Abstract
Background
Left heart valve thickening (LVT) was described in patients with light-chain amyloidosis (AL). This phenomenon reflects likely infiltration of the valve by amyloid proteins. However, the prevalence of LVT and its prognostic value have not been investigated in patients with AL.
Methods and results
Comprehensive transthoracic echocardiography was performed at baseline in 150 patients [median age 68 (33–87) years; 59% male] with confirmed AL. The presence of abnormal mitral and/or aortic valve thickening (>3 mm) was assessed in all included patients. Overall, 42% had LVT at the time of diagnosis. Compared to patients without LVT, those with LVT were older and had a more advanced NYHA functional class (63% in patients with NYHA III-IV vs. 33% in NYHA I–II, p < 0.001). They also had higher left ventricular (LV) wall thickness and mass, larger left atrium, higher mitral annulus E/E’ ratio and systolic pulmonary artery pressures, and lower LV ejection fraction (all p < 0.05). Patients with more advanced Mayo Clinic stage had a higher incidence of LVT: 58% in stage III vs. 45% in stage II and 5% in stage I (p < 0.001). During a median follow-up of 2 years, 79 deaths occurred. The presence of LVT was significantly associated with reduced 5-year survival (32 ± 7 vs. 64 ± 6%). In multivariate analysis, after adjusting for age, gender, NYHA functional class, and LV ejection fraction, LVT remained significantly associated with higher all-cause mortality (hazard ratio 1.90, 95% CI 1.10–3.34, p = 0.02).
Conclusion
Left heart valve thickening is common in patients with AL and is associated with worse functional class, LV systolic and diastolic function, and more advanced stage of the disease. In addition, LVT appears to be a powerful marker of all-cause mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Light-chain amyloidosis (AL) is an infiltrative systemic disease characterized by the extracellular deposition of fibrils in several organs. The accumulation of amyloid protein in the myocardial interstitial tissue is observed in more than 50% of AL patients [1] and this may gradually lead to a restrictive cardiomyopathy. Cardiac involvement in AL is associated with a poor prognosis, particularly in patients with heart failure symptoms, and in the setting of an advanced stage of the Mayo Clinic classification based on cardiac biomarkers [2, 3]. When assessed by echocardiography, amyloid heart disease is associated with increased wall thickness involving both ventricles with normal or small left ventricular (LV) cavity size and either preserved or reduced LV ejection fraction (LVEF). LV systolic dysfunction is most often noted in advanced stages of the disease and is a marker of poor prognosis [4]. Bi-atrial enlargement associated with LV diastolic dysfunction and elevated filling pressures are common and adversely impact patient outcome [5, 6].
Left heart valve thickening (LVT) is known to occur in cardiac amyloidosis, probably as a result of valvular infiltration by amyloid fibrils. However, the prevalence and the prognostic significance of LVT at the time of diagnosis have not been investigated in large series of AL patients yet.
The aim of our study is: (1) to determine the prevalence of LVT+ at diagnosis using transthoracic echocardiography in patients diagnosed with AL; (2) to assess the impact of LVT on 1- and 5-year all-cause mortality. We hypothesized that the presence of LVT is associated with unfavorable outcome.
Methods
Baseline population
The study included 150 patients with confirmed AL, evaluated at our institution between June 1998 and September 2013. The diagnosis of AL was mainly established by peripheral tissue biopsy that demonstrated typical Congo red birefringence under polarized light. Myocardial biopsy was performed only when all other biopsies were non-diagnostic. The amyloid deposits were characterized as AL type by immunofluorescence on frozen tissues; borderline cases were confirmed by immune-electro-microscopy or proteomics. For patients with a compatible phenotype, DNA analyses were used to exclude familial amyloidosis. The presence of monoclonal proteins in the serum and/or a monoclonal population of plasma cells in the bone marrow were documented in all patients to confirm the type of AL [6]. The cardiac involvement and its severity were assessed using the Mayo Clinic staging beginning in 2004 [2, 3]. It includes two biological markers, NT-pro-BNP or BNP, using the different cut-off value: ≥332; ≥100 ng/L, respectively) and troponin (cut-off value: troponin I ≥0.1 µg/L; troponin T ≥0.035 µg/L; troponin Tc us ≥0.07 µg/L). In stage I, both biomarkers are below the cut-off values and patients are considered to have minor cardiac involvement. In stage II, one biomarker is above the cut-off value and cardiac involvement is considered to be moderate. In stage III, severe cardiac involvement is usually present and both biomarkers are elevated beyond the cut-off values. Prior to 2004, patients were considered to have cardiac involvement on the basis of the clinical history, the traditional electrocardiographic findings (low QRS voltage, q-waves in the anterior leads) and echocardiographic features [LV wall thickness greater than 12 mm in the absence of other causes of LV hypertrophy, diastolic LV dysfunction, dilated left atrium (LA), pericardial effusion, and/or thickened right ventricular free-wall]. The involvement of any other organ was determined based on standard criteria for the evaluation of systemic amyloidosis [7].
Data collection
Our study was conducted in a retrospective manner with demographic, clinical, biological, and echocardiographic data were extracted from the echo reports (derived from the 1st baseline echocardiography performed at diagnosis of AL) collected using patients’ charts.
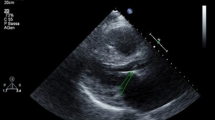
Mortality data were obtained from death certificates, family physician questionnaire, or hospital records. To avoid misclassification of the cause of death, the primary outcome was all-cause mortality regardless of the underlying cause [8]. All cases were performed using harmonic imaging. Echo data were obtained following the available American Society of Echocardiography and the European Association of Cardiovascular Imaging guidelines [9]. These include: (1) LV end diastolic and systolic diameters in the parasternal long-axis view; (2) end-diastolic thickness of the inter ventricular septum and posterior wall; (3) 2D-LVEF using the Simpson method; (4) diastolic LV function using the trans mitral E/A ratio and E/e’ ratio at the lateral mitral annulus; and (5) LA size using the systolic area in the 4-chamber view (LA considered as dilated when the area exceeds 20 cm2) [9]. The 2D maximal LA volume was available in 46% of patients in the 2- and 4-chamber view according to the Simpson method and was considered as significantly dilated when >34 mL/m2 [9]. The mitral and aortic valves were inspected and the presence of LVT (i.e., LVT+: leaflet thickening >3 mm [10, 11] in apical view for the mitral valve and in the long-axis parasternal view for the aortic valve) was reported at the time of echocardiography (Fig. 1). The presence of valve thickening related to leaflet localized nodular or calcification was not considered as LVT+. The presence of any valve regurgitation or stenosis was estimated semi-quantitatively from grade 1 (mild) to grade 4 (severe) according to published guidelines [12]. The systolic pulmonary artery pressure (PAP) was derived from the sum of the right ventricular-right atrial (RA) pressure gradient as estimated using the tricuspid regurgitation jet velocity added to the RA pressure as assessed using the inferior vena cava dimensions and collapsibility with inspiration.
Statistical analysis
Patients were divided into two groups according to the presence or absence of LVT as reported by echocardiography. Continuous data were expressed as median (minimal; maximal) and were compared by Student’s t test or Kruskal–Wallis test as appropriate. Categorical data were given as numbers and percentages and were compared using the Chi-square test. The primary outcome was the overall mortality calculated from the date of the initial echocardiographic study until the date of the last available follow-up or death. The cumulative probability of event was estimated using the Kaplan–Meier method and results are presented at 1 and 5 years. Log-rank test was used to test for significance in survival between the two groups. Univariate Cox proportional-hazard analysis was performed to assess the association between all-cause mortality and the most clinically relevant variables, as well as the echocardiographic parameters known to affect survival in this population.
A multivariable analysis was then performed and included age, gender, and variables that were significantly associated with all-cause mortality in the univariate analysis. Results were reported as hazard ratios with corresponding 95% confidence intervals. A p value <0.05 was considered as statistically significant. Statistical analyses were performed using the JMP software version 10.0.0, 2012 (SAS Institute Inc., Cary, NC, USA).
Results
Our study population included 150 patients who were referred to the National Reference Centre for AL in the Department of Hematology at our institution with a 1st available echocardiographic data obtained at baseline before the beginning of any treatment. The median follow-up was 2.0 (0–18) years for the whole cohort. The follow-up was completed by January 2014 in 99% of patients and only two patients were lost from FU during this period. The median interval between baseline diagnosis and echocardiography was 0 (−1; 2) days.
Baseline characteristics of the patient population
Among our patients, 89 (59%) were men and the median age was 68 (33–87) years. Median LVEF was 61% (25–84%) with a median interventricular septal thickness (IVST) of 14 mm (7–26 mm). Other echocardiographic characteristics are shown in Table 1.
Overall, 63 patients (42%) showed LVT+, of whom 30 (48%) had isolated mitral LVT, 11 (17%) had isolated aortic LVT, and 22 (35%) had both mitral and aortic LVT. No patient had more than grade 2 aortic or mitral regurgitation, and 75 (50%) and 20 (13%) patients had grade 1 and grade 2 mitral regurgitation, respectively. In addition, 38 (25%) patients had grade 1 aortic regurgitation and 1% had grade 2 aortic regurgitation. No significant aortic stenosis or mitral stenosis was found in this cohort.
The Mayo Clinic staging started to be performed after 2004 [2, 3], and thus was available in 113 (75%) patients. However, no significant difference was found between the 113 patients with a Mayo Clinic staging and those without, in terms of age, gender, prevalence of LVT, number or type of organs involvement, NYHA functional class, LVEF, and all other echocardiographic variables.
Twenty (18%), forty (35%), and fifty-three (47%) patients were in Mayo Clinic stages I, II, and III, respectively. Based on standard criteria for the evaluation of affected organs by AL, 88 (59%) had renal involvement.
Comparison of patients according to LVT
The comparison of the two groups is reported in Table 1. Patients with LVT were significantly older [72 (47–86) vs. 66 (33–87) years; p = 0.009]. However, no significant difference was noted between both groups in terms of gender, presence of hypertension, type of monoclonal free light chain involved, and renal or other solid organ involvement (all p > 0.05, Table 1). However, mean baseline LVEF was lower (Fig. 2) and the IVST was higher in patients with LVT. Moreover, almost all diastolic parameters were more altered in the group with LVT as compared to those without (p < 0.05, Table 1).
There was no significant difference in terms of baseline demographic, clinical, biologic, and echocardiographic data between patients with isolated mitral LVT, isolated aortic LVT, or LVT affecting both valves, except for age. Indeed, patients with isolated aortic LVT were significantly older than those with isolated mitral LVT or bi-valvular LVT (respectively, 78 ± 7 vs. 67 ± 9 vs. 71 ± 11 years, p = 0.004).
Relationship between LVT, Mayo clinic stage, and functional class
Patients with LVT were more often in Mayo Clinic stage II and III as compared to those without: 18 (45%) and 31 (58%) vs. 1 (5%) in stage I (p < 0.0001). Moreover, the prevalence of NYHA functional class III–IV was significantly higher in patients with LVT (p < 0.0008).
Comparison according to LVT and stratified for IVST
Table 2 reports baseline demographic, clinical, biological, and echocardiographic data according to the presence of LVT stratified for IVST (i.e. <12 vs. ≥12 mm).
Patients LVT+ and IVST ≥12 mm had more frequently atrial fibrillation and more severe cardiac involvement with significantly more frequent Mayo Clinic stage III (68 vs. 54% in LVT− IVST ≥12 mm group, vs. 33% in LVT+ IVST <12 mm group and vs. 4.5% in LVT− IVST <12 mm group, p < 0.0001). Consistently, patients in LVT+ and IVST ≥12 mm group had higher rate of NYHA functional class ≥III and had significantly higher indexed LV mass and diastolic impairment, lower LV ejection fraction and LV and right ventricular longitudinal function, and higher LA dimensions and systolic PAP, than those in LVT− and IVST <12 mm group. Of note, patients in LVT+ and IVST <12 mm group had significant lower LV ejection fraction, right ventricular longitudinal function, and higher E/A ratio than those in the LVT− and IVST <12 mm group.
Long-term outcome of AL patients according to LVT
During the follow-up period, 79 (53%) patients died. The overall 1- and 5-year survival rates were 69 ± 4 and 51 ± 5%, respectively. In patients with LVT+, the survival rates at 1 and 5 years were, respectively, at 51 ± 4 and 32 ± 7%, which was significantly lower than in those without (70 ± 4 and 64 ± 6%; p = 0.0002, Fig. 3).
Predictors of all-cause mortality
In univariate analyses (Table 3), male gender, NYHA functional class ≥III, Mayo Clinic stage III, IVS thickness, LV ESV, LV indexed mass, LA diameter, LA area, LA volume indexed, E/A ratio, lateral E/e’, systolic PAP, and pericardial effusion, as well as LVT+ were all associated with increased all-cause mortality. Factors associated with reduction in all-cause mortality were increase in higher glomerular filtration rates, LVEF, E-wave deceleration time, and tricuspid annular S-wave velocity.
A very good hematologic response to chemotherapeutic treatment or better was associated with increased survival (at 5 years: 78 ± 10 vs. 46 ± 7%, p = 0.022).
In multivariate analysis (Table 4), after adjusting for age and gender, the presence of LVT remained associated with all-cause mortality. Further adjustments for NYHA functional class, LVEF, and glomerular filtration rates (Model #1) did not modify the independent association between LVT and all-cause mortality. In addition, LVT + remains associated with reduced survival even after adding Nt-pro BNP for adjustment into the Model #1 (Table 4). When adding the troponin level in the Model #1, there was a definite trend for independent relationship between LVT+ and survival (Table 4).
In the subset of patients with available Mayo Clinic staging (n = 113), LVT+ remained associated with all-cause mortality after adjustment for age, gender, and Mayo Clinic staging (hazard ratio 1.83, 95% of confidence of interval 1.00–3.35, p = 0.049).
However, further adjustments for the LV diastolic parameters, systolic pulmonary artery pressure, IVST, and pericardial effusion weakened the statistical significance of the association between LVT+ and overall mortality (Table 4).
After adjustment for the hematologic response, LVT+ remains significantly associated with reduced survival (HR 2.65, 95% CI 1.4–5.1, p = 0.003).
Discussion
Our study includes a large cohort of patients with AL who underwent a baseline comprehensive echocardiographic study prior to the initiation of therapy. Our main findings indicate that LVT+ is common in patients with AL at the time of diagnosis, present in almost one half of patients, irrespective of the presence of significant valve regurgitation or stenosis. In addition, patients with LVT+ demonstrate a more advanced stage of the disease illustrated by higher rates of NYHA functional class, a higher proportion at Mayo Clinic stage II–III, and higher rates of other echocardiographic abnormalities related to cardiac AL involvement, including altered LV systolic and diastolic function and increased wall thickness.
Furthermore, patients with LVT+ have a poorer outcome as compared to those without LVT.
To the best of our knowledge, our report represents the first study to investigate the prognostic value of LVT in patients with AL at the time of diagnosis. Our population was relatively homogeneous, since it did not include patients with other types of amyloidosis (i.e., wild-type senile transthyretin or hereditary amyloidosis) who have a distinct clinical presentation, natural history, prognosis, and management.
Prevalence of LVT in AL patients
We found that 42% of patients demonstrate abnormal mitral or aortic thickening (>3 mm) with 35% of patients showing thickening of both valves at the initial echocardiogram.
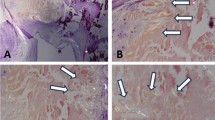
Cardiac involvement in AL patients is manifested by progressive infiltration of the left and right ventricular myocardium, inter atrial septum, and valvular endocardium with amyloid fibrils. The involvement of the valvular endocardium has been described in autopsy studies of AL patients [13]. Another post-mortem study of patients with familial hereditary transthyretin amyloidosis showed evidence of amyloid deposition in all cardiac valves [14]. Macroscopic analysis demonstrated mitral and tricuspid thickening in 50% and aortic valve involvement in 25% of patients. The prevalence of valvular involvement is similar in our patient population. Accordingly, the mitral valve thickening is more common than that of the aortic valve.
Prognostic impact of LVT in AL patients
This study is the first to analyze the prognostic value and impact of LVT+ on outcome in patients with AL. We have found that LVT, a fast and easy echocardiographic marker, was significantly associated with advanced NYHA functional class as well as cardiac involvement in AL. Patients with LVT are at a more advanced stage of the disease, a finding that explains the higher rate of all-cause mortality at 5 years as compared to those without LVT. Despite adjustment for clinically relevant parameters associated with increased all-cause mortality, LVT remains an independent predictor of outcome. However, the adjustments for other hemodynamic echocardiographic parameters weakened the statistical significance of the association between LVT+ and overall mortality. These findings indicate a stronger impact of the hemodynamic alterations as compared to the morphological changes on the outcome of patients with AL. Importantly, the association between LVT+ and prognosis is not related to the severity of valve dysfunction, since LVT was not associated with any significant valve stenosis or regurgitation in this population. Studies in AL patients have shown that LV “hypertrophy” and valvular infiltration may persist for an extended period of time following organ and hematological remission in response to therapy [7]. This phenomenon is due to the delay (sometimes several years) necessary for the elimination of fibrils, whereas the direct toxicity of the light-chain immunoglobulin on the heart has already resolved with treatment [7]. This concept further supports our findings that the hemodynamic alterations have major prognostic implications as compared to the morphological abnormalities that may persist for many years following effective treatment.
Limitations
Our study has a retrospective design with potential limitations inherent to this type of study.
The exact cause of death in our patients was not always available, explaining why we opted to list the total mortality rather than cardiac mortality as outcome [8]. The Mayo clinic staging was not available in 25% of patients. However, we are unable to document any potential selection bias related to the change in the quantification of cardiac involvement in AL. No significant difference was found between baseline characteristics and prevalence of LVT of patients in whom the Mayo Clinic staging was performed and those without this staging system. The LVT is better assessed using transoesophageal echocardiography as it was previously described [10, 11]; but for ethical reasons, TEE could not be offered for our AL patients, because it was not clinically indicated. However, despite its lower sensitivity to detect abnormal thickening, TTE provided important prognostic information as demonstrated in our study.
The LVT may be caused by several other etiologies than amyloid infiltration; and it is difficult to define only by transthoracic echocardiography the exact cause of thickening, that is why, the clinical data are actually very helpful to address differential diagnosis. After reviewing patient’s medical charts, we found no clinical data suggestive for active endocarditis. However, we cannot definitely exclude other causes of LVT (i.e., sclerosis), but the level of traditional risk factors was low, and we cannot consider a systematic bias favouring sclerosis in more advanced stages of the Mayo Clinic classification. In addition, although many efforts have been made to avoid to consider localized valve nodular thickening or calcification as LVT, we can not guarantee the absence of such assessment error. Nonetheless, in light of the low rate of cardiovascular risk factor of this population, these morphological features are very likely infrequent in our cohort.
Finally, assessment of thickening was limited to the left-sided heart valves and did not mention whether right heart valves were also involved in the process of amyloid infiltration. This lack of information regarding tricuspid and pulmonary valves is due to the retrospective design of our study and thus the absence of specific description of the right heart valves in the echocardiographic reports of included patients. Prospective studies are necessary to determine the prevalence and impact of right heart valves on outcome in AL patients.
Conclusion
Left valvular thickening is frequent, present in almost half of AL patients’ naive of treatment, at the baseline echocardiographic study. It is associated with advanced NYHA functional class, Mayo clinic stage, systolic and diastolic dysfunction, as well as increased pulmonary hypertension. Its presence is also associated with an increased risk of death at 5 years. We suggest that valve thickening should be systematically reported as a part of the echocardiographic study in patients with documented or suspected AL. Further prospective studies are required to confirm our findings and to determine whether LVT may be systematically used to detect degree of AL-related cardiac involvement or likelihood of progression/regression of disease after treatment.
References
Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, Falk RH (1998) The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 91(2):141–157
Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, McConnell JP, Litzow MR, Gastineau DA, Tefferi A, Inwards DJ, Micallef IN, Ansell SM, Porrata LF, Elliott MA, Hogan WJ, Rajkumar SV, Fonseca R, Greipp PR, Witzig TE, Lust JA, Zeldenrust SR, Snow DS, Hayman SR, McGregor CG, Jaffe AS (2004) Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood 104(6):1881–1887
Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, Greipp PR, Witzig TE, Lust JA, Rajkumar SV, Fonseca R, Zeldenrust SR, McGregor CG, Jaffe AS (2004) Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol 22(18):3751–3757
Buss SJ, Emami M, Mereles D, Korosoglou G, Kristen AV, Voss A, Schellberg D, Zugck C, Galuschky C, Giannitsis E, Hegenbart U, Ho AD, Katus HA, Schonland SO, Hardt SE (2012) Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol 60(12):1067–1076
Klein AL, Hatle LK, Taliercio CP, Oh JK, Kyle RA, Gertz MA, Bailey KR, Seward JB, Tajik AJ (1991) Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation 83(3):808–816
Lachmann HJ, Booth DR, Booth SE, Bybee A, Gilbertson JA, Gillmore JD, Pepys MB, Hawkins PN (2002) Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med 346(23):1786–1791
Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G (2005) Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 79(4):319–328
Lauer MS, Blackstone EH, Young JB, Topol EJ (1999) Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 34(3):618–620
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270
Crawford MH, Roldan CA (2001) Quantitative assessment of valve thickness in normal subjects by transesophageal echocardiography. Am J Cardiol 87(12):1419–1423
Rajamani K, Chaturvedi S, Jin Z, Homma S, Brey RL, Tilley BC, Sacco RL, Thompson JL, Mohr JP, Levine SR, Investigators P-A (2009) Patent foramen ovale, cardiac valve thickening, and antiphospholipid antibodies as risk factors for subsequent vascular events: the PICSS-APASS study. Stroke 40(7):2337–2342
Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, Scientific Document Committee of the European Association of Cardiovascular Imaging (2013) Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 14(7):611–644
Smith TJ, Kyle RA, Lie JT (1984) Clinical significance of histopathologic patterns of cardiac amyloidosis. Mayo Clin Proc 59(8):547–555
Eriksson A, Olofsson BO, Eriksson P (1986) Heart valve involvement in familial amyloidosis with polyneuropathy. Pathol Res Pract 181(5):563–567
Acknowledgements
We thank very much, Mr. David Lavergne, Ph.D., for his great help for the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors deny any conflict of interest, financial, or otherwise, related to the submitted work.
Rights and permissions
About this article
Cite this article
Mohty, D., Pradel, S., Magne, J. et al. Prevalence and prognostic impact of left-sided valve thickening in systemic light-chain amyloidosis. Clin Res Cardiol 106, 331–340 (2017). https://doi.org/10.1007/s00392-016-1058-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1058-x