Abstract
Background
Insertable cardiac monitor (ICM) increases the detection rate of occult atrial fibrillation (AF) after cryptogenic stroke. The aim of this study was to evaluate the prognostic significance of total atrial conduction time (TACT) assessed by tissue Doppler imaging (PA-TDI interval) to predict AF presence in patients with cryptogenic stroke.
Methods
Ninety patients (57.7 ± 12.3 years, 48 % women) after acute cryptogenic stroke and ICM implantation were prospective recruited at four centers for continuous rhythm monitoring. In all patients, TACT was measured by PA-TDI interval via echocardiography. Patients were followed up (331 ± 186 days) for detection of AF (defined by episode lasting ≥30 s).
Results
AF was detected in 16 patients (18 %) during follow-up (331 ± 186 days). The median period to AF detection was 30 days (q1–q3; 16–62 days). Patients who exhibited occult AF were characterized by significantly longer PA-TDI intervals (154.7 ± 12.6 vs. 133.9 ± 9.5 ms, p < 0.0001). The cut-off value of PA-TDI interval at 145 ms demonstrated sensitivity and specificity for AF detection of 93.8 and 90.5 %, respectively. In multivariate analysis, CHA2DS2–VASc score (HR 1.96 per 1 point, p < 0.01) and longer PA-TDI interval (HR 4.05 per 10 ms, p < 0.0001) were independent predictors of occult AF.
Conclusion
Our data suggest that measurement of TACT could help to predict future AF detection in patients with cryptogenic stroke. The clinical importance of prolonged rhythm monitoring or indication of direct anticoagulation therapy after cryptogenic stroke based on TACT should be further investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and affects over 6 million people in Europe [1]. This arrhythmia is associated with a fivefold higher risk of stroke and responsible for up to 25 % of first strokes [2, 3].
In Germany, about 262,000 people experienced a new or recurrent stroke each year, and stroke per se is responsible for the fourth leading cause of death [4].
However, it has been also known that 12–40 % of strokes remain unexplained after standard diagnostic, so-called cryptogenic stroke [3, 5].
Recently, insertable cardiac monitor (ICM) increased the detection rate of occult AF in patients with cryptogenic stroke in a range between 8.9 and 30 % [6–10].
To improve the detection rate of AF, predictors of AF are of great clinical interest. Atrial fibrosis is central to AF and associated with thromboembolic risk [11–14]. Increasing age, prolonged PR interval and atrial premature beats have been proposed as risk markers for future AF in patients with cryptogenic stroke [15–17].
Recently, total atrial conduction time (TACT) determined by tissue Doppler imaging (PA-TDI interval) via transthoracic echocardiogram was demonstrated to be a reliable predictor of new-onset and recurrent AF [18–21]. Furthermore, PA-TDI interval showed an association with CHADS2 score, peak velocity of left atrial appendage and stroke in patients with AF [22, 23].
In light of the above-mentioned studies, we hypothesized that measuring TACT could facilitate the detection of occult AF in patients with cryptogenic stroke. Thus, this study sought to assess the validity of an echocardiographic index, termed PA-TDI interval, in predicting the occurrence of future AF in patients presenting with cryptogenic stroke.
Methods
Study design and patient recruitment
In this prospective multicenter trial, we studied a total of 90 patients with acute cryptogenic stroke. Patients were enrolled at four cardiology centers between March 2013 and April 2015. Patients classified with acute cryptogenic stroke according to the Trial of Org 10 172 in Acute Stroke Treatment (TOAST) criteria [24]. Extensive clinical work-up included 12-lead electrocardiogram (ECG), 72-h ECG monitoring at stroke unit, an additional 24-h ECG monitoring and transesophageal echocardiography. Detailed brain and vascular imaging included magnetic resonance imaging (MRI) scan with diffusion weighted image (DWI) and computer tomography (CT) angiogram were conducted.
Patients were eligible for enrollment if they were ≥18 years and received the diagnosis of cryptogenic stroke within the previous 60 days. Patients were excluded if they had a history of AF or atrial flutter (over 30 s), a permanent indication or contraindication for anticoagulation at enrollment, and an indication for implantation of a pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization therapy device.
The study protocol was approved by all relevant institutional ethics committees and informed written consent was obtained from all study patients.
Echocardiography
All patients underwent transthoracic echocardiography using a GE Vivid 7 Echocardiograph according to the standards of the American Society of Echocardiography criteria. Left atrial diameter (LAD) was measured in parasternal long axis view at end systole. Left ventricular systolic ejection fraction (LV-EF) was assessed by Simson’s method.
To estimate TACT, PA-TDI interval was assessed two times per patient (to calculate the mean value PA-TDI interval of every patient) by measuring the time interval between the onset of P-wave in lead II of the ECG (on echocardiographic images) to the peak A´-wave of the lateral atrial wall on the tissue Doppler tracing (Fig. 1). Reproducibility of PA-TDI interval measurement was previously reported [21]. Mean values were calculated from the results of measurements.
ECG monitoring
All patients received a validated ICM (Reveal XT; Medtronic, Minneapolis, MN) for continuous ECG monitoring. The ICM was implanted subcutaneous in a left parasternal position. Sensitivity was programmed at 0.05 mV according to the manufacture’s recommendation. Detection of AF was defined as any episode of AF lasting longer than ≥30 s. A cardiologist who was blinded to the results of transthoracic echocardiography work-up made diagnosis of AF.
Statistical analysis
Data were analyzed with JMP11.0 software package (SAS Institute, Inc, Cary, NC). Numeric values were expressed as mean ± standard deviation. χ 2 test, Student’s t test, or 1-way analysis of variance was performed when appropriate to test for statistical differences. p < 0.05 was considered statistically significant. Event rate curves were plotted according to the Kaplan–Meier method and were analyzed with the log-rank test. Univariate and multivariate Cox regression were performed to assess predictive values of factors for subsequent cardiac events.
Results
A total of 90 patients with acute cryptogenic stroke were analyzed. The baseline clinical characteristics of the study population are summarized in Table 1. The mean age of the study population was 57.7 ± 12.3 years with 48 % women. Among the study population, 70 % had hypertension, 18 % had diabetes mellitus, 7 % had coronary artery disease, and 6 % had chronic kidney disease stage ≥ III. The virtual CHA2DS2–VASc score of the study population was 3.4 ± 1.7.
During a mean follow-up of 331 ± 186 days, 16 patients (18 %) experienced detection of AF.
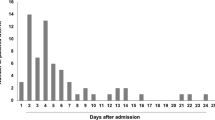
The first documented AF episode occurred after a median of 30 days (mean period to AF detection: 40.7 ± 42.2 days) with a range from day 1 to day 176 (Fig. 2). Notably, 8 patients with new diagnosed AF (50 %) developed AF during the first 30 days of continuous rhythm monitoring.
Regarding baseline demographics, there was no significant difference between patients with and without AF during follow-up, except for hypertension and virtual CHA2DS2–VASc score (Table 1). In detail, patients with new diagnosed AF during follow-up had a significantly higher CHA2DS2–VASc score compared to patients without AF detection (4.5 ± 1.2 vs. 3.1 ± 1.7, p < 0.01). Patients with AF detection during follow-up had a significant larger left atrial diameter (LAD) (40.3 ± 6.4 vs. 37.0 ± 4.2 mm, p < 0.05). The maximum P-wave duration (Pmax) on surface ECG was significantly higher in patients with AF detection during follow-up vs. patients without AF detection (114 ± 15.1 vs. 96.5 ± 15.9 ms, p < 0.0001). The best discriminating value of Pmax was 110 ms, which was characterized by 81.3 % sensitivity and 71.7 % specificity (area under ROC curve (AUC) 0.79).
The PA-TDI interval ranged between 108 and 177 ms. Patients with AF detection had a significant longer PA-TDI interval as compared to those without AF (Fig. 3: 154.7 ± 12.6 vs. 133.9 ± 9.5 ms, p < 0.0001). Moreover, Pmax and PA-TDI interval showed a significant correlation (p < 0.0001, r = 0.475) (see online supplement).
At an optimal cut-off value of >145 ms, the sensitivity and specificity in identifying occult AF via PA-TDI interval were 93.8 and 90.5 %, respectively (Fig. 4a).
Kaplan–Meier analysis demonstrated that the patients with PA-TDI interval >145 ms had significantly higher incidence of AF detection as compared to the patients with PA-TDI interval under or equal to 145 ms (Fig. 4b: log-rank, p < 0.0001).
On the other hand, at an optimal cut-off value of 144 ms, the sensitivity and specificity in predicting non-AF detection were 93.8 and 90.5 %, respectively (Fig. 4b).
A univariate regression analysis revealed that PA-TDI interval was the most powerful predictor of occult AF (hazard ratio [HR] = 2.73 per 10 ms PA-TDI prolongation, p < 0.0001). In addition, CHA2DS2–VASc score (HR = 1.58, p = 0.0023 per 1 point) and Pmax (HR = 1.89, p < 0.0001 per 10 ms) were also significant predictors of AF detection. CHADS2 score, a dilatation of LA diameter, the presence of hypertension, and the use of beta-blocker therapy showed significant association with the documented AF.
A multivariate regression analysis demonstrated that PA-TDI interval was the most powerful independent predictor of occult AF during follow-up period (HR = 3.51 per 10 ms, p < 0.0001) (Table 2).
Discussion
In this study, we found that total atrial conduction time (TACT) measured via PA-TDI interval is an independent predictor of future AF detection in patients with cryptogenic stroke and implanted ICM. To the best of our knowledge, this is the first prospective study to examine the relationship between TACT and occurrence of AF in patients with cryptogenic stroke.
Several studies found that continuous rhythm monitoring is superior to standard ECG monitoring for detection of AF after cryptogenic stroke. This advancement of continuous rhythm monitoring with ICM revealed a substantial percentage of AF detection in patients with cryptogenic stroke in up to 30 % [6–10].
In this study, 16 patients (18 %) experienced detection of occult AF during follow-up. New diagnosed AF in all these patients resulted in initiation of oral anticoagulation therapy for secondary prevention.
However, the detection rate of AF in cryptogenic stroke showed a wide variance of 12–30 % in the literature, even under use of ICM. Asymptomatic AF without anticoagulation therapy can result in recurrences of stroke; therefore, identification of patients at high risk for AF after cryptogenic stroke is crucial to determine patients who will benefit from intensive rhythm monitoring and further oral anticoagulation therapy.
AF is associated with atrial fibrosis, the hallmark of structural atrial remodeling [11, 12]. Of note, Marrouche and colleagues found that atrial fibrosis detected by MRI was associated with thromboembolic milieu on transesophageal echocardiography and previous stroke in patients with AF [13, 14].
Kochhäuser et al. found that numerous supraventricular premature beats and short supraventricular runs in 24-h Holter-ECG were associated with future AF in patients with cryptogenic stroke [16]. The results from the CRYSTAL AF trail, published by Thijs et al., showed that age and a prolonged PR interval were associated with AF detection after cryptogenic stroke [15]. They suggested that increased age as well as prolonged PR interval contributed to atrial fibrosis formation, which may be a culprit mechanism in the genesis of AF.
PA-TDI interval is a user-friendly and inexpensive echocardiographic index. Previously, our research group found a correlation between PA-TDI interval and atrial fibrosis in patients undergoing cardiac surgery [21]. De Vos and colleagues first identified PA-TDI interval as an independent predictor of the development of new onset AF [18]. Studies utilizing PA-TDI interval have also shown a correlation of PA-TDI interval with peak velocity of left atrial appendage, CHADS2 score and stroke in patients with AF [22, 23].
According to this, our study demonstrated that CHA2DS–VASc score, Pmax, and PA-TDI interval were independently associated with AF detection after cryptogenic stroke.
The measurement of Pmax is universally feasible based on the 12-lead ECG, which enables the first-line risk stratification. In this study, Pmax showed significant correlation to PA-TDI interval (r = 0.475) and was also an independent predictor of AF detection. However, Merckx et al. illustrated that the PA-TDI interval was superior in the measurement of TACT compared to the Pmax on surface ECG (r = 0.91 vs. r = 0.64) [25].
Our results highlight that PA-TDI interval was the most powerful independent predictor for occult AF in patients with cryptogenic stroke.
Notably, 8 of 16 patients with new diagnosed AF developed the arrhythmia within the first 30 days. All 8 patients with AF documentation within the first 30 days of continuous rhythm monitoring had PA-TDI interval ≥145 ms. In contrast, at a cut-off point of PA-TDI interval <144 ms, the specificity and sensitivity to predict non-AF detection were 93,8 and 90,5 %, respectively. Thus, the cut-off points to predict AF detection and non-AF detection suggests that a PA-TDI value of 144–145 ms should be a good cut-off point for risk stratification in patients with cryptogenic stroke.
The atrial size can also reflect atrial fibrosis and affect atrial conduction time; therefore, we have additionally assessed the association between PA-TDI interval/maximal left atrial diameter (LAD) and the AF incidence. However, the PA-TDI interval/maximal LAD ratio showed no significant difference between patients with and without AF detection (39.1 ± 5.9 vs. 36.8 ± 5.4 ms/cm, p = 0.13).
Further studies have to prove if PA-TDI interval could help to select patients who mostly benefit from prolonged rhythm monitoring in order to initiate prompt oral anticoagulation therapy for secondary prevention after AF detection or even to judge an indication direct oral anticoagulation therapy after cryptogenic stroke.
Study limitations
This study has several limitations. Although all patients had stated no known history of AF, prior episodes of asymptomatic AF could not be excluded. Despite the multicenter and prospective study design, the number of patient is relatively small. However, this study intended to evaluate the utility of PA-TDI interval measurement for predicting AF occurrence. Therefore, the cut-off value of PA-TDI was not prospectively assessed.
Although reproducibility of PA-TDI interval measurement was previous reported of our laboratory [21], inter-observer variability cannot be ruled out. It is true that for the measurement of PA-TDI interval, the investigator should accumulate some experiences; however, based on the results of this study, we believe that PA-TDI interval results in better risk stratification “on top.”
The present findings should be validated in larger prospective studies to substantiate the benefit of PA-TDI interval measurement in predicting AF after cryptogenic stroke into clinical routine.
Conclusion
This study confirmed the important role of measuring total atrial conduction time via PA-TDI interval in patients with cryptogenic stroke. We demonstrated that PA-TDI interval was an independent predictor of future AF detection in patients with acute cryptogenic stroke. Systematic evaluation of PA-TDI interval in the setting of cryptogenic stroke may help to select patients who benefit from prolonged rhythm monitoring to detect occult AF.
Abbreviations
- AF:
-
Atrial fibrillation
- CT:
-
Computer tomography
- ECG:
-
Electrocardiogram
- DWI:
-
Diffusion weighted image
- ICM:
-
Insertable cardiac monitor
- LAD:
-
Left atrial diameter
- LV-EF:
-
Left ventricular systolic ejection fraction
- MRI:
-
Magnetic resonance imaging
- Pmax:
-
Maximum P-wave duration
- TACT:
-
Total atrial conduction time
- TDI:
-
Tissue Doppler imaging
- TOAST:
-
Trial of Org 10 172 in Acute Stroke Treatment
References
Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S et al (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31:2369–2429
Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk for stroke: the Framingham Study. Stroke 22:983–988
White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL (2005) Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 111:1327–1331
Kolominsky-Rabas PL, Sarti C, Heuschmann PU, Graf C, Siemonsen S, Neundoerfer B, Katalinic A, Lang E, Gassmann KG, von Stockert TR (1998) A prospective community-based study of stroke in Germany—The Erlangen Stroke Project (ESPro) incidence and case fatality at 1, 3, and 12 months. Stroke 29:2501–2506
Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener HC (2001) Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 32:2559–2566
Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J, CRYSTAL AF Investigators (2014) Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 26:2478–2486
Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O’Donnell M, Laupacis A, Côté R, Sharma M, Blakely JA, Shuaib A, Hachinski V, Coutts SB, Sahlas DJ, Teal P, Yip S, Spence JD, Buck B, Verreault S, Casaubon LK, Penn A, Selchen D, Jin A, Howse D, Mehdiratta M, Boyle K, Aviv R, Kapral MK, Mamdani M, EMBRACE Investigators and Coordinators (2014) Atrial fibrillation in cryptogenic stroke. N Engl J Med 26:2467–2477
Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH, ASSERT Investigators (2012) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2:120–129
Ritter M, Kochhäuser S, Duning T, Reinke F, Pott C, Dechering DG, Eckardt L, Ringelstein EB (2013) Occult atrial fibrillation in cryptogenic stroke: detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke 44:1449–1452
Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ (2013) Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 17:1546–1550
Nattel S, Burstein B, Dobrev D (2008) Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 1:62–73
Burstein B, Nattel S (2008) Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 51:802–809
Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, Macleod RS, Marrouche NF (2011) Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cadiol. 7:831–838
Akoum N, Fernandez G, Wilson B, Mcgann C, Kholmovski E, Marrouche N (2013) Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol 10:1104–1109
Thijs VN, Brachmann J, Morillo CA, Passman RS, Sanna T, Bernstein RA, Diener HC, Di Lazzaro V, Rymer MM, Hogge L, Rogers TB, Ziegler PD, Assar MD (2016) Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurol 86:261–269
Kochhäuser S, Dechering DG, Dittrich R, Reinke F, Ritter MA, Ramtin S, Duning T, Frommeyer G, Eckardt L (2014) Supraventricular premature beats and short atrial runs predict atrial fibrillation in continuously monitored patients with cryptogenic stroke. Stroke 3:884–886
Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS (2015) Thorpe KE; EMBRACE Steering Committee and Investigators. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke 4:936–941
De Vos CB, Weijs B, Crijns HJ, Cherlex EC, Palmans A, Habets J, Prins MH, Pisters R, Nieuwlaat R, Tielema RG (2009) Atrial tissue Doppler Imaging for prediction of new-onset atrial fibrillation. Heart 95:835–840
Antoni ML, Bertini M, Atary JZ, Delgado V, ten Brinke EA, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Bax JJ, van de Veire NR (2010) Predictive value of total atrial conduction time estimated with tissue Doppler imaging for the development of new-onset atrial fibrillation after acute myocardial infarction. Am J Cardiol 106:198–203
Bertini M, Borleffs CJ, Delgado V, Ng AC, Piers SR, Shanks M, Antoni ML, Biffi M, Boriani G, Schalij MJ, Bax JJ, Van de Veire NR (2010) Prediction of atrial fibrillation in patients with an implantable cardioverter-defibrillator and heart failure. Eur J Heart Fail 12:1101–1110
Müller P, Hars C, Schiedat F, Bösche LI, Gotzmann M, Strauch J, Dietrich JW, Vogt M, Tannapfel A, Deneke T, Mügge A, Ewers A (2013) Correlation between total atrial conduction time estimated with tissue Doppler imaging (PA-TDI interval), structural atrial remodeling an new-onset of atrial fibrillation after cardiac surgery. J Cardiovasc Electrphysiol. 24:626–631
Chao TF, Sung SH, Wang KL, Tsao HM, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Liu SH, Wu TJ, Yu WC, Chen SA (2011) Atrial electromechanical interval can identify patients with paroxysmal atrial fibrillation and is associated with CHADS2 score and peak velocity of left atrial appendage. J Cardiovasc Electrophysiol 22:1325–1330
Chao TF, Lin YJ, Tsao HM, Chang SL, Lo LW, Hu YF, Tuan TC, Li CH, Chang HY, Wu TJ, Yu WC, Chen SA (2013) Prolonged atrial electromechanical interval is associated with stroke in patients with atrial fibrillation after catheter ablation. JCE 24:375–380
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1:35–41
Merckx K, De Vos CB, Palmans A, Habets J, Cherlex EC, Crijns HJ, Tieleman RG (2005) Atrial activation time determined by transthoracic Doppler tissue Imaging can be used as an estimate of the total duration of atiral electrical activation. J Am Soc Echocardiogr 18:940–944
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Human rights statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000(5). Informed consent was obtained from all patients for being included in this study.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Müller, P., Ivanov, V., Kara, K. et al. Total atrial conduction time to predict occult atrial fibrillation after cryptogenic stroke. Clin Res Cardiol 106, 113–119 (2017). https://doi.org/10.1007/s00392-016-1029-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1029-2