Abstract
Objectives
This comparative case-matched analysis investigated feasibility and safety of direct transfemoral (TF) transcatheter aortic valve implantation (TAVI) without pre-dilatation using balloon-expandable devices.
Background
Presently, balloon aortic valvuloplasty (BAV) is considered mandatory preceding transfemoral TAVI with balloon-expandable devices. However, procedural severe adverse events may be associated with BAV.
Methods
26 consecutive patients (study group) received direct TF-TAVI using Edwards Sapien XT (n = 17) or Sapien 3 (n = 9) devices (61.5 % female, 81.3 ± 6.3 years, logEuroSCORE I 15.3 ± 13.2 %). A control group of patients after conventional TF-TAVI was retrieved from our database containing 1153 TAVI patients and matched to the study group regarding baseline and procedural data. Data reporting adheres to VARC-2 definitions.
Results
Device success was 96.2 % (25/26) and 92.3 % (24/26) in study and control groups, respectively (p = 1.00). Procedure time (60.0 ± 54.0 vs. 70.0 ± 29.1 min; p = 0.41), fluoroscopy time (13.3 ± 5.8 vs. 17.8 ± 6.9 min; p = 0.01) and amount of contrast agent (118.7 ± 47.9 vs. 153.0 ± 53.2 ml; p = 0.02) were lower in the study group. All-cause 30-day mortality was 7.7 % (2/26) in both groups, disabling stroke was observed in 3.8 % (1/26) and 7.7 % (2/26) in study and control groups, respectively. Resultant transvalvular mean gradient and effective orifice area (EOA) were 11 ± 5 vs. 11 ± 5 mmHg and 1.6 ± 0.3 vs. 1.5 ± 0.3 cm2. Paravalvular leakage ≥grade II was observed in 0 and 7.7 % (2/26; p = 0.49).
Conclusions
TF-TAVI without pre-dilatation was feasible and safe in this consecutive series of patient regardless of aortic valve morphology, for example. extent of valvular calcification or baseline EOA. This technique resulted in significantly lower fluoroscopy times and amounts of contrast agent while yielding non-inferior hemodynamic and clinical outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Following controlled randomized trials [1, 2] and extensive clinical experience as captured in major registries [3, 4], TAVI has become clinical routine and treatment of choice for inoperable or high-risk patients with severe, symptomatic aortic stenosis after interdisciplinary heart team consensus. TAVI has meanwhile been incorporated in international guidelines [5, 6].
Currently, BAV is considered a mandatory prerequisite prior to TF-TAVI using balloon-expandable transcatheter heart valves (THV) [7]. BAV is being performed to facilitate subsequent retrograde insertion of delivery catheter and crimped THV into the native aortic annulus. Also, BAV is thought to promote adequate apposition of the THV stent to the annulus with optimal stent expansion and good functional results without paravalvular leakage (PVL). Furthermore, BAV has been advocated to predict displacement of coronary leaflets during TAVI or as a sizing tool [8] even though multi-slice contrast-enhanced computed tomography is increasingly being recognized as the planning modality of choice [9, 10].
On the other hand, specific risks inherent in BAV have been identified [11–14]. Distal embolization resulting in cerebral ischemia may occur if calcific particles are mobilized from aortic valve leaflets. Low cardiac output can be a consequence of rapid ventricular pacing (RVP) or of aggravation of aortic regurgitation (AR) especially in cases of impaired left ventricular function. Other complications such as conduction disturbances, annular rupture or aortic dissection have also been directly attributed to BAV alone. Even though severe complications are not very frequent after BAV they may still have an adverse impact on the elderly and frail patient population typically considered for TAVI.
Recently, feasibility of TF-TAVI without pre-dilatation using the self-expandable Medtronic CoreValve THV has been demonstrated [15]. Regarding balloon-expandable devices, direct TAVI without pre-dilatation is increasingly being performed when choosing an antegrade transapical access (TA) [16–18]. For the TF route, however, only limited reports exist in selected patients [19–22].
This study reports first experience in a consecutive series of patients undergoing direct TF-TAVI without pre-dilatation using the Edwards Sapien XT or Sapien 3 THV. Results are compared to a control group derived after logistic regression and the “nearest neighbor matching” method. Primary endpoints of the study were acute clinical and hemodynamic outcomes as adjudicated by the updated standardized Valve Academic Research Consortium (VARC-2) definitions [23].
Methods
Patients
A consecutive series of 26 patients received direct TF-TAVI without pre-dilatation using Edwards Sapien XT or Sapien 3 THV for treatment of severe symptomatic calcified aortic stenosis (study group). Allocation of patients to TAVI followed current international recommendations [5] after consensus of the local dedicated heart team. Patients unsuitable for a retrograde TF approach were excluded from analysis. For comparative assessment, a matched control group of 26 patients treated by conventional TF-TAVI including BAV and using the same devices was retrieved from our hospital database containing a total of 1153 patients. Written informed consent was obtained from all patients before the procedure.
Diagnostic work-up and study procedure
By routine, all patients received preoperative transthoracic (TTE) and transesophageal echocardiography (TEE) for evaluation of cardiac functional status. Furthermore, diagnostic work-up included contrast-enhanced, electrocardiogram-gated multislice computed tomography (MSCT). Datasets were analyzed using the 3mensio Medical Imaging Software (3mensio, Medical Imaging, Bilthoven, Netherlands) for calculation of native aortic annulus dimensions and determination of adequate THV size as well as assessment of aortic root anatomy and morphology (e.g. distribution and severity of valvular calcification, aortic root dimensions or height of coronary ostia take-off), prediction of optimal c-arm angulation and assessment of aorto-iliac and peripheral vascular status.
TAVI procedures were performed under general anesthesia in a specially equipped hybrid operating suite by a dedicated team of cardiologists and cardiac surgeons. Percutaneous vascular access was gained using the Prostar vascular closure system (Abbott Vascular, Santa Clara, CA, USA). A temporary, transvenous pacing lead and an aortic root pigtail catheter were placed from the contralateral groin. Retrograde passage of the stenotic aortic valve was followed by placement of a pre-shaped, stiff guidewire in the left ventricular apex. In the study group, the THV mounted on the Ascendra+ or Commander delivery catheters were now directly inserted and advanced to the descending thoracic aorta followed by loading of the crimped valve onto the deployment balloon. After flexing of the delivery catheters, the aortic arch was crossed and the THV positioned in the native aortic annulus. After retraction of the pusher and angiographic confirmation of correct implantation height, THV were slowly deployed under RVP, maximum inflation maintained for 3 s, balloon deflated and RVP terminated. THV function was subsequently assessed by TEE and aortic root angiography. Control group procedures followed standard protocols.
Statistical analysis
Baseline, intraprocedural and acute follow-up data up to 30 days were prospectively collected and entered into a standardized database and retrospectively analyzed. Clinical endpoints were adjudicated in accordance with the updated standardized VARC-2 definitions. Data are presented as absolute numbers and percentages for categorical variables and mean values and standard deviation for continuous variables unless stated otherwise. Dichotomous variables were compared by Fisher´s exact test and continuous variables by t tests. P values were reported without correction for multiple testing. A level of significance was set to two-tailed p < 0.05.
To evaluate the effect of a treatment in a non-randomized setting, 1:1 matching (drawing without replacement) was conducted by logistic regression and nearest neighbor matching as the measure of proximity. In a first step matching pairs of all complete cases from the treatment group were identified for the following 19 variables: valve type, valve size, age, gender, logEuroSCORE I, STS Score, New York Heart Association (NYHA) functional class, left ventricular ejection fraction, pulmonary hypertension >60 mmHg, peripheral artery disease, creatinine at baseline, chronic obstructive pulmonary disease >Gold II, previous cardiac surgery, previous stroke, coronary artery disease, diabetes mellitus, arterial hypertension, malignant disease. In consecutive steps all remaining pairs were identified in case of missing data. All computation was carried out by the statistical software R and the R-package MatchIt [24, 25].
Results
Baseline demographics and matching results
26 consecutive patients (study group) received direct TF-TAVI using Edwards Sapien XT (n = 17) or Sapien 3 (n = 9) devices (61.5 % female, 81.3 ± 6.3 years, logEuroSCORE I 15.3 ± 13.2 %). Matching yielded a control group of 26 patients receiving conventional TF-TAVI with BAV who were similar to the study group with regard to important baseline and procedural parameters. No significant inter-group differences were present after matching. Detailed patient demographics are summarized in Table 1.
Periprocedural data
There were no significant differences between study and control groups regarding baseline hemodynamic parameters, proportion of valve type or valve sizes. Device success according to VARC-2 definitions was achieved in 96.2 % (25/26) and 92.3 % (24/26) in the study and control groups, respectively (p = 1.00). Reasons for failure to reach this composite endpoint were: sequential valve-in-valve implantation in one patient for malfunction of a correctly positioned THV with moderate intravalvular aortic regurgitation in the study group. In the control group, intervention was classified as unsuccessful for: procedural mortality in one patient (see below for details) and sequential valve-in-valve procedure in a second patient for valve malpositioning.
Procedure time (p = 0.41), fluoroscopy time (p = 0.01) and the amount of contrast agent used (p = 0.02) were lower in the study group. No patient in the study group required postdilatation of deployed THV while in the control group postdilatation was performed in three cases (in two patients for residual moderate PVL, in one patient for elevated transvalvular gradient). Detailed periprocedural data are summarized in Table 2.
Echocardiographic outcome data
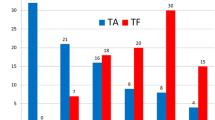
In the study group, peak and mean transvalvular gradients as determined by TTE prior to discharge decreased from 67 ± 25 to 25 ± 18 mmHg and 38 ± 14 to 11 ± 5 mmHg (both p < 0.01). EOA increased from 0.8 ± 0.2 to 1.6 ± 0.3 cm2 (p < 0.01) compared to baseline values. Corresponding data in the control group were: decrease of peak and mean transvalvular gradients from 72 ± 27 to 42 ± 17 mmHg and 22 ± 11 to 11 ± 5 mmHg (both p < 0.01), increase of EOA from 0.7 ± 0.2 to 1.5 ± 0.3 cm2 (p < 0.01). There were no significant inter-group differences regarding resultant peak and mean transvalvular gradients (p = 0.49 and p = 1.00) or resultant EOA (p = 0.25; Table 3; Fig. 1).
In both groups, 11 patients had no residual PVL and AR grade I was present in 11/24 (45.8 %) patients in both groups. In the control group, two patients had PVL grade AR II. In one patient from the study group no discharge TTE was available (Fig. 2).
Clinical outcome data
All-cause 30-day mortality was 7.7 % (2/26) in both groups. In the study group, one patient died on postoperative day 6 due to progressive right heart failure after pacing lead perforation and surgical revision via sternotomy. A second patient died due to multi-organ failure on postoperative day 6 after a severe bleeding complication from the access vessel. In the control group, one patient died from annular rupture during the procedure and a second patient died following an uneventful procedure due to electromechanical dissociation on postoperative day 1. Regarding all further VARC-2 adjudicated clinical endpoints, no significant differences were found between the two groups. Pacemaker (PM) implantation rate was 15.4 % in both groups. Indications were new onset LBBB or AVB I° or higher. Detailed clinical outcome data are summarized in Table 3.
Discussion
Main findings
This study provides evidence of safety and feasibility of TF-TAVI without prior BAV. Acute clinical and functional outcomes were non-inferior compared to a well-matched control group of patients receiving conventional TF-TAVI including prior BAV. Moreover, fluoroscopy time and amount of contrast agent were significantly reduced.
Traditionally, preparatory BAV preceding valve implantation has been considered a mandatory technical step for all types of THV or access routes. Preliminary study results suggest BAV to be dispensable when performing antegrade TA-TAVI [16–18] with balloon-expandable THV. These studies have triggered initiation of the multicenter, prospective observational EASE-IT trial (NCT02127580) comparing conventional TA-TAVI with BAV to direct TA-TAVI without BAV using balloon-expandable devices. Regarding retrograde TF-TAVI, feasibility of direct implantation without prior BAV has been demonstrated using self-expandable THV [15]. However, to date only limited evidence for this policy exists regarding retrograde TF-TAVI with balloon-expandable devices. Previous retrospective study suggests the technique to be feasible. However, no matching of patients in study and control groups was performed and the Edwards Sapien XT device was used exclusively [22, 26].
In our study, safety and feasibility was also demonstrated for use of the Sapien 3 THV. To date, no other publications exist regarding this technique with this particular type of THV. As the Sapien 3 THV is one of the most frequently used types of THV and the TF access route is also considered the primary technique by most centers, demonstration of safety and feasibility of this novel implantation technique with this type of THV may be of interest to many physicians.
Both the Novaflex+ delivery catheter for the Edwards Sapien XT THV and the Commander delivery catheter for the Edwards Sapien 3 THV feature specific nose cones enabling smooth and atraumatic retrograde passage even of severely calcified aortic valves. In contrast to other groups who use partial balloon inflation before retrograde valve passage as a sort of “wedge manoeuvre” [21] we did not encounter any difficulty in advancing the crimped THV into the native aortic valve within the presented series of cases. Also, both delivery catheters allowed for retraction of valves should they have been advanced too far into the left ventricle. In our preliminary experience, correct positioning of THV was thus well feasible without hemodynamic compromise in all cases. Both THV, the Sapien XT and the Sapien 3, caused no difficulties when expanded in a non-predilated annulus. Feasibility of this procedure was shown for the Sapien XT but not yet for the Sapien 3, which has an increased stent height, an asymmetrical foreshortening of the stent and a novel outer polyethylene terephthalate cuff for enhanced paravalvular sealing, when compared to the older THV version. Theoretically, the reduced need for oversizing when using the Sapien 3 THV should lead to decreased PM rates when compared to the older Sapien XT. We found no decrease of the overall PM rate, which is backed by the work of Tarantini et al. [27]. Likely, the issue requires further investigation in larger patient numbers.
Furthermore, we found no need for postdilatation in any patient from the study group suggesting sufficient radial force during the deployment process to allow for adequate apposition of THV stent to the native aortic annulus with non-inferior results regarding degree of PVL when compared to the control group.
Sparing patients one phase of RVP (i.e. for BAV) may also contribute to the safety of the procedure even though this hypothesis could not be supported by the presented data. However, RVP has recently been demonstrated to be associated with microcirculatory arrest and delayed recovery of tissue perfusion [28] and it appears possible that with larger patient numbers this phenomenon may translate into clinically relevant effects.
As stated above, adverse events have been well recognized as associated with BAV as a stand-alone treatment [29–31]. Whether similar rates of complications have to be expected when performing preparatory BAV prior to TAVI remains unclear since clinical sequelae often become apparent only during the further course of the patient making discrimination of causal relation difficult. Since traditionally, when performing preparatory BAV, contrast agent was concomitantly administered for confirmation of annular and aortic root dimensions, the total amount of contrast agent was naturally significantly lower in the study group. As the potential adverse impact of contrast induced nephropathy is well described in the current literature [32, 33], this circumstance may improve clinical outcome. However, outcomes in larger patient cohorts will have to be awaited to confirm this theoretical advantage.
Limitations
The present study represents a retrospective, single-center experience with limited patient numbers. Patients were not randomized to the respective treatment groups and even though analysis of baseline patient characteristics did not reveal statistically significant inter-group differences, results may have been biased by hidden confounders. Furthermore, patients in study and control groups were treated during different time periods. Thus, results may reflect learning curve effects even though the majority of patients in the control group were treated less than 6 months preceding cases in the study group and all but two cases in the control group were treated more than 3 years after initiation of the TAVI program. Conclusions have to be drawn with caution due to the descriptive, retrospective nature of this single-center analysis in a limited number of patients.
Conclusions
In conclusion, preliminary experience in a limited number of patients suggests TF-TAVI using balloon-expandable devices is feasible and safe. Regarding valve function and rates of residual PVL as well as regarding important clinical outcome parameters, this technique proved non-inferior compared to conventional TF-TAVI with prior BAV. It has therefore become our default technique in patients receiving TF-TAVI with balloon-expandable devices. Possible advantages regarding the incidence of periprocedural adverse events will need to be investigated in larger patient numbers before general recommendations can be made.
Abbreviations
- BAV:
-
Balloon-aortic valvuloplasty
- EOA:
-
Effective orifice area
- logEuroSCORE:
-
Logistic European system for cardiac operative risk evaluation I
- MSCT:
-
Multislice computed tomography
- NYHA:
-
New York Heart Association
- PM:
-
Pacemaker
- PVL:
-
Paravalvular leakage
- RVP:
-
Rapid ventricular pacing
- TA:
-
Transapical
- TAVI:
-
Transcatheter aortic valve implantation
- TEE:
-
Transesophageal echocardiography
- TF:
-
Transfemoral
- TTE:
-
Transthoracic echocardiography
- VARC:
-
Valve Academic Research Consortium
References
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, PARTNER Trial Investigators (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 21:1597–1607
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364:2187–2198
Walther T, Thielmann M, Kempfert J, Schroefel H, Wimmer-Greinecker G, Treede H, Wahlers T, Wendler O (2013) One-year multicentre outcomes of transapical aortic valve implantation using the SAPIEN XTTM valve: the PREVAIL transapical study. Eur J Cardiothorac Surg 43:986–992
Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O (2010) Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 122:62–69
Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Lung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Schwedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M, ESC Committee for Practice Guidelines (CPG); Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS) (2012) Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 42:S1–S44
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajia P, Sundt TM 3rd, Thomas JD (2014) 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol [Epub ahead of print]
Vahanian A, Himbert D (2011) Transcatheter aortic valve implantation: could it be done without prior balloon valvuloplasty? JACC Cardiovasc Interv 4:758–759
Patsalis PC, Al-Rashid F, Neumann T, Plicht B, Hildebrandt HA, Wendt D, Thielmann M, Jakob HG, Heusch G, Erbel R, Kahlert P (2013) Preparatory balloon aortic valvuloplasty during transcatheter aortic valve implantation for improved valve sizing. JACC Cardiovasc Interv 6:965–971
Cerillo AG, Mariani M, Berti S, Glauber M (2012) Sizing the aortic annulus. Ann Cardiothorac Surg 1:245–256
Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA (2012) SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 6:366–380
Ben-Dor I, Pichard AD, Satler LF, Goldstein SA, Syed AI, Gaglia MA Jr, Weissman G, Maluenda G, Gonzalez MA, Wakabayashi K, Collins SD, Torguson R, Okubagzi P, Xue Z, Kent M, Lindsay J, Waksman R (2010) Complications and outcome of balloon aortic valvuloplasty in high-risk or inoperable patients. JACC Cardiovasc Interv 3:1150–1156
Tissot CM, Attias D, Himbert D, Ducrocq G, Iung B, Dilly MP, Juliard JM, Lepage L, Détaint D, Messika-Zeitoun D, Nataf P, Vahanian A (2011) Reappraisal of percutaneous aortic balloon valvuloplasty as a preliminary treatment strategy in the transcatheter aortic valve implantation era. EuroIntervention 7:49–56
Doguet F, Godin M, Lebreton G, Eltchaninoff H, Cribier A, Bessou JP, Litzler PY (2010) Aortic valve replacement after percutaneous valvuloplasty—an approach in otherwise inoperable patients. Eur J Cardiothorac Surg 38:394–399
Kahlert P, Al-Rashid F, Döttger P, Mori K, Plicht B, Wendt D, Bergmann L, Kottenberg E, Schlamann M, Mummel P, Holle D, Thielmann M, Jakob HG, Konorza T, Heusch G, Erbel R, Eggebrecht H (2011) Cerebral embolization during transcatheter aortic valve implantation: a transcranial doppler study. Circulation 126:1245–1255
Grube E, Naber C, Abizaid A, Sousa E, Mendiz O, Lemos P, Kalil Filho R, Mangione J, Buellesfeld L (2011) Feasibility of transcatheter aortic valve implantation without balloon pre-dilation. JACC Cardiovasc Interv 4:751–757
Conradi L, Seiffert M, Schirmer J, Koschyk D, Blankenberg S, Reichenspurner H, Diemert P, Treede H (2014) Transapical transcatheter aortic valve implantation without prior balloon aortic valvuloplasty: feasible and safe. Eur J Cardiothorac Surg 46:61–66
Wendler O, Dworakowski R, Monaghan M, MacCarthy PA (2012) Direct transapical aortic valve implantation: a modified transcatheter approach avoiding balloon predilatation. Eur J Cardiothorac Surg 42:734–736
Kempfert J, Meyer A, Kim WK, van Linden A, Arsalan M, Blumenstein J, Möllmann H, Walther T (2014) First experience without pre-ballooning in transapical aortic valve implantation: a propensity score-matched analysis. Eur J Cardiothorac Surg [Epub ahead of print]
García E, Martin P, Hernandez R, Rodríguez V, Fernández A, Gama V, Almería C, Macaya C (2014) Feasibility and safety of transfemoral implantation of Edwards SAPIEN XT prosthesis without balloon valvuloplasty in severe stenosis of native aortic valve. Catheter Cardiovasc Interv 83:791–795
García E, Almeria C, Unzué L, Jiménez P, Cuadrado A, Macaya C (2014) Transfemoral implantation of Edwards Sapien XT aortic valve without previous valvuloplasty: role of 2D/3D transesophageal echocardiography. Catheter Cardiovasc Interv [Epub ahead of print]
Davies WR, Bapat VN, Hancock JE, Young CP, Redwood SR, Thomas MR (2014) Direct TAVI using a balloon-expandable system: a novel technique to eliminate pre-deployment balloon aortic valvuloplasty. EuroIntervention 10:248–252
Möllmann H, Kim WK, Kempfer J, Blumenstein J, Liebetrau C, Nef H, van Linden A, Walther T, Hamm C (2014) Transfemoral aortic valve implantation of Edwards SAPIEN XT without predilatation is feasible. Clin Cardiol [Epub ahead of print]
Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB, Valve Academic Research Consortium (VARC)-2 (2012) Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg 42:S45–S60
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Ho DE, Imai K, King G, Stuart EA (2011) MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 42:1–28
García E, Almería C, Unzué L, Jiménez-Quevedo P, Cuadrado A, Macaya C (2014) Transfemoral implantation of Edwards Sapien XT aortic valve without previous valvuloplasty: role of 2D/3D transesophageal echocardiography. Catheter Cardiovasc Interv 84(6):868–876
Tarantini G, Mojoli M, Purita P, Napodano M, D’Onofrio A, Frigo A, Covolo E, Facchin M, Isabella G, Gerosa G, Iliceto S (2014) Unravelling the (arte)fact of increased pacemaker. EuroIntervention [Epub ahead of print]
Selle A, Figulla HR, Ferrari M, Rademacher W, Goebel B, Hamadanchi A, Franz M, Schlueter A, Lehmann T, Lauten A (2014) Impact of rapid ventricular pacing during TAVI on microvascular tissue perfusion. Clin Res Cardiol [Epub ahead of print]
Isner JM (1991) Acute catastrophic complications of balloon aortic valvuloplasty. The Mansfield Scientific Aortic Valvuloplasty Registry Investigators. J Am Coll Cardiol 17:1436–1444
McKay RG (1991) The Mansfield Scientific Aortic Valvuloplasty Registry: overview of acute hemodynamic results and procedural complications. J Am Coll Cardiol 17:485–491
Serruys PW, Luijten HE, Beatt KJ, DiMario C, deFeyter PJ, Essed CE, Roelandt JR, van den Brand M (1988) Percutaneous balloon valvuloplasty for calcific aortic stenosis. A treatment ‘sine cure’. Eur Heart J 9:782–794
Konigstein M, Ben-Assa E, Abramowitz Y, Steinvil A, Leshem Rubinow E, Havakuk O, Arbel Y, Halkin A, Keren G, Banai S, Finkelstein A (2013) Usefulness of updated valve academic research consortium-2 criteria for acute kidney injury following transcatheter aortic valve implantation. Am J Cardiol 112(11):1807–1811
Khawaja MZ, Thomas M, Joshi A, Asrress KN, Wilson K, Bolter K, Young CP, Hancock J, Bapat V, Redwood S (2012) The effects of VARC-defined acute kidney injury after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention 8(5):563–570
Conflict of interest
Lenard Conradi, Hendrik Treede, Ulrich Schäfer and Patrick Diemert are consultants to Edwards Lifesciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Conradi and A. Schaefer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Conradi, L., Schaefer, A., Seiffert, M. et al. Transfemoral TAVI without pre-dilatation using balloon-expandable devices: a case-matched analysis. Clin Res Cardiol 104, 735–742 (2015). https://doi.org/10.1007/s00392-015-0836-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0836-1