Abstract
The C-reactive protein (CRP), first described as a serum component capable of precipitating the C-polysaccharide of pneumococci, is one of the most important proteins because the serum concentration rises in the acute phase reaction. The acute phase reaction is the nonspecific reaction of the body to noxious stimuli of the most varied kinds, such as infections, burns, neoplasms and tissue trauma. The CRP is synthesized in liver parenchymal cells by cytokines which are derived from stimulated leucocytes and released into the circulation. Because of its molecular structure and in synergy with the complement system, it is able to precipitate and/or lyse microorganisms, thereby rendering them harmless. Measurement of the serum CRP concentration can provide important information with respect to the diagnosis and monitoring of treatment. Due to immunosenescence in geriatric patients the synthesis of CRP appears to be limited to inflammatory stimuli; however, this phenomenon does not appear to be of major clinical relevance. Despite the introduction of new parameters of the acute phase reaction, sometimes with better performance, such as interleukin-6, procalcitonin and the soluble endotoxin receptor sCD14, measurement of CRP for diagnosis and treatment monitoring is still justified even in geriatric patients as testing is rapid, economic and nearly ubiquitously available round the clock. Biochemical markers of the acute phase reaction should always be interpreted together with the clinical picture and their specific limitations.
Zusammenfassung
Das C-reaktive Protein (CRP) – erstmals beschrieben als Serumkomponente, welche in der Lage ist, das C-Polysaccharid der Pneumokokken zu präzipitieren – ist eines der wichtigsten Proteine, dessen Serumkonzentration im Rahmen der Akute-Phase-Reaktion ansteigt. Die Akute-Phase-Reaktion ist die unspezifische Reaktion des Körpers auf Noxen verschiedenster Art, wie beispielsweise Infektionen, Verbrennungen, Neoplasien und Gewebstraumen. CRP wird durch Zytokine, die aus stimulierten Leukozyten stammen, in den Leberparenchymzellen synthetisiert und in die Blutzirkulation abgegeben. Dort vermag es u. a. aufgrund seiner Molekülstruktur in Synergie mit dem Komplementsystem Mikroorganismen zu präzipitieren bzw. zu lysieren und damit unschädlich zu machen. Die Messung der CRP-Konzentration im Serum kann wichtige Informationen bezüglich Diagnostik und Therapiemonitoring bereitstellen. Aufgrund der Immunseneszenz beim geriatrischen Patienten scheint die CRP-Synthese auf einen inflammatorischen Reiz hin eingeschränkt zu sein. Dieses Phänomen scheint jedoch klinisch nicht von größerer Relevanz zu sein. Damit ist die Messung des CRP auch beim geriatrischen Patienten zur Diagnostik und zum Therapiemonitoring – trotz der Einführung neuer Messgrößen der Akute-Phase-Reaktion mit z. T besserer Performance, wie Interleukin-6, Procalcitonin und dem löslichen Endotoxinrezeptor sCD14 – aufgrund der schnellen, fast ubiquitär rund um die Uhr verfügbaren ökonomisch günstigen Messmöglichkeiten immer noch berechtigt. Grundsätzlich sollten biochemische Marker der Akute-Phase-Reaktion immer zusammen mit dem klinischen Bild und ihren spezifischen Limitationen interpretiert werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Discovery, structure and function of C-reactive protein
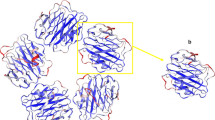
In 1930 Tillet and Francis described the finding of a precipitation reaction between the serum of pneumonia patients and the somatic C-polysaccharide of pneumococci [46] and 11 years later Abernethy and Avery isolated the substance responsible for the precipitation and classified this precipitation factor as a protein [1]. Since that time this protein has been called C-reactive protein (CRP) on account of its reactivity with the C-polysaccharide of pneumococci. In 1977 the primary structure of CRP was demonstrated [35]. The amino acid sequence of this non-glycosylated molecule is coded on the long arm of chromosome 1 (1q23.2). This molecule appears to be so important for the body that no functionally relevant genetic defects have been described to date in humans. The intact molecule (molecular mass 115,135 Dalton) consists of five subunits (monomers) (Fig. 1). Another molecule with this so-called pentameric structure is serum amyloid P (SAP). This is the CRP equivalent, i.e. the main reactant of the acute phase reaction, in phylogenetically primitive animals, such as the horseshoe crab and also in representatives of mammals, for example mice [38]. In humans SAP is constitutionally present but is not included in the acute phase proteins [39]. Because of their structural and therefore functional relationship CRP and SAP are included in the family of pentraxins [15]. In special binding domains of the monomers a large group of endogenous and exogenous ligands, such as phospholipids, nuclear ribonucleic acid and histones can be bound and inactivated [14, 16, 51]. After binding to the ligands, the molecule is also able to bind to components of the complement system such as C1q in a calcium-dependent reaction and activate the classical complement metabolic pathway through factor C3. This leads to the precipitation of bacterial pathogens, such as pneumococci and initiate lysis of the pathogens by forming the terminal C5-C9 membrane attack complex [32, 50]. Besides the complement fixation to the pathogens with subsequent lysis or complement-mediated uptake into phagocytes, CRP also causes binding of the CRP-bacteria complex to the Fc receptors of leukocytes because of its immunoglobulin-like protein domains [6, 31, 47] with subsequent activation of cytotoxicity mediated by leukocyte components (e.g. cytokines and proteases). The molecular function is derived from its properties. It is one of the most important reactants of what is known as the acute phase reaction.
Model of the C-reactive protein molecule consisting of five monomers (courtesy of T.J. Greenhough and coworkers, Keele University UK. Image copyright Keele University) [42]
The acute phase reaction is the body’s nonspecific reaction triggered by local or generalized tissue damage due to infectious, chemical or physical causes, ischemic necrosis, malignant neoplasms and immunological and allergic reactions. It is clinically characterized by local reactions, such as erythema, swelling and pain. Generalized reactions include malaise, fever and leukocytosis. The purpose of the acute phase reaction is to limit the damage locally and prepare the body for a spread of the damage by a generalized reaction. To fulfil this task, various biochemical reactions take place in the body. How the body deals with the pathogens of a bacterial infection can be used to illustrate the biochemical sequence of an acute phase reaction. Following entry of the pathogens, the bacterial lipopolysaccharides are recognized by the monocyte-macrophage system. The bacterial lipopolysaccharides become associated with lipopolysaccharide-binding protein, which is produced by the liver. The complex of lipopolysaccharide and lipopolysaccharide-binding protein is then bound to the endotoxin receptor CD14 (cluster of differentiation, CD14). This complex then reacts on the cell surface with the toll-like receptors (TLR) and here especially with TLR-4. The TLR family plays an important part in the innate immune response, which is concerned with the rapid and effective elimination of pathogens. The innate immune system contrasts with the specific immune system, which only responds after a few days following exposure to the pathogen, e.g. by production of specific antibodies. Activation of TLR-4 now leads via an intracellular signaling cascade to transcription and translation of the genes for tumor necrosis factor alpha (TNF-α), interleukin-1β and interleukin-6 (IL-6) (Fig. 2). An article by Rossol et al. provides a good overview of these mechanisms [40]. The cytokines reach the liver through the circulation where they induce synthesis of acute phase proteins in the hepatocytes, which are released into the circulation (Fig. 3). In this process the plasma concentration of CRP, which is an important representative of the acute phase proteins, can rise to 10,000 times the normal concentration. These cytokines, however, also cause activation of various other cell systems in the organism, such as endothelial cells with subsequent release of interleukin 6 (IL-6) (Fig. 3) to provide the body with optimal conditions for dealing with the pathogens.
Induction pathway to cytokine synthesis via the cluster of differentiation 14 (CD14) receptor and toll-like receptor 4 (TLR-4). Activation of nuclear factor kappaB (NF-κB) via TLR. The ternary complex consisting of lipopolysaccharide (LPS), lipopolysaccharide-binding protein (LBP) and CD14 activates TLR-4 which recruits the myeloid differentiation primary response protein (MYD88). This is followed by an activation of interleukin-1 receptor-associated kinase (IRAK), TNF-receptor associated factor (TRAF6) and Iκkinase (IKK). Activated IKK phosphorylates IκB (inhibitor of NF-κB) which dissociates from the complex with NF-κB. NF-κB diffuses into the nucleus and activates gene promotors [33] (reprinted with kind permission of Springer Science+Business Media).
The systemic action of the cytokines interleukin 1 (IL-1), IL-6 and tumor necrosis factor alpha (TNF-α) in the induction of acute phase proteins such as C-reactive protein (CRP) [20] (reprinted with kind permission of Springer Science+Business Media)
Although initiation of the acute phase reaction in humans, e.g. by bacterial pathogens, leads to a rise in the most varied, reciprocally inhibiting or reinforcing cytokine mediators, studies have shown that IL-6 is able to induce CRP synthesis without synergy with other mediators. This was demonstrated in a study in which IL-6 was tested as a thrombopoiesis-stimulating medication. Patients were subcutaneously injected with this protein in the form of a recombinant drug prior to chemotherapy to shorten the duration of chemotherapy-induced thrombocytopenia [3]. After treatment with recombinant human IL-6, a rise of the CRP was apparent as a side effect of the cytokine therapy. The serum concentration of TNF-α remained unchanged during the IL-6 treatment, rendering synergy between TNF-α and IL-6 in the induction of CRP synthesis unlikely in this special situation [3, 5].
Time kinetics and diagnostic significance of CRP
Tissue trauma and microbial infections are important initiators of the acute phase reaction. The postoperative period is an example of the high clinical value of CRP measurements, when tissue damage with subsequent infection represents an important pathological situation. The CRP increase occurs to remove the cell detritus generated during the operative trauma. This postoperative increase also prepares the nonspecific immune system for potential infections. The CRP time kinetics after surgical trauma is demonstrated in the following by means of data from pediatric cardiac surgery. During the early postoperative phase there is a slight increase within 12 h up to 10 mg/l followed by a sharp increase to CRP values between 100–150 mg/l. The maximum is reached on the second postoperative day. After 3–4 days there is a fall in the CRP level. Deviation from these kinetics can indicate a postoperative complication due to infection [17]. With respect to the time-dependent kinetics, the following can be stated: the liver begins CRP synthesis approximately 6 h after a bacterial infection and the peak concentration in peripheral blood is reached approximately 48 h after the start of the infection [37]. Elimination of the CRP with a half-life of 19 h is by hepatic clearance [23]. This clearance is constant and is not influenced by comorbidities [49] so that the effectiveness of therapeutic measures can be easily monitored. For the sake of completeness it should be noted that CRP synthesis can also be demonstrated extrahepatically, e.g. in microglia [25], lymphocytes [27], alveolar macrophages [13] and fat cells [10]; however, this extrahepatic component only minimally contributes to the CRP concentration in the circulation.
Measurement of CRP is also important in monitoring acute exacerbations of chronic diseases. Proinflammatory cytokines are released from the focus of inflammation, which then reach the liver via the circulation, where they trigger CRP synthesis. For instance, Crohn’s disease activity can be detected by measuring the CRP serum concentration. In ulcerative colitis, another chronic inflammatory bowel disease, the rise in CRP concentration is less marked, probably because the inflammation is limited to the mucosa and is not transmural [48].
Measurement of the CRP concentration is suitable not only for monitoring inflammatory disease activity but also for rapidly assessing the success of treatment of inflammatory diseases. In polyarteritis nodosa, an immunological vasculitis, a drop in the CRP in the first days after initiation of immunosuppressive therapy indicates a response to the treatment [22].
Measurements of CRP in geriatric patients
From what has already been described, it is apparent that measurement of the CRP concentration in the circulation is an important component in establishing a diagnosis and monitoring the course of various diseases. Does this also apply for geriatric patients, particularly in view of the ageing immune system? This process of ageing of the immune system is described by the term immunosenescence, which is associated with dysregulation of the immune system. Although functional changes take place in the innate immune system as part of the ageing process, these are less pronounced than in the acquired immune system. Good review articles include those by Desai et al. [12], Gomez et al. [19] and Opal et al. [36].
Larger scale studies, especially prospective studies regarding the relevance of CRP as a biomarker in geriatric patients with an aged immune system are rare but necessary as a recent study has shown that vital parameters, such as body temperature only have limited value for the detection of infections in elderly patients [43]. In a retrospective study Wester et al. investigated 890 patients who had a positive blood culture for S. pneumoniae and E. coli from 1994–2004 [53]. The patients were divided into 3 age groups: < 65 years (300 patients), 65–84 years (443 patients) and > 85 years (147 patients). In these groups the CRP levels on the day the blood culture was taken were compared 2–3 days later and again 4–7 days later. A statistically significant negative correlation was found between patient age and the CRP level at the first measurement time. The median CRP levels at the first two blood sampling times were significantly higher in the youngest group; however, no statistically significant differences were found in sensitivity related to age. The authors came to the conclusion that a weakened CRP response with increasing age was seen in the study but that this is hardly of clinical relevance. Another study on the value of CRP measurement in patients of a geriatric clinic was prospectively conducted by Liu et al. for 3 months in patients who were initially hospitalized in the geriatric department as emergency admissions [30]. The study included a total of 232 patients (135 women, mean age 82.6 years, range 70–99 years and 97 men, mean age 82.6 years, range 69–95 years). In 83 patients infections were diagnosed using the criteria of the International Sepsis Definitions Conference. A significant difference in the mean CRP level (21.3 mg/l vs. 150.5 mg/l) was found between patients with and without infections. The optimal sensitivity and specificity values for the diagnosis of a bacterial infection were found at a cut-off value of 60 mg/l (sensitivity 80.7 %, specificity 96 %, positive predictive value 91.9 % and negative predictive value 89.9 %). Higher sensitivity and specificity values were found for the CRP and body temperature measurements than for the leukocyte count and neutrophil count. The authors came to the conclusion that the CRP is a suitable and useful biomarker for predicting bacterial infections in elderly patients. Talebi-Taher et al. investigated 150 consecutive patients, 50 patients with sepsis, 50 patients with severe inflammatory response syndrome (SIRS) and 50 healthy control persons, who were admitted to the emergency department with SIRS or sepsis [45]. The mean age of all study participants was 74.3 years. The CRP, procalcitonin (PCT), IL-6, erythrocyte sedimentation rate (ESR) and leukocyte count were measured on the first day. In the ROC analysis, the greatest area under the curve (AUC) for CRP was found at 0.88 for differentiating between sepsis and SIRS. At the cut-off level of 12 mg/l the sensitivity was 98 %, specificity was 72 %, the positive predictive value was 63.6 % and the negative predictive value was 98.6 %. For differentiating between SIRS and the control group, the IL-6 values demonstrated the best AUC level of 0.75. In a recent meta-analysis, the diagnostic accuracy of the infection marker PCT for identification of systemic bacterial infections in elderly patients was investigated by Lee et al. [29]. Unlike CRP, PCT is produced in many body cells in the case of bacterial infections. The authors found a specificity and sensitivity of 83 % for PCT and for CRP a sensitivity of 91 % but a specificity of only 36 %. They also found no evidence of an influence of age on the PCT value in the sense of immunosenescence. The authors concluded from the results that the PCT level might represent a possible marker for excluding sepsis in elderly patients; however, the results should always be interpreted in the clinical context. A general synoptic overview regarding the different humoral markers and effectors of the acute phase reaction is presented in Table 1. The question finally arises as to whether the reference ranges for CRP should be adapted for elderly patients. There is evidence for a tendency to higher CRP values in elderly patients, especially those with frailty or chronic infections [52]; however, elderly persons in general tend to demonstrate a greater range within the reference ranges because of individual heterogeneous previous illnesses [28]. Specific questions should be considered with the individual course of laboratory results with awareness of cut-off values from studies rather than being orientated to age-adapted reference ranges.
Conclusion
The studies cited show that despite the introduction and approval of newer diagnostic markers for identifying and monitoring bacterial infections, such as PCT [21], the soluble subtype of the endotoxin receptors CD14 [4] and IL-6 with sometimes better diagnostic validity compared with CRP, measurement of CRP is still justified in geriatric patients because of its low cost and because it is available round the clock in most hospitals. Biochemical markers of the acute phase reaction should always be interpreted together with the clinical picture and their specific limitations.
References
Abernethy TJ, Avery OT (1941) The occurrence during acute infections of a protein not normally present in the blood : I. distribution of the reactive protein in patients’ sera and the effect of calcium on the flocculation reaction with C polysaccharide of pneumococcus. J Exp Med 73:173–182
Ball EM, Gibson DS, Bell AL et al (2014) Plasma IL-6 levels correlate with clinical and ultrasound measures of arthritis in patients with systemic lupus erythematosus. Lupus 23:46–56
Banks RE, Forbes MA, Patel PM et al (2000) Subcutaneous administration of recombinant glycosylated interleukin 6 in patients with cancer: pharmacokinetics, pharmacodynamics and immunomodulatory effects. Cytokine 12:388–396
Behnes M, Bertsch T, Lepiorz D et al (2014) Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care 18:507
Bertsch T, Banks RE, Forbes MA et al (1996) Phospholipase A2 activity in serum is induced during treatment with recombinant human interleukin-6 in patients with cancer. Ann Clinl Biochem 33:565–567
Bodman-Smith KB, Melendez AJ, Campbell I et al (2002) C-reactive protein-mediated phagocytosis and phospholipase D signalling through the high-affinity receptor for immunoglobulin G (FcgammaRI). Immunology 107:252–260
Boenisch S, Fae P, Drexel H et al (2013) Are circulating levels of CRP compared to IL-6 and PCT still relevant in intensive care unit patients? (English Version). J Lab Med. doi:10.1515/labmed-2013-0029
Boenisch S, Fae P, Drexel H et al (2013) Spielen CRP-Spiegel neben IL-6 und PCT noch eine Rolle für Patienten auf Intensivstationen?/Are circulating levels of CRP compared to IL-6 and PCT still relevant in intensive care unit patients? (German Version). J Lab Med 37:1–11. doi:10.1515/labmed-2012-0010
Brunkhorst R, Eberhardt OK, Haubitz M et al (2000) Procalcitonin for discrimination between activity of systemic autoimmune disease and systemic bacterial infection. Intensive Care Med 26(Suppl 2):S199–201
Calabro P, Chang DW, Willerson JT et al (2005) Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol 46:1112–1113
Chun HY, Chung JW, Kim HA et al (2007) Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol 27:461–466
Desai A, Grolleau-Julius A, Yung R (2010) Leukocyte function in the aging immune system. J Leukoc Biol 87:1001–1009
Dong Q, Wright JR (1996) Expression of C-reactive protein by alveolar macrophages. J Immunol 156:4815–4820
Du Clos TW (1989) C-reactive protein reacts with the U1 small nuclear ribonucleoprotein. J Immunol 143:2553–2559
Du Clos TW (2013) Pentraxins: structure, function, and role in inflammation. ISRN Inflamm 2013:379040
Du Clos TW, Marnell L, Zlock LR et al (1991) Analysis of the binding of C-reactive protein to chromatin subunits. J Immunol 146:1220–1225
Ehrich JH, Krull F, Peltner U et al (1986) [Significance of C-reactive protein in pediatric diagnosis]. Monatsschr Kinderheilkd 134:840–846
Gaitonde S, Samols D, Kushner I (2008) C-reactive protein and systemic lupus erythematosus. Arthritis Rheum 59:1814–1820
Gomez CR, Nomellini V, Faunce DE et al (2008) Innate immunity and aging. Exp Gerontol 43:718–728
Heinrich PC, Müller M, Graeve L (2014) Löffler/Petrides Biochemie und Pathobiochemie. Springer-Verlag, Berlin
Heppner HJ, Bertsch T, Alber B et al (2010) Procalcitonin: inflammatory biomarker for assessing the severity of community-acquired pneumonia - a clinical observation in geriatric patients. Gerontology 56:385–389
Hind CR, Winearls CG, Pepys MB (1985) Correlation of disease activity in systemic vasculitis with serum C-reactive protein measurement. A prospective study of thirty-eight patients. Eur J Clin Invest 15:89–94
Hutchinson WL, Noble GE, Hawkins PN et al (1994) The pentraxins, C-reactive protein and serum amyloid P component, are cleared and catabolized by hepatocytes in vivo. J Clin Invest 94:1390–1396
Illei GG, Tackey E, Lapteva L et al (2004) Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum 50:2048–2065
Juma WM, Lira A, Marzuk A et al (2011) C-reactive protein expression in a rodent model of chronic cerebral hypoperfusion. Brain Res 1414:85–93
Knudsen LS, Klarlund M, Skjodt H et al (2008) Biomarkers of inflammation in patients with unclassified polyarthritis and early rheumatoid arthritis. Relationship to disease activity and radiographic outcome. J Rheum 35:1277–1287
Kuta AE, Baum LL (1986) C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. J Exp Med 164:321–326
Lapin A, Bohmer F (2005) Laboratory diagnosis and geriatrics: more than just reference intervals for the elderly. Wien Med Wochenschr 155:30–35
Lee SH, Chan RC, Wu JY et al (2013) Diagnostic value of procalcitonin for bacterial infection in elderly patients - a systemic review and meta-analysis. Int J Clin Pract 67:1350–1357
Liu A, Bui T, Van Nguyen H et al (2010) Serum C-reactive protein as a biomarker for early detection of bacterial infection in the older patient. Age Ageing 39:559–565
Marnell LL, Mold C, Volzer MA et al (1995) C-reactive protein binds to Fc gamma RI in transfected COS cells. J Immunol 155:2185–2193
Mold C, Gewurz H, Du Clos TW (1999) Regulation of complement activation by C-reactive protein. Immunopharmacology 42:23–30
Müller-Esterl W (2010) Biochemie - Eine Einführung für Mediziner und Naturwissenschaftler. Spektrum Akademischer Verlag, Heidelberg
Ohl K, Tenbrock K (2011) Inflammatory cytokines in systemic lupus erythematosus. J Biomed Biotechnol 2011:432595
Oliveira EB, Gotschlich EC, Liu TY (1977) Primary structure of human C-reactive protein. Proc Natl Acad Sci U S A 74:3148–3151
Opal SM, Girard TD, Ely EW (2005) The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis 41(Suppl 7):S504–512
Pepys MB, Hirschfield GM (2003) C-reactive protein: a critical update. J Clin Invest 111:1805–1812
Pepys MB, Baltz M, Gomer K et al (1979) Serum amyloid P-component is an acute-phase reactant in the mouse. Nature 278:259–261
Pepys MB, Dash AC, Markham RE et al (1978) Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol 32:119–124
Rossol M, Heine H, Meusch U et al (2011) LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol 31:379–446
Shrivastava AK, Singh HV, Raizada A et al (2015) Inflammatory markers in patients with rheumatoid arthritis. Allergol Immunopathol (Madr) 43:81–87
Shrive AK, Cheetham GM, Holden D et al (1996) Three dimensional structure of human C-reactive protein. Nat Struct Biol 3:346–354
Singler K, Bertsch T, Heppner HJ et al (2013) Diagnostic accuracy of three different methods of temperature measurement in acutely ill geriatric patients. Age Ageing 42:740–746
Stuart RA, Littlewood AJ, Maddison PJ et al (1995) Elevated serum interleukin-6 levels associated with active disease in systemic connective tissue disorders. Clin Exp Rheumatol 13:17–22
Talebi-Taher M, Babazadeh S, Barati M et al (2014) Serum inflammatory markers in the elderly: are they useful in differentiating sepsis from SIRS? Acta Med Iran 52:438–442
Tillett WS, Francis T (1930) Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med 52:561–571
Tron K, Manolov DE, Rocker C et al (2008) C-reactive protein specifically binds to Fcgamma receptor type I on a macrophage-like cell line. Eur J Immunol 38:1414–1422
Vermeire S, Van Assche G, Rutgeerts P (2006) Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 55:426–431
Vigushin DM, Pepys MB, Hawkins PN (1993) Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest 91:1351–1357
Volanakis JE (1982) Complement activation by C-reactive protein complexes. Ann N Y Acad Sci 389:235–250
Volanakis JE, Wirtz KW (1979) Interaction of C-reactive protein with artificial phosphatidylcholine bilayers. Nature 281:155–157
Walston J, Mcburnie MA, Newman A et al (2002) Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 162:2333–2341
Wester AL, Blaasaas KG, Wyller TB (2008) Is the concentration of C-reactive protein in bacteraemia associated with age? Immun Ageing 5:8
Acknowledgement
The CRP image (Fig. 1) was kindly provided by Professor Trevor J. Greenhough.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Bertsch, J. Triebel, C. Bollheimer, M. Christ, C. Sieber, K. Fassbender and H.J. Heppner declare that there are no conflicts of interest.
This article does not contain any studies with human or animal subjects.
Additional information
Thomas Bertsch and Jakob Triebel contributed equally to this article.
Rights and permissions
About this article
Cite this article
Bertsch, T., Triebel, J., Bollheimer, C. et al. C-reactive protein and the acute phase reaction in geriatric patients. Z Gerontol Geriat 48, 595–600 (2015). https://doi.org/10.1007/s00391-015-0938-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00391-015-0938-4