Abstract
Aims
Heart failure (HF) continues to be a leading cause of morbidity and mortality in industrialized countries. Data on the epidemiology of HF are largely lacking for Germany. The aims of this study were to estimate the incidence and prevalence of HF in Germany, to estimate 1-year all-cause mortality in patients who received their first diagnosis of HF in hospital and to assess related risk factors.
Methods
The study was based on data for the years 2004–2006 from three German statutory health insurance providers, comprising data of more than six million people. The study sample was not restricted to a specific age group. The incidence rate of HF in 2006 was assessed in patients who did not have a diagnosis of HF or had not received medications for HF in the previous 2 years. One-year all-cause mortality in patients who received their first diagnosis of HF in hospital was analysed using Kaplan–Meier method and Cox proportional hazard model. Case identification was based on confirmed outpatient diagnoses, main and secondary hospital discharge diagnoses as well as medications for HF.
Results
The age- and sex-standardized incidence rate of HF was 2.7 per 1000 person years. Age- and sex-standardized prevalence of HF was 1.7 % in 2004, 1.9 % in 2005 and 1.7 % in 2006. The 1-year all-cause mortality was 23 % among patients who received their first HF diagnosis during a hospitalization in 2006.
Conclusion
Our study revealed an incidence and prevalence of HF in Germany which were largely comparable to those from other countries. Due to the poor prognosis of HF, high readmission rates and an aging society, HF remains highly relevant in the context of health care planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is one of the most common, severe and costly diseases in industrialized countries [1]. While several studies, e.g. from the USA or Sweden, have shown a prevalence of HF ranging from 2–3 % in adults [2, 3], data on the prevalence of HF in Germany are scarce. Analyses based on health insurance data of the AOK showed a prevalence of 2.9 % for 2004 [4]. However, this estimate may not apply to Germany as a whole since the AOK population is not considered representative for the German population. Many AOK insurance members belong to the lower social class and are more often diseased than patients insured in the so-called “Ersatzkassen” in Germany [5]. Estimates of the incidence of HF for Germany are lacking overall. For other industrialized countries, studies suggested an incidence of HF of 2–5 per 1000 person years [2].
In Germany, HF is the leading cause of hospital admissions if birth-associated codes are ignored. In 2011, 380000 persons were hospitalized because of HF [6]. Overall, 1.3 % of direct costs in the German health care system are due to HF [7]. The prognosis of HF is poor but has improved over the past years in industrialized countries [2, 8]. Within their first year after diagnosis 20–30 % of patients with HF die [2]. The prognosis is worse in patients who are first diagnosed with HF in hospital. In these patients, a 1-year mortality of 21–44 % has been reported [9–11]. Data on the mortality of patients with HF is lacking for Germany.
The aim of this study was (1) to estimate incidence and prevalence of HF in Germany, (2) to estimate all-cause mortality in patients who received their first HF diagnosis in hospital and (3) to identify factors associated with mortality in these patients.
Methods
Data source
This study was based on data from three German statutory health insurance providers (SHI) who provided data to the German Pharmacoepidemiological Research Database (GePaRD) and gave permission to use their data for this research project. Overall, more than six million persons between 2004 and 2006 were included in the study, since no more recent data were available to us at the time of the analysis. GePaRD has been described in more detail elsewhere [12, 13]. In brief, the database contains demographic characteristics of the health insurance members, information on their hospital admissions, outpatient physician visits and outpatient prescriptions. The hospital data holds information on the dates of admission and discharge, the admission diagnosis, main and secondary hospital discharge diagnoses, diagnostic and therapeutic procedures with their respective dates, as well as the reason for hospital discharge. Outpatient data contain information on outpatient diagnoses, treatments and procedures. Outpatient diagnoses can only be related to a quarter of the year and are distinguishable into confirmed diagnoses, suspected diagnoses, diagnoses ruled out and status post-diagnoses. All diagnoses are coded according to the German Modification of the International Classification of Diseases 10th Revision (ICD-10-GM) [14]. Outpatient prescription data contain the dates of prescription and dispensation, the specialty of the prescribing physician, the generic and brand name of the drug, as well as drug strength, anatomical-therapeutical-chemical code, defined daily dose and packaging size.
Study population
The study population consisted of people who were insured for at least six months during the study period and had valid information on sex, year of birth and place of residence. Based on this study population, different subpopulations were defined for incidence, prevalence and mortality estimation which had to fulfil further criteria (see below).
Ascertainment of heart failure cases
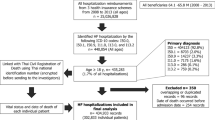
Patients were identified as HF cases if they were diagnosed with one of the following ICD codes as main hospital discharge diagnosis, secondary hospital discharge diagnosis or confirmed outpatient diagnosis: HF (I50.-), hypertensive heart disease with (congestive) HF (I11.0-), hypertensive heart and renal disease with (congestive) HF (I13.0-), hypertensive heart and renal disease with both (congestive) HF and renal disease (I13.2-) or other functional disturbances following cardiac surgery (incl. HF) (I97.1-). Unless a patient died in the quarter of the diagnosis of HF or in one of the two subsequent quarters, it was further required that a drug used in the treatment of HF was prescribed during this time period to eliminate cases with miscoding of HF. The following drug classes were considered in this definition: angiotensin-converting-enzyme inhibitors, beta blockers, angiotensin II receptor antagonists, high-ceiling diuretics, low-ceiling diuretics, potassium-sparing agents, and cardiac glycosides. The individual drugs considered are listed in the supplementary material.
Ascertainment of mortality
Patients were identified as having died, if the reason for the end of the last insurance period or the reason for discharge from hospital was “death”. The date of death was defined as the end of the last insurance period or the end of the hospital stay ending with “death”. If these dates differed, the end of the hospital stay was considered as the date of death.
Ascertainment of comorbidities
Comorbidities which were selected to describe the study population and the HF cases were assessed using ICD-10-GM codes related to main hospital discharge diagnoses, secondary hospital discharge diagnoses or confirmed outpatient diagnoses during the respective study year (ICD codes are displayed in the supplementary material).
Incidence estimation of heart failure
The incidence of HF was assessed in the year 2006 using a retrospective cohort design. Persons with an insurance period in 2006 and without diagnoses of or prescriptions for HF in the preceding 2 years were eligible for inclusion in the cohort. Persons entered the cohort on the first day of 2006 that was preceded by a 2-year insurance period. Cohort exit was defined as the date of the HF diagnosis, the end of the insurance period, death or the end of the study period (31.12.2006), whichever came first.
The incidence rate of HF was calculated by dividing the number of HF cases by the accumulated person-time in the cohort stratified by sex and age. The incidence rate was given per 1000 person years. Corresponding 95 % confidence intervals (CIs) were calculated according to the substitution method [15].
Estimation of the period prevalence of heart failure
The period prevalence of HF was estimated for each of the years 2004–2006 in cross-sectional analyses. Separate study populations for each study year were defined. To be included in the given study population, persons had to be insured for at least 1 day during the respective study year. The annual period prevalence of HF was calculated by dividing the cumulative number of HF cases in a given year by the mid-year population of that year. The period prevalence was described stratified by sex and age (0–9, 10–19,…, >90 years). The Wilson-Score method was used to calculate corresponding 95 % CIs [16].
Standardization of prevalence and incidence estimates
Prevalence and incidence estimates were directly standardized according to the age distribution of the old Europe standard population [17]. 95 % CIs for age-standardized estimates were calculated following the method proposed by Fay and Feuer which is based on the gamma distribution [18].
One-year all-cause mortality in patients who received a new diagnosis of heart failure during a hospitalization
The 1-year all-cause mortality was studied in patients who fulfilled the criteria for case identification and received their first diagnosis of HF as main or secondary discharge diagnosis during hospitalization in 2006 preceded by 2 years without diagnoses of or prescriptions for HF. For this analysis, patients were followed for one more year after their discharge from hospital. Patients remained in the cohort until the end of the insurance period, death or the end of the study period, whichever came first. The 1-year all-cause mortality of these patients was calculated based on the Kaplan–Meier method stratified by age. Differences between the age groups were evaluated using the log rank test (p ≤ 0.05). To determine factors associated with mortality, a Cox proportional hazard model was used. Independent variables which were included in the model were: Sex, age (0–49, 50–59, …, 90 years and older) and selected comorbidities (as shown in Table 3).
Results
Characteristics
The study population which was defined to estimate the prevalence of HF in 2006 comprised 6,284,194 insurants, of whom 48.2 % were women (Table 1). Patients with HF were substantially older than the study population. Furthermore, HF cases showed a more pronounced cardiovascular risk profile with arterial hypertension and diabetes mellitus as the most prevalent diseases.
Incidence of heart failure
In 2006, the age-standardized incidence rate of HF was 2.3 per 1000 person-years in women and 3.1 per 1000 person-years in men. In the age group 50–59 years, the incidence of HF was 1.5 per 1000 person years in women and 3.2 per 1000 person-years in men and more than doubled in each of the higher age categories in both sexes (Fig. 1). The age-specific incidence rates of HF were consistently higher in men than in women across all age groups.
Prevalence of heart failure
Throughout the study period, the age-standardized prevalence of HF was stable in women and slightly increased in men. In 2006, the age- and sex-standardized prevalence of HF was 1.7 % and was slightly lower in women (1.6 %) than in men (1.8 %) (Table 2). A considerable increase of the prevalence of HF with advancing age was observed (Fig. 2). In 2006, the prevalence of HF was 0.9 % in women and 1.7 % in men in the age group 50–59 years and increased to 51.2 % in women and 50.8 % in men in the age group ≥90 years. Sex-specific differences in the prevalence of HF reversed in patients aged 80 years or older.
One-year all-cause mortality in patients who received a new diagnosis of heart failure during a hospitalization
The 1-year all-cause mortality of the 3395 patients newly diagnosed with HF in hospital was 23 % and was lower in men (21 %) than in women (26 %). Mortality was highest in the first month after the HF diagnosis in hospital in all age groups (Fig. 3). The 1-year all-cause mortality increased with advancing age in men and women. The log-rank test indicated significant differences in the mortality between the different age groups and between men and women (p < 0.0001 and p = 0.002, respectively).
The multivariable Cox proportional hazard model showed that increasing age was significantly associated with death within 12 months after the first diagnosis (Table 3). Furthermore, patients with cancer, chronic obstructive pulmonary disease, chronic renal failure, and diabetes mellitus had an increased mortality risk. The presence of arterial hypertension, atrial fibrillation, obesity, prior acute myocardial infarction and sleep apnea were associated with a decreased mortality risk.
Discussion
Incidence and prevalence of HF increased with advancing age and were higher in men than in women; however, the sex-specific difference in the prevalence of HF reversed in patients aged 80 years or older. Mortality of patients who received a new diagnosis of HF during a hospitalization increased with advancing age and was higher in men than in women in the highest age-categories.
The incidence of HF in our study is slightly lower than that of a recently published Swedish study based on administrative data from the Stockholm region [3]. This study comprised all consultations in primary and secondary care of more than 2.1 million inhabitants from 2003 onwards. For 2006, an age-standardized HF incidence rate of 3.6 and 3.9 per 1000 person years was calculated for men and women, respectively. In the same year, the age-standardized incidence rates for men and women in our study were 3.1 and 2.3 per 1000 person years. Our study applied a more conservative algorithm for case identification than the Swedish study, since HF drug treatment was additionally required for case identification. This may explain the somewhat lower incidence rates in our study.
The Rotterdam study, a population-based cohort study from 1989–2000 with several follow up examinations in the region of Rotterdam, provides further data for comparison [19]. However, since this study included only persons aged 55 years and older, only age-specific incidence rates could be compared. These were somewhat higher in our study than in the Rotterdam study, especially in the highest age categories. In patients aged 60–69 years and 80–89 years, incidence rates in the Rotterdam study were 4.3 and 34.5 per 1000 person years, respectively, while we observed incidence rates of 5.7 and 43.0 per 1000 person years in these age groups. Reasons for the differences of the results between the two studies remain unclear. The voluntary participation in the Rotterdam study might have led to a healthier study population and therefore lower prevalence estimates.
Our age-standardized incidence of HF is somewhat higher than that reported by Cowie et al. [20]. This study included all eligible persons registered with 31 general practices (82 General Practitioners (GPs) in Hillingdon, West London. GPs referred all suspected HF cases to a rapid access clinic in Hillingdon hospital or to the hospital’s Accident and Emergency Department where HF cases were further diagnosed according to strict diagnostic criteria by a cardiologist. In this study a crude incidence of 1.4 cases in men and 1.2 cases in women per 1000 persons per year was reported. Differences between the incidence estimates of these two studies might be explained by the regional UK study sample, stricter diagnostic criteria in the UK study and the requirement of a diagnosis of HF by a cardiologist, but also some under-ascertainment of HF cases in the UK study which the authors discuss themselves. Furthermore, persons might have sought treatment outside of the respective geographical area [20].
The age-standardized prevalence of HF in our study is in good accordance with the Swedish study from the Stockholm region [3]. In 2006, the age-standardized prevalence was 1.8 and 1.9 % in Swedish men and women, respectively, compared to 1.8 and 1.6 % in German men and women. Again, the slightly lower prevalence in our study might be explained by our more conservative algorithm for identification of HF cases. Conversely, the age-specific prevalence of HF in our study was somewhat higher than that in the Rotterdam study [19] although a comparison is difficult due to the different age-bounds used in both studies. Since, the Rotterdam study was mainly conducted in the 1990’s [19], the higher prevalence observed in our study might be explained by a positive secular trend of the prevalence of HF in the last decades [2].
Our prevalence estimates compare well with those of another German study which applied a case definition similar to ours, i.e. also requesting medication for case identification [4]. That study was based on data from one SHI (AOK) which traditionally insures people with a low to middle socioeconomic status and thus is not considered representative for the German population. In that German study, the prevalence was evaluated in persons aged 35 years and older. For persons older than 35 years, an age- and sex-standardized prevalence of HF of 2.9 % was observed [4], while our study showed an age- and sex-standardized prevalence of HF of 2.4 % for the same age range. The higher prevalence among men than among women in younger age groups and conversely higher among women than among men at older age was also seen in the other German study [4]. In the Swedish study no reversed effect was seen but the prevalence of HF was more similar in both sexes in higher age groups [3]. This effect accompanied by the lack of difference in incidence might generally reflect the better prognosis of HF in women [21].
The 1-year all-cause mortality of hospitalized patients who received their first HF diagnosis during hospitalization estimated in our study was comparable to or somewhat lower than that reported in other studies [9–11, 22, 23]. Seventy-seven percent of patients in our study survived the first year compared to 56–80 % of patients in other studies. Since survival estimation in most of the other studies was based on newly hospitalized, but not necessarily newly diagnosed HF patients, these study populations also included patients with prevalent HF [9–11, 22]. Therefore, it can be assumed that these patients were in a more advanced stage of their disease which likely contributed to the poorer survival compared to our results. Furthermore, the somewhat lower mortality in our study might result from the younger age of our hospitalized study population (65.6 years; SD = 13.7), since the survival of patients with HF strongly depends on the patients’ age [11]. In the other studies which provided information on age and showed a higher mortality than our study, the mean age ranged from 72 to 77 years [9, 11]. In addition, the other studies were mainly conducted in the 1980’s and 1990’s [9–11]. Since the survival of patients with HF improved over the last decades [1], a better survival in our study was to be expected. In contrast to the other studies, the single-centre cohort study of Leong et al. [22], conducted in Singapore in 2003 and 2004, included patients at ages similar to our study population (68.7 years; SD = 12.0). This study revealed a 1-year all-cause mortality of 20.8 % which is close to that in our study. However, it was based on only a small sample of patients (N = 173) and included a different ethnic group [22]. A comparison of the age-specific mortality with that of other studies was not possible since the other studies did either not provide age-specific mortality estimates [9, 22] or analysed mortality in different age bounds [10, 11].
The decreased mortality risk observed for some cardiovascular comorbidities might reflect a better physicians’ and patients’ adherence to the guideline recommended drug therapy in this comorbid population which probably leads to an improved short-term survival compared to those without the respective comorbidity.
Strengths and limitations
The major strength of our study is the large, unselected and supraregional sample which allowed robust estimations of the incidence, prevalence and mortality of HF. Further, recall and selection bias could be avoided since this study was based on routine data of SHIs and did not depend on patient recall or informed consent of patients for participation. Our study included information on both outpatient and inpatient data which facilitated the analysis of mortality in hospitalized patients with newly diagnosed HF due to the inclusion of a more homogeneous group. Since we included persons from three different SHIs which insure different populations with respect to sociodemographic factors [5], a higher external validity of our results can be assumed in comparison to the previous German study based on data from one insurance only [4].
Some limitations of our study have to be mentioned. First, outpatient data on individual level are collected by the insurance companies only since 2004, therefore only data from 2004 onwards were available for the analysis. Thus, when identifying incident cases of HF in 2006, prevalent cases could have been misclassified as incident cases if these patients received HF diagnoses or medications prior to 2004. However, we assume that the resulting overestimation of the incidence of HF is probably neglectable since a 2-year interval without physician visits in patients with HF is unlikely.
Second, the administrative nature of health insurance data might have led to misclassification of patients with HF and therefore to an overestimation or underestimation of the epidemiological estimates [24]. For data protection reasons, it was not possible to validate cases coded as HF against clinical records. However, we expect that the magnitude of misclassification due to inaccuracy in coding diagnoses of HF is diminished by the case identification algorithm also requiring medications for the treatment of HF. Therefore, our incidence and prevalence estimates are rather conservative. Generally, it was shown that hospital diagnoses and outpatient prescriptions of patients included in GePaRD are in good accordance with external data [25, 26]. Furthermore, a high validity of e.g. mortality related information was shown for GePaRD data [27].
In conclusion, our study revealed an incidence and prevalence of HF in Germany which was largely comparable to those from other countries. Due to an improving survival of HF, the strong age association of HF and the aging society, the number of patients with HF is likely to increase in the future. In this context, high hospitalization and readmission rates will present an increasing burden on health care systems.
References
McMurray JJ, Pfeffer MA (2005) Heart failure. Lancet 365(9474):1877–1889
Bui AL, Horwich TB, Fonarow GC (2011) Epidemiology and risk profile of heart failure. Nat Rev Cardiol 8(1):30–41
Zarrinkoub R, Wettermark B, Wandell P, Mejhert M, Szulkin R, Ljunggren G, Kahan T (2013) The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 15(9):995–1002
Gerste B, Günster C, Heller G, Hilfer S (2007) Sektorenübergreifende Leistungsanalysen. Inanspruchnahme von Gesundheitsleistungen durch Patienten mit koronarer Herzkrankheit und Herzinsuffizienz, Bonn
Hoffmann F, Icks A (2012) Structural differences between health insurance funds and their impact on health services research: results from the Bertelsmann Health-Care Monitor. Gesundheitswesen 74(5):291–297
Statistisches Bundesamt (2011) Gesundheit: Diagnosedaten der Patienten und Patientinnen in Krankenhäusern, Wiesbaden
Statistisches Bundesamt (2010) Gesundheit: Krankheitskosten, 2002, 2004, 2006 and 2008, Wiesbaden
Cleland JG, Gemmell I, Khand A, Boddy A (1999) Is the prognosis of heart failure improving? Eur J Heart Fail 1(3):229–241
Blackledge HM, Tomlinson J, Squire IB (2003) Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001. Heart 89(6):615–620
Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV (2002) Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med 162(15):1689–1694
MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, Redpath A, Pell JP, McMurray JJ (2000) Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 102(10):1126–1131
Garbe E, Suling M, Kloss S, Lindemann C, Schmid U (2011) Linkage of mother-baby pairs in the German pharmacoepidemiological research database. Pharmacoepidemiol Drug Saf 20(3):258–264
Pigeot I, Ahrens W (2008) Establishment of a pharmacoepidemiological database in Germany: methodological potential, scientific value and practical limitations. Pharmacoepidemiol Drug Saf 17(3):215–223
Deutsches Institut für Medizinische Dokumentation un Information (2011) Internationale Statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme: 10. Revision: Version 2008. https://www.dimdi.de/static/de/klassi/icd-10-gm/kodesuche/onlinefassungen/htmlgm2008/index.htm. Accessed 26 Jan 2014
Daly LE (1998) Confidence limits made easy: interval estimation using a substitution method. Am J Epidemiol 147(8):783–790
Newcombe R, Altman D (2000) Proportions and their differences. In: Altman D, Machin D, Bryant T, Gardner M (eds) Statistics with confidence. British Medical Journal Books, Bristol, pp 45–56
International Agency for Research on Cancer (1976) Cancer incidence in five continents. Volume IIV, Lyon
Fay MP, Feuer EJ (1997) Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 16(7):791–801
Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH (2004) Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 25(18):1614–1619
Cowie MR, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Suresh V, Sutton GC (1999) Incidence and aetiology of heart failure; a population-based study. Eur Heart J 20(6):421–428
Regitz-Zagrosek V, Oertelt-Prigione S, Seeland U, Hetzer R (2010) Sex and gender differences in myocardial hypertrophy and heart failure. Circ J 74(7):1265–1273
Leong KT, Goh PP, Chang BC, Lingamanaicker J (2007) Heart failure cohort in Singapore with defined criteria: clinical characteristics and prognosis in a multi-ethnic hospital-based cohort in Singapore. Singap Med J 48(5):408–414
Cleland JG, McDonagh T, Rigby AS, Yassin A, Whittaker T, Dargie HJ, National Heart Failure Audit Team for E, Wales (2011) The national heart failure audit for England and Wales 2008–2009. Heart 97(11):876–886
Khand AU, Shaw M, Gemmel I, Cleland JG (2005) Do discharge codes underestimate hospitalisation due to heart failure? Validation study of hospital discharge coding for heart failure. Eur J Heart Fail 7(5):792–797
Schink T, Garbe E (2010) Assessment of the representativity of in-patient hospital diagnoses in the German Pharmacoepidemiological Research Database. Pharmacoepidemiol Drug Saf 19:S178–S179
Schink T, Garbe E (2010) Representativity of dispensations of non-steroidal anti-inflammatory drugs (NSAIDs) in the German Pharmacoepidemiological Research Database. Pharmacoepidemiol Drug Saf 19:S294
Ohlmeier C, Schmedt N, Hillebrand K, Langner I, Mikolajczyk R, Garbe E (2013) Validation of mortality related information in the German Pharmacoepidemiological Research Database (GePaRD). Pharmacoepidemiol Drug Saf 22(S1):306
Acknowledgments
The authors are grateful to all statutory health insurances that provided data for this study, namely the AOK Bremen/Bremerhaven, the Techniker Krankenkasse (TK), and the hkk. This work was supported by the Robert Koch-Institute, [Grant Number 1362/1-922].
Conflict of interest
The authors had complete autonomy for the process of establishing the protocol, carrying out the analyses and interpreting the results. This also includes the full right to publish the results without limitation. Rafael Mikolajczyk reports grants from Bayer Pharma, grants from Sanofi Pasteur, outside the submitted work. Wilhelm Haverkamp reports speaker’s bureau activities for Bayer HealthCare, Boehringer Ingelheim, Daiichi Sankyo and Berlin Chemie.
Ethics
Use of the data for research purposes needs to be approved by the contributing SHIs and by their governing local or federal authorities. In accordance with the Code of Social Law (SGB X), informed consent of the insurants was not required. Since the study was based on routinely collected pseudonymized data and persons were not contacted, a vote of the ethics committee was not needed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ohlmeier, C., Mikolajczyk, R., Frick, J. et al. Incidence, prevalence and 1-year all-cause mortality of heart failure in Germany: a study based on electronic healthcare data of more than six million persons. Clin Res Cardiol 104, 688–696 (2015). https://doi.org/10.1007/s00392-015-0841-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0841-4