0.81; 95% CI 0.44–1.50). No meta-analysis was carried out.
Six RCT comparisons [30, 31, 33,34,35] and 1 FU [22] reported MI as an outcome. The primary analysis included 5 RCTs comparisons (n = 737) and 1 FU (n = 300) (one RCTs [34] was the same as the FU [14]) and the treatment duration ranged from 8 weeks to 6 months. Pooled results showed a decrease in the risk of MI with the use of SSRI (RR 0.54, 95% CI 0.34–0.86; p = 0.01; I2 = 0%; Fig. 2b). A sensitivity (leave one out) analysis showed that the statistical significance was still present, even if any one of the study was excluded (supplemental material).
In an exploratory analysis excluding the high risk of bias RCT, the use of SSRI was still associated with reduced risk of MI (RR 0.59, 95% CI 0.36–0.96; p = 0.04; I2 = 0%; supplemental material), as was in an exploratory analysis only including studies with only post-ACS patients, (RR 0.56, 95% CI 0.35–0.90; p = 0.02; I2 = 0%; supplemental material). The exploratory analysis, including only post-ACS patients, but excluding the high bias risk study (RR 0.62, 95% CI 0.37–1.02; p = 0.06; I2 = 0%), and the several exploratory analyses, including only RCTs showed a non-statistically significant trend towards lower MI risk in the SSRI group (supplemental material).
Secondary outcomes
Our metanalyses showed no statistically significant difference in angina (RR 0.76, 95% CI 0.54–1.05; p = 0.10; I2 = 0%), stroke (RR 1.27, 95% CI 0.30–5.26; p = 0.75; I2 = 0%), hospitalizations (RR 0.57, 95% CI 0.29–1.12; p = 0.10; I2 = 43.31%), or congestive heart failure (RR 0.59, 95% CI 0.08–4.47; p = 0.61; I2 = 31.88%) risk between the SSRI and placebo groups. Only one study [14] reported PCI as an outcome (RR 0.64, 95% CI 0.38–1.09). The analyses for these outcomes can be seen in the supplementary material.
Assessment of the quality of the evidence
The GRADE framework assessment of the quality of the evidence can be seen in Table 2, and is further detailed in the supplemental material. We considered the quality of the evidence for all outcomes to be low, except for the outcome of angina, which was considered to be very low.
Discussion
Our analysis found (1) that SSRIs significantly reduced the risk of myocardial infarction in depressed patients with CAD; (2) no statistically significant difference in all-cause mortality, cardiovascular mortality, stroke incidence, hospitalization, congestive heart failure, or angina.
Despite the high prevalence of depression in patients who have ACS and the proven efficacy of SSRIs in treating depressive symptoms in that population, the effects on cardiovascular events have remained unclear. The mechanisms involved in the potential cardiovascular benefits of SSRIs are still a matter of discussion. Several mechanisms have been proposed: treatment of depression/depressive symptoms; inhibition of serotonin-mediated platelet aggregation, inflammation reduction; improved endothelial function, and increased medication adherence [36, 37]. SSRIs may also have side effects, such as serotonin syndrome, QT prolongation (for some SSRIs), bleeding, and increased risk of suicide during the early stages of treatment, among other side effects, that may contribute to mortality or morbidity [38].
Our analysis showed that the use of SSRIs in the studies population was associated with a significant reduction in MI risk. Given the magnitude of the effect (RRR 46%) and the high prevalence and risk associated with depression in post-ACS patients, we consider this finding to be clinically relevant. Our conclusion is also supported by a sensitivity (leave-one-out jack-knife test) and by an exploratory analysis that excluded the RCT with a high risk of bias. When only post-ACS patients were included, we still found a statistically significant risk reduction in MI incidence (RRR 44%), which reiterates the validity of our analysis for the depressed post-ACS population. The remaining exploratory analyses showed a non-statistically significant tendency of SSRI reduction in MI risk, which may be explained by the need for longer follow-up periods and or a higher number of events to be able to have enough study power to assess the effects of SSRIs on MI. Further RCTs on this topic are necessary to better clarify and support our results.
The magnitude of the effect we found in our review is similar to that of the long-term follow-up of EsDEPACS [14] (HR 0.54; 95% CI 0.27–0.96; p = 0.04, in the non-adjusted analysis) and in the non-randomized use of SSRIs in the ENRICHD trial [39] (HR 0.53; 95% CI 0.32–0.90 for the adjusted analysis), which corroborates our results on MI incidence reduction. Moreover, it is also in line with a previous systematic review [22] that showed a reduction in the risk of MI (RR 0.59; 95% CI 0.37–0.93) with the use of SSRIs in depressed patients with cardiovascular disease (post-ACS, post-stroke, and HF patients).
Our analysis on mortality failed to show statistically significant benefit with the use of SSRIs, which is in line with the findings of SADHART [15] and EsDEPACS [14], as well as with the findings of a systematic review [22] that included post-ACS, post-stroke, and HF patients with depression. A previous systematic review [21] that focused on depressed post-ACS patients had found that SSRIs reduced the risk of all-cause mortality, but that meta-analysis included a study where the use of SSIR was not randomized [39], which was excluded from our review. Our analyses did not show any statistically significant benefit in the rates of hospitalization, stroke, angina, or congestive heart failure. Appropriately powered RCTs are needed to clarify the effect of SSRIs on these outcomes.
Given that all but one study included only patients in the post-ACS setting with depression, and given the exploratory analyses carried out, our results should be considered valid only for that population. In terms of SSRI choice, dosage, and treatment duration, not enough studies were included to allow for meaningful comparative analyses. However, given that most patients in our review were given either sertraline or escitalopram, and that the 2 largest (totaling 89% of patients included in the post-ACS meta-analysis of MI risk) and the longest studies included had an intervention duration of 6 months, with clinically titrated dosages based on the depressive symptoms reduction and tolerability, we consider that our results should not be extrapolated to other conditions. Escitalopram might be preferred over sertraline given that it was the only SSRI able to prove cardiovascular benefit in an individual study [14]; however, whether some SSRIs are superior to others, the effect on MI incidence reduction is dosage dependent, treatment duration dependent, continuous-treatment dependent, or carryover effect dependent warrants further research.
Limitations
The main limitations of this systematic review include: (1) being a review of aggregated study data and not of individual patient data; (2) the inclusion of RCTs at a high risk of bias; (3) the inclusion of observational follow-up analysis of RCTs; (4) clinical heterogenicity of the studies included; (5) the absence of subgroup analysis by severity of depression, SSRI choice, dosage, and treatment duration; (6) the short follow-up duration of most studies included and the low rates of some of the outcomes analyzed. Further limitations can be seen in the supplemental material.
The absence of raw individual patient data made it impossible to carry out exploratory subgroup analyses stratified by or adjusted for severity of depression, remission status, severity of the coronary disease (STEMI, NSTEMI, or UA; Killip class; Canadian Cardiovascular Society Angina Class; LVEF; previous MI) and for co-morbidities. The analyses stratified by the severity of depression and remission status would be particularly relevant given that the severity and non-remission of depression, and the presence of general anxiety disorders have been linked to worse cardiovascular outcomes [14, 15, 40].
Two studies [31, 32] were considered to be at a high risk of bias. One [32] of them did not influence the quantitative analyses, because no non-null relevant outcomes were reported. To minimize the impact of the other high-risk study [31], exploratory analyses excluding it were carried out.
Given the observational nature of the extended follow-up period, the inclusion of FUs limits the strength of this review; however, due to the short-term duration and the low rate of events of the RCTs, we decided to include FUs in order to increase the statistical power of our analyses. None of the FU reported the percentage of antidepressant use in the different groups during the observational follow-up phase, which is a further limitation. As a result, we are not able to definitely ascertain whether the possible beneficial effects of SSRIs found in the observational phase are a result of a direct effect (for e.g., inhibition of platelet aggregation, inflammation reduction, or improved endothelial function [41]) or a result of a carryover effect.
There was also some clinical heterogenicity between the studies included: differences in the cardiac inclusion criteria (stable CAD, post-ACS, or post-MI), ACS treatment; location, ethnicity; severity of the depression; time since the latest cardiac event, treatment duration, follow-up time, SSRI choice, and equivalent dosages of SSRI treatment. These covariates should be studied in future RCTs. Regarding the outcome of angina, there is a possibility of clinical heterogenicity, given that the studies did not specify if they were reporting stable angina or unstable angina, which may decrease the scientific validity of the analysis of this outcome.
The short duration time of most of the RCTs included (at most 6 months), the low rate of occurrence of some outcomes and the fact that around 50% of the events for each primary outcomes were pooled from a follow-up study (KimFU) are major limitations in terms of cardiovascular outcome analyses. Furthermore, most of the studies included were not designed nor powered to analyze changes in cardiovascular outcomes and none of the metanalyses carried out met the optimal information size criteria as defined by the GRADE Handbook [26].
Conclusion
The use of SSRIs in post-ACS patients with depression was associated with a 44% relative risk reduction of MI. No statistically significant difference was found regarding mortality, stroke, hospitalization, CHF, or angina incidence. The overall quality of the evidence available was low. Further long-term RCTs are necessary to determine the effect of SSRIs on mortality and cardiovascular events in this population.
Availability of data and material (data transparency)
Any missing dada or material used in this article will be made available to readers upon reasonable request.
Abbreviations
- 95% CI:
-
95% Confidence interval
- ACS:
-
Acute coronary syndrome
- AD:
-
Antidepressant
- BDI:
-
Beck's depression inventory
- CAD:
-
Coronary artery disease
- CHF:
-
Congestive heart failure
- DSM-III-R:
-
Diagnostic and Statistical Manual of Mental Disorders III R
- DSM-IV:
-
Diagnostic and Statistical Manual of Mental Disorders IV
- F:
-
Female
- FU:
-
Extended follow-up analysis of an RCT
- GRADE:
-
Grading of recommendations, assessment and evaluation
- HAMD-17:
-
17 Item Hamilton rating scale for depression
- HAM-D-24:
-
24 Item Hamilton rating scale for depression
- HDRS:
-
Hamilton depression rating scale
- HF:
-
Heart failure
- HR:
-
Heart rate
- IDD:
-
Inventory to diagnose depression
- IPT:
-
Interpersonal psychotherapy
- LVEF:
-
Left ventricle ejection fraction
- M:
-
Male
- MDD:
-
Major depressive disorder
- mDD:
-
Minor depressive disorder
- MI:
-
Myocardial infarction
- NSTEMI:
-
Non-ST elevation myocardial infarction
- P:
-
Placebo
- PCI:
-
Percutaneous coronary intervention
- RCT:
-
Randomized controlled trial
- RoB2:
-
Cochrane revised tool for risk of bias in randomized trials
- RR:
-
Risk ratio
- RRR:
-
Risk ratio reduction
- SCL-90 DS:
-
90-Item symptom check list depression scale
- SCL-90-R DS:
-
90-Item symptom check list revised depression subscale
- SDS:
-
Self-rating depression scale
- SSRI:
-
Selective serotonin reuptake inhibitor
- STEMI:
-
ST elevation myocardial infarction
- UA:
-
Unstable angina
References
Thombs BD, Bass EB, Ford DE et al (2006) Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med 21(1):30–38. https://doi.org/10.1111/j.1525-1497.2005.00269.x
Kang H-J, Stewart R, Bae K-Y et al (2015) Predictors of depressive disorder following acute coronary syndrome: Results from K-DEPACS and EsDEPACS. J Affect Disord 181:1–8. https://doi.org/10.1016/j.jad.2015.04.004
Amin AA, Jones AMH, Nugent K, Rumsfeld JS, Spertus JA (2006) The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 152(5):928–934. https://doi.org/10.1016/j.ahj.2006.05.006
Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P (2011) Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry 33(3):203–216. https://doi.org/10.1016/j.genhosppsych.2011.02.007
Osler M, Mårtensson S, Wium-Andersen IK et al (2016) Depression after first hospital admission for acute coronary syndrome: a study of time of onset and impact on survival. Am J Epidemiol 183(3):218–226. https://doi.org/10.1093/aje/kwv227
Lichtman JH, Froelicher ES, Blumenthal JA et al (2014) Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 129(12):1350–1369. https://doi.org/10.1161/CIR.0000000000000019
Vaccarino V, Badimon L, Bremner JD et al (2019) Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J. https://doi.org/10.1093/eurheartj/ehy913
Albus C, Waller C, Fritzsche K, Gunold H, Haass M, Hamann B, Kindermann I, Köllner V, Leithäuser B, Marx N, Meesmann M, Michal M, Ronel J, Scherer M, Schrader V, Schwaab B, Weber CS, Herrmann-Lingen C (2019) Significance of psychosocial factors in cardiology: update 2018. Clin Res Cardiol 108(11):1175–1196
Bush DE, Ziegelstein RC, Tayback M et al (2001) Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol 88(4):337–341. https://doi.org/10.1016/S0002-9149(01)01675-7
Frasure-Smith N, Lespérance F (2008) Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry 65(1):62. https://doi.org/10.1001/archgenpsychiatry.2007.4
Celano CM, Huffman JC (2011) Depression and cardiac disease. Cardiol Rev 19(3):130–142. https://doi.org/10.1097/CRD.0b013e31820e8106
Barth J, Schumacher M, Herrmann-Lingen C (2004) Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 66(6):802–813. https://doi.org/10.1097/01.psy.0000146332.53619.b2
van Melle JP, de Jonge P, Spijkerman TA et al (2004) Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 66(6):814–822. https://doi.org/10.1097/01.psy.0000146294.82810.9c
Kim J-MJ-WH, Stewart R, Lee Y-S et al (2018) Effect of escitalopram vs placebo treatment for depression on long-term cardiac outcomes in patients with acute coronary syndrome. JAMA 320(4):350. https://doi.org/10.1001/jama.2018.9422
Glassman AH, Bigger JT, Gaffney M (2009) Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression. Arch Gen Psychiatry 66(9):1022. https://doi.org/10.1001/archgenpsychiatry.2009.121
Smolderen KG, Buchanan DM, Gosch K et al (2017) Depression treatment and 1-year mortality after acute myocardial infarction. Circulation 135(18):1681–1689. https://doi.org/10.1161/CIRCULATIONAHA.116.025140
National Institute for Health and Clinical Excellence (2009) Depression: the treatment and management of depression in adults (update). (Clinical guideline 90). https://www.nice.org.uk/guidance/CG90. Accessed 21 May 2020
Lichtman JH, Bigger JT, Blumenthal JA et al (2008) Depression and coronary heart disease. Circulation 118(17):1768–1775. https://doi.org/10.1161/CIRCULATIONAHA.108.190769
Post-Myocardial Infarction Depression Clinical Practice Guideline Panel (2009) AAFP guideline for the detection and management of post-myocardial infarction depression. Ann Fam Med 7(1):71–79. https://doi.org/10.1370/afm.918
Thombs BD, de Jonge P, Coyne JC et al (2008) Depression screening and patient outcomes in cardiovascular care. JAMA 300(18):2161. https://doi.org/10.1001/jama.2008.667
Pizzi C, Rutjes AWS, Costa GM, Fontana F, Mezzetti A, Manzoli L (2011) Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. Am J Cardiol 107(7):972–979. https://doi.org/10.1016/j.amjcard.2010.11.017
Kim Y, Lee YS, Kim MG et al (2018) The effect of selective serotonin reuptake inhibitors on major adverse cardiovascular events. Int Clin Psychopharmacol. https://doi.org/10.1097/YIC.0000000000000238
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339(1):b2535–b2535. https://doi.org/10.1136/bmj.b2535
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/bmj.l4898
Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH (2012) Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. https://doi.org/10.18637/jss.v049.i05
Schünemann H, Brożek J, Guyatt G, Oxman A (eds) (2013) GRADE handbook for grading the quality of evidence and strength of recommendations. The GRADE Working Group. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 21 May 2020
GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org. Accessed 21 May 2020
Strik JJMH, Honig A, Lousberg R et al (2000) Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med 62(6):783–789. https://doi.org/10.1097/00006842-200011000-00007
McFarlane A, Kamath MV, Fallen EL, Malcolm V, Cherian F, Norman G (2001) Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. Am Heart J 142(4):617–623. https://doi.org/10.1067/mhj.2001.116766
Glassman AH (2002) Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 288(6):701. https://doi.org/10.1001/jama.288.6.701
Mohapatra PK, Kar N, Kar GC, Behera M (2005) Effectiveness of sertraline in treatment of depression in a consecutive sample of patients with acute myocardial infarction: six month prospective study on outcome. Clin Pract Epidemiol Ment Health 1:26. https://doi.org/10.1186/1745-0179-1-26
Kennedy S (University of TOC) (2006) Synopsis-study 10413: a double-blind, multicentre, randomised, parallel-group, placebo-controlled study assessing the efficacy and safety of escitalopram in post-myocardial infarction patients suffering from depressive symptoms. https://www.clinicaltrialsregister.eu/ctr-search/trial/2004-000990-78/results. Accessed 21 May 2020
Lespérance F, Frasure-Smith N, Koszycki D et al (2007) Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease. JAMA 297(4):367. https://doi.org/10.1001/jama.297.4.367
Kim J-M, Bae K-Y, Stewart R et al (2015) Escitalopram treatment for depressive disorder following acute coronary syndrome. J Clin Psychiatry 76(01):62–68. https://doi.org/10.4088/JCP.14m09281
Tian X, Wang Q, Guo R, Xu L, Chen QM, Hou Y (2016) Effects of paroxetine-mediated inhibition of GRK2 expression on depression and cardiovascular function in patients with myocardial infarction. Neuropsychiatr Dis Treat 12:2333–2341. https://doi.org/10.2147/NDT.S109880
Chittaranjan A, Chethan KB, Sandarsh S (2013) Cardiovascular mechanisms of SSRI drugs and their benefits and risks in ischemic heart disease and heart failure. Int Clin Psychopharmacol 28(3):145–155. https://doi.org/10.1097/YIC.0b013e32835d735d
Pizzi C, Santarella L, Bugiardini R (2014) Epidemiology and the physiopathological link between depression and cardiovascular disease. IJC Metab Endocr 5:52–55. https://doi.org/10.1016/j.ijcme.2014.10.004
Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA (2016) The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom 85(5):270–288. https://doi.org/10.1159/000447034
Taylor CB (2005) Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 62(7):792. https://doi.org/10.1001/archpsyc.62.7.792
Fang XY, Spieler D, Albarqouni L, Ronel J, Ladwig KH (2018) Impact of generalized anxiety disorder (GAD) on prehospital delay of acute myocardial infarction patients. Findings from the multicenter MEDEA study. Clin Res Cardiol 107(6):471–478. https://doi.org/10.1007/s00392-018-1208-4
Peikert A, Kaier K, Merz J, Manhart L, Schäfer I, Hilgendorf I, Hehn P, Wolf D, Willecke F, Sheng X, Clemens A, Zehender M, von Zur MC, Bode C, Zirlik A, Stachon P (2020) Residual inflammatory risk in coronary heart disease: incidence of elevated high-sensitive CRP in a real-world cohort. Clin Res Cardiol 109(3):315–323. https://doi.org/10.1007/s00392-019-01511-0
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JJF had speaker and consultant fees with Grünenthal, Fundação MSD (Portugal), TEVA, MSD, Allergan, Medtronic, GlaxoSmithKline, Novartis, Lundbeck, Solvay, BIAL, Merck-Serono, Merz, Ipsen, Biogen, Acadia, Allergan, Abbvie, Sunovion-Pharmaceuticals. FJP had consultant and speaker fees with Astra Zeneca, Bayer, BMS, Boehringer Ingelheim, and Daiichi Sankyo. DC has participated in educational meetings and/or attended conferences or symposia (including travel, accommodation, and/or hospitality) with Bristol-Myers Squibb, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Merck Serono, Ferrer, Pfizer, Novartis, and Roche. The remaining authors do not have interests to disclose.
Code availability
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernandes, N., Prada, L., Rosa, M.M. et al. The impact of SSRIs on mortality and cardiovascular events in patients with coronary artery disease and depression: systematic review and meta-analysis. Clin Res Cardiol 110, 183–193 (2021). https://doi.org/10.1007/s00392-020-01697-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-020-01697-8