Abstract
Background
Inflammation is the driving force in atherosclerosis. One central strategy in the treatment of peripheral arterial disease (PAD) is the promotion of angiogenesis. Here, proangiogenic Tie-2 expressing monocytes (TEM) and circulating angiogenic cells (CAC) play a crucial role. Exercise training (ET) is recommended in PAD patients at Fontaine stage II to promote angiogenesis.
Methods
40 patients with intermittend claudication (IC) [2 groups: supervised ET (SET) vs. non-supervised ET (nSET), each n = 20] and 20 healthy controls were included in the study. Analysis of TEM and CAC was performed from whole blood by flow-cytometry. TEM were identified via CD45, CD86, CD14, CD16 and analysed for the expression of Tie-2. CAC were identified via their expression of CD45 (CD45dim), CD34 and VEGF-R2 (CD309/KDR). Follow up was performed after mean of 7.65 ± 1.62 months.
Results
In comparison to healthy controls, we found increased proportions of CAC (p < 0.0001) and similar TEM numbers in both ET groups. At follow-up (FU) TEM poroportions increased (p < 0.001) and CAC proportions decreased (p < 0.01), but both more significantly in SET (p < 0.001) than nSET (p = 0.01). Only in SET fibrinogen levels decreased and VEGF-A increased (both p < 0.05). Finally, we found in both ET groups a significant increase in absolute walking distance but with a higher individual increase in SET (p < 0.01). TEM and CAC proportions correlated inversely with the absolute walking distance (CAC: r = −0.296, p = 0.02; TEM: r = −0.270, p = 0.04) as well as with ABI (CAC: r = −0.394, p < 0.01; TEM: r = −0.382, p < 0.01).
Conclusions
ET influences the distribution of CAC and TEM proportions. nSET, although still effective in regard to an improved walking distance, is less effective in the influence of proangiogenic cells and inflammatory burden than SET. Our results indicate SET to be a more preferential exercise form, supporting the necessity to establish more SET programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral arterial disease (PAD) in associated with a high cardiovascular mortality [1], due to a high prevalence of further atherosclerotic manifestations in the coronary and cerebral circulation [2, 3]. Inflammation is the driving force in atherosclerotic diseases [4]. Especially PAD represents a generalized form of atherosclerosis, with a high inflammatory status [5], thus explaining the high cardiovascular mortality rate in PAD [1].
Therefore, it is essential to treat PAD patients consequently as early as possible in dependence of each patient’s disease state. According to international guidelines, for patients in Fontaine state I or II A/B (Rutherford 1–3) a conservative treatment with best medical treatment and exercise training is recommended [6, 7]. The exercise training is most effective under professional supervision; however, in the absence of a structured and supervised exercise program, a non-supervised home based exercise training is recommended, although inferior to the supervised training [8]. It is well known, that physical training has a positive effect on inflammation, immune cells and atherosclerosis [9, 10]. Part of the responsible mechanisms are related to the mobilisation of circulating angiogenic cells (CAC) stimulated by either moderate [11–13] or intensive, strenuous exercise [14–16].
Since the first description of endothelial progenitor cells (EPC) by Asahara in 1997 [17] an incredible amount of research has been performed. These EPC are of hematopoetic origin and are frequently characterized by the surface marker molecules CD34 and vascular endothelial growth factor (VEGF)-Receptor 2 (CD133, KDR) [18]. CAC in contrast are in fact of myeloid origin, but also express CD34 and VEGF-R2 [18]. Nevertheless these cells do not represent endothelial precursors [19], but a controversial discussion still continues in particular because myelomonocytic cells share several characteristics with endothelial cells [20]. Current consensus therefore defines two major classes of angiogenic cells, but only specifically after in vitro culture. Firstly the CAC (also called early EPC), which appear after a few days (early) in culture after adherence to fibronectin, and secondly the endothelial colony forming cells (ECFC, or “outgrowth endothelial cells” (OEC) or late EPC). These can be detected after 2 weeks (late) of culture on collagen [21]. A third type of proangiogenic cells are called colony forming (CFU)-Hill EPC, derived from re-plated peripheral blood mononuclear cells (PBMC), but these have been shown to be mixed colonies of monocytes and T-lymphocytes [22, 23].
Hypoxia/ischemia is thought to be the primary stimulus to mobilize CAC from the bone marrow to home in the sites of ischemia, which is mainly influenced by VEGF [24]. CAC are a rare group of cells in the peripheral circulation consisted of about below 1 % [25]. Much more prominent in the peripheral circulation are proangiogenic Tie-2 expressing monocytes (TEM), representing about 1–2 % of the PBMC population, but represent up to 20 % of the monocyte population [26, 27]. TEMs have been well investigated for their pro-angiogenic role in cancer [28], however little is known about their pro-angiogenic involvement in PAD. TEM belong to the intermediate/non-classical monocytes subpopulation and are activated similarly to CAC by hypoxia/ischemia and divers pro-angiogenic cytokines (VEGF, Angiopoietin-2) [26, 27].
The effects of exercise training in PAD on CAC and TEM are not well investigated so far. The aim of the present pilot study was therefore to analyse the influence of a supervised or non-supervised exercise training (SET, nSET respectively) on the proportion of CAC and TEM to gain information from their distribution under exercise for potential future monitoring, i.e. success/efficiency of a performed exercise training program.
Materials and methods
Study population
The present study was designed as a pilot study and carried out in accordance with the principles outlined in the Declaration of Helsinki and was approved by the Ethics Committee of the University of Mainz and the State of Rheinland-Pfalz, Germany. Participation was voluntary. Each participant gave written informed consent. We enrolled 40 patients with known PAD admitted to the 2nd Medical Department, Institute for Vascular Medicine of the Johannes Gutenberg University Mainz with intermittent claudication (IC; Rutherford 1-3, Fontaine II A/B). Patients with known cancer, autoimmune disease, end stage renal disease (ESRD), orthopedic maladies (chronic arthrosis of hip or knee, spinal canal stenosis) not allowing them to train properly, age under 18 or pregnancy were excluded from the study. Patients who had received antihypertensive treatment or who had received a diagnosis of hypertension (blood pressure above 140/90 mm Hg) were considered to have arterial hypertension. Smoking was classified as current smoking, past smoking (stopped between >4 weeks and <40 years ago), or never smoking. Diabetes mellitus was diagnosed in patients who had previously undergone dietary treatment or had received oral antidiabetic or insulin medication or who had a current fasting blood glucose level >125 mg/dl or HB1Ac levels >6.5 %. Family history of premature atherosclerosis was attributed to patients with a documented case of PAD, atherosclerotic stroke or coronary artery disease (CAD) in a first-degree relative before the age of 65 years. Hyperlipoproteinemia was diagnosed in patients who had been given lipid-lowering medication or had a history of cholesterol levels >240 mg/dl. The age median of the total study population was 69.5 years [62.25; 76]. The distribution of the cardiovascular risk factors and medication taken by the study participants are given in Table 1. Follow up was performed after 7.65 ± 1.62 months.
Exercise training was performed according to the recommendations of the present guidelines [6, 7]. For the study, patients were asked whether they preferred to perform an exercise training under supervision with accredited vascular physiotherapists or to perform a home based exercise training under their own responsibility. All patients were informed to walk at least 30 min up to 60 min per day, at least on 3–5 days a week. They were asked to walk with an intensity to nearly reach their typical claudication sensations, then to rest for up to 5 min and repeat the same distance with a lower intensity. This protocol is in accordance with standard exercise recommendations [29]. In the SET group supervised training was performed once per week on top on the regular exercise during the week. In addition, patients in the SET group were given a “home work” plan, with exercises to be performed at rest. Written information, on how to perform the home based exercise training under self-management in the nSET group with the same exercises at rest was handed out at the beginning of the study. Patients controlled their weekly walking distance with the aid of a pedometer.
The pain free and absolute walking distance of each patient at the beginning and at follow up appointment of the study was measured after standard constant load protocol on a treadmill with 12 % elevation at a speed of 3.2 km/h. The same elevation and speed was used at both treadmill tests.
Controls (n = 20) were recruited from patients present at the Medical Department for different reasons at the time of the study, being invited as healthy individuals in regard to CAD and PAD to participate in the study as control subjects. Of the individuals enrolled to the study, we selected those with an ankle-brachial index (ABI) above 0.9 after exclusion of CAD by coronary angiography, which was performed earlier because of clinical signs of atherosclerosis in history, in ECG or stress test. Due to the presence of common cardiovascular risk factors the control population has to be regarded as controls at risk.
Preparation of blood samples
From each participant blood was drawn by venopuncture after a fasting period of at least 12 h. For the different analysis whole blood samples were either heparinised or drawn in EDTA or sodium citrate. In dependence of the designated tests the samples were transported immediately to our lab or the hospitals core facility for routine blood analysis (Institute for Clinical Chemistry and Laboratory Medicine), either on ice or at room temperature.
Flow cytometry of monocytes and CAC
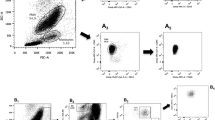
Flow cytometric analysis was performed after standard protocol we used in adaption of the protocol of two recent publications by Hristov and Schmidt-Lucke, respectively [30, 31]. In brief, after identifying live cells being 7-AAD negative (Biolegend, San Diego, USA) only CD45 (clone J.33) (Beckman-Coulter Inc., Krefeld, Germany) positive cells (Fig. 1a1) were analysed. From these CAC (a2) and monocytes (b1) were identified by different gating strategies. The CAC population was identified by the marker molecule CD34 (clone 581) (Beckman-Coulter Inc., Krefeld, Germany) in the SSC diagram (a2). From this population the CD45 low expressing population (CD45dim) (a3) was then analysed for CD34 and VEGF-R2 (CD309, KDR) (clone 89106) (R&D Systems, Wiesbaden, Germany) (a4). The monocyte population was identified by size and granulation in the forward scatter/sideward scatter diagram (FSC/SSC) (a1), as well as by the marker molecules CD45 and CD86 (b1) (clone HA5.2B7) (Beckman-Coulter Inc. Krefeld, Germany). Monocytes were analysed for CD14 (clone M5E2) (Biolegend, San Diego, USA), CD16 (clone 3G8) (bd-biosciences, Heidelberg, Germany) (b2/b3), and Tie-2 (clone 83715)(R&D Systems, Wiesbaden, Germany) (b4).
Flow cytometric analysis of CD14/CD16 monocyte subpopulations, TEM and CAC. a 1 Cell populations were identified in the forward scatter/sideward scatter diagram (FSC/SSC) after excluding dead and only CD45 positive cells. a 2 The CAC population was identified by the marker molecule CD34 in the SSC diagram. a 3 CD45 low expressing population (CD45dim) was then analysed for (a 4 ) CD34 and VEGF-R2 (CD309, KDR). b 1 The monocyte population was identified by size and granulation in the FSC/SSC diagram and by the marker molecules CD45 and CD86. (b 2 /b 3 ) Monocytes were analysed for CD14, CD16 and (b 4 ) Tie-2
For the analysis of peripheral blood monocytes and CAC 100 µl of sodium citrate anticoagulated whole venous blood/per sample was used from each participant. The samples were transferred to 5 ml FACS tubes (BD Falcon) and washed twice before erythrocytes were then lysed with BD lysing-solution (bd-biosciences) for 10 min at room temperature. Cells were then stained with directly fluorochrome conjugated antibodies. Staining conditions for each monoclonal antibody were preliminarily determined. Data are presented as percentage of positive cells corrected for background fluorescence, determined by unstained sample measurements.
Routine laboratory methods
Blood samples were drawn from each participant under standardized conditions (see above). Serum was centrifuged at 4000g for 10 min, immediately divided into aliquots, and frozen at −80 °C until analysis. Lipid serum levels (total-, HDL-, LDL-cholesterol, triglycerides), blood glucose, HbA1c, fibrinogen and cellular counts were measured immediately after transfer to the hospitals core facility for routine blood analysis (Institute for Clinical Chemistry and Laboratory Medicine) under their current standardized conditions. C-reactive protein (CRP) was determined by a highly sensitive, latex particle–enhanced immunoassay (detection range 0–20 mg/dL; Roche Diagnostics). Measurement of VEGF-A (R&D Systems, Wiesbaden, Germany) was performed by ELISA.
Cell counts
Total leukocyte and monocyte count/ml, as well as total monocyte proportion was determined by routine blood work analysis. The proportion of total CAC was determined by flow cytometry. To achieve the absolute CAC count/ml, we set the above determined CAC-proportions in relation to the total leucocyte count/ml.
Statistical analysis
For data management and statistical analysis we used the Prism V5.0f statistical software package (Graphpad, San Diego, CA, USA). Data are given as median [25th; 75th percentile] for continuous variables. A value of p < 0.05 was considered to be significant. The three groups (Controls, SET, nSET) were contrasted using one-way ANOVA and Dunn’s multiple comparison tests. Comparison of Adm. to FU inside each group was performed using paired-t test. To avoid multiple testing concerning the three groups at admission and follow up we used Bonferroni’s multipe correction test as a post hoc analysis. Correlation of continuous data among the general study population was performed using Spearman correlation coefficients (r).
Results
Analysis of the study population
With regard to male sex and age we did not find differences between PAD patients in general and controls at risk. PAD patients, though, presented more often manifest cardiovascular risk factors (Table 1), like hypertension or hyperlipidemia. Controls presented a history of strict non-smoking compared to PAD patients, and those controls who had smoked or were at present active smokers had smoked less severe in the past years regarding their amount of pack years. Concerning the medication, we did find differences with regard to ACE inhibitors or AT1 receptor blockers. Although nearly one third of the healthy controls received aspirin with an intention of primary prophylaxis, all PAD patients received significantly more aspirin than controls. For statins we did not find differences between the three groups (Table 1).
Naturally, the absolute walking distance as well as the claudication onset distance was significantly lower in PAD patients and in consequence, a significantly lower ABI was measured in PAD patients than in controls.
Total and LDL cholesterol levels were similar between the three groups, but HDL-cholesterol levels were decreased in patients. PAD patients showed a significant increase of PBMC/ml, whereas relative and absolute monocyte numbers were equal between all 3 groups. Furthermore, similar levels of acute phase proteins such as CRP and fibrinogen were measured (see Table 2).
At FU, we observed an increase of the absolute walking (WD) and claudication onset distance (CD) in SET (WD: p = 0.02; CD: p = 0.005) and nSET (WD: p = 0.06; CD: p = 0.02) patients, though still significantly lower than in controls. The individual increase in CD (ΔCD) was similar between SET and nSET, nevertheless, for SET we observed a much higher individual increase in WD (ΔWD) than in nSET. ABI or LV-EF did not change over the training period in all three groups. IMT of nSET patients though was higher at FU than in controls. Concerning markers of inflammation, we found a decrease for CRP and fibrinogen in the SET group, whereas for nSET we observed significant higher levels of CRP and fibrinogen compared to controls (Table 2).
Analysis of CD14/CD16 and Tie-2 monocyte (TEM) subpopulations
Concerning the distribution of the three CD14/CD16 monocyte subpopulations, we found no differences between the three groups. The classical CD14++CD16− subpopulation, the intermediate CD14 ++CD16+ subpopulation, as well as the non-classical CD14+CD16++ subpopulation did not reveal differences between SET, nSET or controls neither at admission, nor at FU (Table 3).
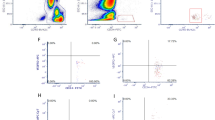
Partly, the CD16+ subpopulation contains proangiogenic TEM. In the present study population, we did not find a significant difference at admission between SET, nSET or controls (Fig. 2a), however after the training period of 6 months we observed a significant increase of the TEM proportion in both the SET and, to a lower extend, in the nSET group compared to controls (Fig. 2a). In addition, we found the individual changes in TEM proportions (ΔTEM) in SET and nSET patients to be significantly higher than in controls. In SET, ΔTEM was higher than in nSET, but not significantly (Fig. 2b). The results are presented in Table 3.
Analysis of CD34+CD45dimVEGF-R2 CAC
Next, we analysed circulating CAC in the study population. In contrast to the TEM subpopulation, we found the CAC proportion to be significantly increased in both PAD groups before training (Fig. 3a). Similarly, total CAC numbers/ml were significantly higher in PAD patients than controls at admission (Fig. 3b). At FU, we observed a decrease of CAC proportions in SET to a comparable level of controls, whereas for nSET a less prominent decrease was measurable, leading to still significant higher percentages of circulating CAC in comparison to controls, though to a lower extend than at admission (Fig. 3a). Thus, similar results were found for total CAC numbers/ml at FU (Fig. 3b) (Table 3). Individual changes in relative and absolute CAC numbers (ΔCAC%, ΔCAC/ml) were then significantly higher in the SET than in the nSET group (Fig. 3c, d).
Analysis of VEGF-A
Angiogenesis is regulated by divers cytokines from the VEGF family. We have therefore analysed the serum levels of VEGF-A before and after the training period. We found no difference for VEGF-A between controls and patients before training (see Table 2). At FU, however, we observed a significant increase VEGF-A levels for SET patients, whereas for nSET patients VEGF-A levels were comparable to the levels before the training (see Table 2).
Correlation of TEM and CAC with walking distance, ABI and markers of inflammation and angiogenesis
At FU, we found an inverse correlation for of CAC and TEM with the absolute walking distance (CAC: r = −0.296, p = 0.02; TEM: r = −0.270, p = 0.04) and at least for TEM with the claudication onset distance (CAC: r = −0.245, p = 0.06; TEM: r = −0.259, p = 0.05). With ABI, CAC and TEM correlated inversely in a similar way (CAC: r = −0.394, p < 0.01; TEM: r = −0.382, p < 0.01). Neither CAC nor TEM correlated with fibrinogen (CAC: r = −0.038, p = 0.77; TEM: r = 0.172, p = 0.19), CRP (CAC: r = 0.109, p = 0.41; TEM: r = 0.201, p = 0.13) or VEGF-A (CAC: r = 0.122, p = 0.36; TEM: r = −0.026, p = 0.95).
Nevertheless, we observed an inverse correlation of the absolute walking distance with fibrinogen (r = −0.429; p < 0.001), but not with CRP (r = −0.120; p = 0.37). The ABI correlated inversely with fibrinogen (r = −0.319; p = 0.01) or CRP (r = −0.244; p = 0.06) in a similar way.
Correlation of individual changes at follow up
Because of the difference in the performed exercise program, we were interested, whether individual changes (Δ) in TEM and CAC proportions at FU have been influenced by changes in inflammatory (fibrinogen, CRP) or pro-angiogenic stimuli (VEGF-A), then influencing individual changes in walking. We found changes in fibrinogen (ΔFibrinogen) correlated inversely with changes of CAC (r = −0.334; p = 0.01) but not TEM proportions (r = 0.186; p = 0.16), as was true for the correlation of ΔCRP with both ΔCAC (r = −0.064; p = 0.63) and ΔTEM (r = 0.188, p = 0.15). Individual changes in VEGF-A however correlated with both ΔCAC (r = -0.477; p < 0.001) and ΔTEM inversely (r = −0.409; p = 0.001). Nevertheless, only individual changes in CAC proportions corresponded inversely to individual changes in absolute walking distance (ΔCAC: r = −0.312; p = 0.02; ΔTEM: r = 0.190; p = 0.15).
Discussion
The stimulation of angiogenesis is a central point in the treatment of PAD [32]. Especially for patients with intermittend claudication in the Fontaine stages II stimulation of neo-angiogenesis through exercise training represents in most cases a first-line therapy, considering international guidelines [6, 7].
In the present study, we performed a supervised exercise training as well as a non-supervised training with PAD patients, who suffered from similar cardiovascular co-morbidities and had a comparable severity of the disease. The training lasted for 6 months. At follow up we observed an increase in the absolute as well as the claudication onset walking distance in both groups to a similar extent in total. However, only in the SET group we were able to observe a higher individual benefit of the participants concerning the prolongation of their walking distance. IMT increased in the nSET group, LVEF and ABI did not change. These results correspond very well to the general opinion, regarding SET to be more effective than nSET [8, 29]. Furthermore, the possibly more intense training in the SET group resulted in an amelioration of the inflammatory state (i.e. fibrinogen, CRP) as well as an increase in VEGF-A concentrations, then possibly contributing to the observed changes in TEM and CAC. For TEM we found similar proportions for patients and controls at admission but a significant increase at FU for both training groups, but higher in the SET group. In contrast CAC were higher in both patient groups at the beginning of the study than in controls and decreased significantly at FU, again more intense in the SET group.
Monocytes are a ubiquitous cell population, rapidly and abundantly increased in case of need, like infection and/or inflammation. They are capable to migrate to the site of inflammation and evade into the tissue where necessary. Furthermore monocytes still harbour the potential to differentiate to more mature and professional cell types, like Dendritic cells [33, 34], macrophages and even CAC like cells [35]. These are usually differentiated from the proangiogenic Tie2+ subpopulation within the intermediate CD14++CD16+ subpopulation [27, 28, 36]. TEM are thought to prepare the hypoxic environment for CAC and attract them to the site of ischemia [37]. CAC then might facilitate and promote angiogenesis by other proangiogenic chemokines to induce the sprouting of collateral vessels. Our result might be interpreted in a similar way: an increased TEM population has prepared the site of hypoxia/ischemia for CAC and has attracted these to migrate into the vessel walls, thus, a reduced number of circulating CAC is observed. On the other hand, due to the efficient exercise training, a reduced need for CAC might also explain our results, at least for the CAC population. Nevertheless, a reduced CAC proportion is surprising, in regard to the recent literature on the influence of exercise on monocytes and CAC proportions.
It has been shown that exercise training might diminish proinflammatory CD14+CD16+ monocytes possibly through its anti-inflammatory effects [38]. Furthermore, a supervised exercise training over 12 months has been found to increase CAC significantly [39].
These two studies contradict our results only at first glance. Our approach to identify the CD14 and CD16+ monocytes subpopulations was more detailed, since we first identified the total monocyte population in regard to a double positive CD45/CD86 staining, and secondly we differentiated monocytes in three subpopulations, which is the standard approach nowadays. Therefore the CD16+ monocyte subpopulation might have been overestimated, since the gating without initial CD45/CD86 identification bears the risk of positioning the gates inadequately [40].
Next to that, our study concept and performance are not comparable with Schlagers’ study. First of all the training duration was different (6 vs. 12 months), and most importantly the patients’ disease severity was different. Patients in Schlagers study were less able to walk (Median 101.5 m) at the beginning compared to our SET patients (Median 401 m). At follow up the increase in walking distance was sufficient but most patients possibly still had to be categorized in Fontaine stage II B (walking below 200 m). The increase in CAC fits to this observation. A recent publication of our group reports of higher proportions of CAC in the more severe stages of stable PAD [41], possibly showing the ongoing need for CAC to intensively promote angiogenesis.
Limitations
It remains unclear, whether the lower numbers of circulating CACs at FU are related either to an evasion to the ischemic tissue, therefore not being detectable in the peripheral blood circulation, or due to a reduced evasion from the bone marrow after the ischemic and inflammatory stimulus was reduced. The lack of tissue samples to prove this has to be acknowledged as a methodological limitation of our study. In addition, it is uncertain, whether these observations are related to the intensity of the training. Furthermore, one has to admit, that being actively selected for a training program under study conditions or not, already might motivate study participants to exercise more often or intense [42]. However, from a clinical point of view the higher motivation is actively used in supervised training programs. This probably contributes further to the superior efficiency of SET in contrast to a home-based nSET, independently of a possible better training concept.
In order to better assess our findings for their clinical relevance, data on the further clinical outcome would be preferable, especially in regard to a comparison of further training versus no training. Future studies to address this question need then to be performed, moreover since the relatively small number of the study patients (n = 40), requires the current data to be validated in other large cohorts.
Conclusions
To our best knowledge, we report here for the first time that an effective training in a supervised form can influence both the proportion of TEM as well as of CAC. Furthermore, supervised exercise training is more effective in ameliorating the inflammatory state, as well as in preparing the basis for a sufficient angiogenesis. Data from our study further strengthens the necessity to establish a broad disposability in supervised exercise training programs.
References
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K (2007) Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 33(Suppl 1):S1–75. doi:10.1016/j.ejvs.2006.09.024
Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PW (2006) International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 295(2):180–189. doi:10.1001/jama.295.2.180
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B (2006) ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113(11):e463–654. doi:10.1161/CIRCULATIONAHA.106.174526
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340(2):115–126. doi:10.1056/NEJM199901143400207
Fischer MA, Stedman MR, Lii J, Vogeli C, Shrank WH, Brookhart MA, Weissman JS (2010) Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med 25(4):284–290. doi:10.1007/s11606-010-1253-9
Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Rother J, Sievert H, van Sambeek M, Zeller T (2011) ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 32(22):2851–2906. doi:10.1093/eurheartj/ehr211
Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK (2013) Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127(13):1425–1443. doi:10.1161/CIR.0b013e31828b82aa
Bendermacher BL, Willigendael EM, Teijink JA, Prins MH (2006) Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2:CD005263. doi:10.1002/14651858.CD005263.pub2
Volaklis KA, Halle M, Koenig W, Oberhoffer R, Grill E, Peters A, Strasser B, Heier M, Emeny R, Schulz H, Ladwig KH, Meisinger C, Thorand B (2015) Association between muscular strength and inflammatory markers among elderly persons with cardiac disease: results from the KORA-Age study. Clin Res Cardiol 104(11):982–989. doi:10.1007/s00392-015-0867-7
Dopheide J, Scheer M, Doppler C, Obst V, Stein P, Vosseler M, Abegunewardene N, Gori T, Münzel T, Daiber A, Radsak M, Espinola-Klein C (2015) Change of walking distance in intermittent claudication: impact on inflammation, oxidative stress and mononuclear cells: a pilot study. Clin Res Cardiol. doi:10.1007/s00392-015-0840-5
Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G (2004) Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109(2):220–226. doi:10.1161/01.CIR.0000109141.48980.37
Jaumdally RJ, Goon PK, Varma C, Blann AD, Lip GY (2010) Effects of atorvastatin on circulating CD34+/CD133+/CD45- progenitor cells and indices of angiogenesis (vascular endothelial growth factor and the angiopoietins 1 and 2) in atherosclerotic vascular disease and diabetes mellitus. J Intern Med 267(4):385–393. doi:10.1111/j.1365-2796.2009.02151.x
Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R (2004) Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol 24(4):684–690. doi:10.1161/01.ATV.0000124104.23702.a0
Mobius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, Adams V (2009) Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol (1985) 107(6):1943–1950. doi:10.1152/japplphysiol.00532.2009
Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Bohm M, Kindermann W, Nickenig G (2005) Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil 12(4):407–414
Volaklis KA, Tokmakidis SP, Halle M (2013) Acute and chronic effects of exercise on circulating endothelial progenitor cells in healthy and diseased patients. Clin Res Cardiol 102(4):249–257. doi:10.1007/s00392-012-0517-2
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275(5302):964–967
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95(4):343–353. doi:10.1161/01.RES.0000137877.89448.78
Yoder MC (2009) Defining human endothelial progenitor cells. J Thromb Haemost JTH 7(Suppl 1):49–52. doi:10.1111/j.1538-7836.2009.03407.x
Schmeisser A, Strasser RH (2002) Phenotypic overlap between hematopoietic cells with suggested angioblastic potential and vascular endothelial cells. J Hematother Stem Cell Res 11(1):69–79. doi:10.1089/152581602753448540
Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA (2007) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109(5):1801–1809. doi:10.1182/blood-2006-08-043471
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348(7):593–600. doi:10.1056/NEJMoa022287
van Beem RT, Noort WA, Voermans C, Kleijer M, ten Brinke A, van Ham SM, van der Schoot CE, Zwaginga JJ (2008) The presence of activated CD4(+) T cells is essential for the formation of colony-forming unit-endothelial cells by CD14(+) cells. J Immunol 180(7):5141–5148
Chavakis E, Dimmeler S (2011) Homing of progenitor cells to ischemic tissues. Antioxid Redox Signal 15(4):967–980. doi:10.1089/ars.2010.3582
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95(3):952–958
Murdoch C, Tazzyman S, Webster S, Lewis CE (2007) Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. Journal of immunology 178(11):7405–7411
Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L (2007) Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 109(12):5276–5285. doi:10.1182/blood-2006-10-053504
De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8(3):211–226. doi:10.1016/j.ccr.2005.08.002
Gardner AW, Poehlman ET (1995) Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 274(12):975–980
Hristov M, Schmitz S, Schuhmann C, Leyendecker T, von Hundelshausen P, Krotz F, Sohn HY, Nauwelaers FA, Weber C (2009) An optimized flow cytometry protocol for analysis of angiogenic monocytes and endothelial progenitor cells in peripheral blood. Cytometry A 75(10):848–853. doi:10.1002/cyto.a.20772
Schmidt-Lucke C, Fichtlscherer S, Aicher A, Tschope C, Schultheiss HP, Zeiher AM, Dimmeler S (2010) Quantification of circulating endothelial progenitor cells using the modified ISHAGE protocol. PLoS One 5(11):e13790. doi:10.1371/journal.pone.0013790
Haas TL, Lloyd PG, Yang HT, Terjung RL (2012) Exercise training and peripheral arterial disease. Compr Physiol 2(4):2933–3017. doi:10.1002/cphy.c110065
Xu H, Kramer M, Spengler HP, Peters JH (1995) Dendritic cells differentiated from human monocytes through a combination of IL-4, GM-CSF and IFN-gamma exhibit phenotype and function of blood dendritic cells. Adv Exp Med Biol 378:75–78
Zhou LJ, Tedder TF (1996) CD14 + blood monocytes can differentiate into functionally mature CD83 + dendritic cells. Proc Natl Acad Sci USA 93(6):2588–2592
Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG (2001) Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res 49(3):671–680
Czepluch FS, Bernhardt M, Kuschicke H, Gogiraju R, Schroeter MR, Riggert J, Hasenfuss G, Schafer K (2014) In vitro and in vivo effects of human monocytes and their subsets on new vessel formation. Microcirculation 21(2):148–158. doi:10.1111/micc.12100
Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE (2000) Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res 87(5):378–384
Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD (2008) Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol 84(5):1271–1278
Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S (2011) Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis 217(1):240–248. doi:10.1016/j.atherosclerosis.2011.03.018
Zawada AM, Rogacev KS, Schirmer SH, Sester M, Bohm M, Fliser D, Heine GH (2012) Monocyte heterogeneity in human cardiovascular disease. Immunobiology 217(12):1273–1284. doi:10.1016/j.imbio.2012.07.001
Dopheide J, Geissler P, Rubrech J, Trumpp A, Zeller G, Bock K, Dorweiler B, Dünschede F, Münzel T, Radsak M, Espinola-Klein C (2015) Inflammation is associated with a reduced number of pro-angiogenic Tie-2 monocytes and endothelial progenitor cells in patients with critical limb ischemia. Angiogenesis. doi:10.1007/s10456-015-9489-y
Brehm W, Wagner P, Sygusch R, Schonung A, Hahn U (2005) Health promotion by means of health sport–a framework and a controlled intervention study with sedentary adults. Scand J Med Sci Sports 15(1):13–20. doi:10.1111/j.1600-0838.2003.00369.x
Acknowledgments
This paper contains in part data reported in the medical thesis of Jennifer Rubrech, Amelie Trumpp and Philip Geissler. The authors like to thank Ursel Petrat, Sabine Wolk and Andrea Drescher for excellent technical support, as well as Arnhild Meyer-Gürsoy and Khaled El-Sadany for their excellent exercise training concept and for the supervision of the SET group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Dopheide, J.F., Geissler, P., Rubrech, J. et al. Influence of exercise training on proangiogenic TIE-2 monocytes and circulating angiogenic cells in patients with peripheral arterial disease. Clin Res Cardiol 105, 666–676 (2016). https://doi.org/10.1007/s00392-016-0966-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-0966-0