Abstract
Purpose
Elective sigmoid resection is proposed as a treatment for symptomatic diverticular disease for the possible improvement in quality of life achievable. Albeit encouraging results have been reported, recurrent diverticulitis is still a concern deeply affecting quality of life. The aim of this study is to determine the rate of recurrent diverticulitis after elective sigmoid resection and to look for possible perioperative risk factors.
Methods
Patients who underwent elective resection for DD with at least a 3-year follow-up were included. Postoperative recurrence was defined as left-sided or lower abdominal pain, with CT scan-confirmed findings of diverticulitis.
Results
Twenty of 232 (8.6%) patients developed CT-proven recurrent diverticulitis after elective surgery. All the 20 recurrent diverticulitis were uncomplicated and did not need surgery. Eighty-five percent of the recurrences occurred in patients with a preoperative diagnosis of uncomplicated DD, 70% in patients who had at least 4 episodes of diverticulitis, and 70% in patients with a history of diverticulitis extended to the descending colon. Univariate analysis showed that recurrence was associated with diverticulitis of the sigmoid and of the descending colon (p = 0.04), with a preoperative diagnosis of IBS (p = 0.04) and with a longer than 5 years diverticular disease (p = 0.03). Multivariate analysis was not able to determine risks factors for recurrence.
Conclusion
Our study showed that patients with a preoperative diagnosis of IBS, diverticulitis involving the descending colon, and a long-lasting disease are more likely to have recurrent diverticulitis. However, these variables could not be assumed as risk factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic elective sigmoid resection is proposed as a treatment for symptomatic diverticular disease (DD) [1]. Patients recovering from an episode of acute complicated diverticulitis might be indicated for elective surgery even though such a behavior is not widely adopted [2]. Similarly for patients suffering from acute recurrent diverticulitis, smoldering diverticulitis, or symptomatic uncomplicated diverticular disease (SUDD), the decision on elective surgery is taken on a tailored approach [3].
The question still remains open, whether or not to perform elective surgery for symptomatic diverticular disease. The main concern is nowadays related to the possible improvement in quality of life achievable with planned surgery. Moreover, it is desirable to be able to avoid future complicated attacks and also to prevent patients from any kind of recurrent diverticulitis [4]. However, elective surgery might be unable to fully prevent future symptomatic diverticular disease.
Therefore, it is crucial to corroborate the effectiveness of elective surgery by describing the postoperative recurrence rate of acute diverticulitis [5].
This retrospective analysis aims to determine the rate of recurrent diverticulitis after elective sigmoid resection and to look for possible perioperative risk factors.
Materials and methods
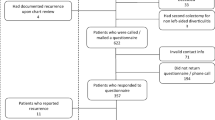
This is a retrospective analysis of a prospectively maintained single institution dedicated database. Patients with a diagnosis of diverticular disease have been prospectively enrolled in a specific database since 2013. Patients who underwent elective colon resection for DD with at least a 3-year follow-up were included in the analysis. All patients included received a preoperative CT scan reporting the presence of DD and a full colonoscopy.
Demographic data included age, gender, the American Society of Anesthesiologists (ASA) classification, smoking habits, diagnosis of irritable bowel syndrome (IBS), whether it was SUDD, smoldering DD, acute recurrent diverticulitis or complicate diverticulitis, the number of episodes of acute diverticulitis, description of the surgical operation, and pathology examination.
Smoldering diverticulitis was defined as prolonged (3 months) left lower quadrant abdominal pain associated with inflammation at the blood examination not responding to therapy with CT scan proved DD [6].
SUDD was defined as the presence of colonic diverticulosis both at CT scan and colonoscopy associated with persistent localized pain and diarrhea/constipation without evidence of inflammation at the blood examination [7].
Recurrent, uncomplicated diverticulitis was defined as the combination of multiple episodes of left lower quadrant abdominal pain, fever, leukocytosis, and evidence of inflammation on CT [5, 6].
Complicated diverticular disease was defined as pericolic abscesses, colonic perforation, diverticular bleeding, colonic diverticular stenosis, or colonic fistula [8].
All patients were operated by four colorectal surgeons with more than 10-year experience.
Mandatory technical steps that were reported in all surgical procedures were:
-
Distal resection at the level of the upper rectum (the distal sigmoid was completely removed).
-
Creation of an end-to-end double-staple Knight-Griffen anastomosis.
-
Splenic flexure mobilization was not performed routinely during sigmoidectomy.
-
Vascular ligation was performed proximal or distal to the left colic artery take-off.
-
During sigmoidectomy, the level of the colonic transection was at the colonic-sigmoid junction.
-
During left hemicolectomy, the distal descending colon was removed after splenic flexure mobilization.
Postoperative recurrent diverticulitis was defined as left-sided or lower abdominal pain, with CT scan-confirmed findings of diverticulitis. This study was approved by the Institutional Review Board of the medical center. All patients were enrolled in the Diverticular Disease Registry (DDR Trial) ClinicalTrials.gov (NCT04907383) [9].
Statistical analysis
Categorical variables were reported as frequencies (percentages), while continuous variables were reported as median (interquartile range) or mean ± standard deviation, as appropriate. The χ2 test was used for categorical variables, and the Student t tests were used for continuous variables comparing patients with and without recurrence. Cox regression was used to perform a multivariate analysis of the variables found significant at the univariate analysis. The hazard ratios with 95% confidence interval were presented. Statistical significance was set at a p value of < 0.05. All statistical analyses were performed using the statistical software Statistical Package for the Social Sciences (SPSS) software (version 22, SPSS, Chicago, IL, USA).
Results
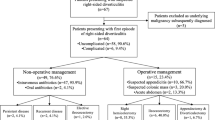
The patient’s characteristics are listed in Table 1. One hundred ninety-eight patients (198/232; 85%) had 3 or fewer episodes of diverticulitis, and 79% (184/232) had a preoperative diagnosis of uncomplicated diverticular disease. One hundred forty-six patients (62.8%) had a history of diverticular disease longer than 5 years. Nineteen patients (8.2%) had a preoperative diagnosis of irritable bowel syndrome (IBS); 87.9% (204/232) of patients had diverticulitis of the sigmoid colon, while in 12.1% (28/232), the disease was involving the descending colon. 85.3% (198/232) of patients underwent sigmoidectomy, while 14.7% (34/232) underwent left hemicolectomy. 86.2% of patients (200/232) underwent a total laparoscopic procedure, conversion was recorded in 12/232 patients (5.2%) due to intraoperative bleeding (5 patients) and massive adhesions (7 patients), and open procedures were 20/232 (8.6%) for anesthesiologic contraindication to pneumoperitoneum. During surgery, 65.5% of patients (152/232) had the inferior mesenteric artery (IMA) ligated and 34.5% (80/232) preserved. After 30 days postoperatively, no mortality occurred; anastomotic leak was recorder in 4 patients (1.7%) requiring reintervention, anastomotic bleeding in 14 patients (6%) that required endoscopic hemostasis in cases, and only hemotransfusion in 6 cases; and postoperative ileus in 13 patients treated conservatively (5.6%) (Table 2).
In our prospective cohort, 20/232 (8.6%) patients developed CT-proven recurrent diverticulitis after elective surgery. The mean time to recurrence was 31 months (range, 4–86 months). Ten recurrences (50%) occurred within the first 3 years (4 in the first year, 3 in the second year, and 3 in the third one). All the 20 recurrent diverticulitis were uncomplicated and did not need surgery. Eighty-five percent (17/20) of the recurrences occurred in patients with a preoperative diagnosis of uncomplicated DD, 70% (14/20) in patients who had at least 4 episodes of diverticulitis preoperatively, and 70% (14/20) in patients with a history of diverticulitis extended to the descending colon.
A comparison was made between patients with recurrence and those without recurrence (Table 3). Univariate analysis showed that recurrence of diverticulitis after elective surgery was associated with diverticulitis of the sigmoid and of the descending colon (p = 0.04), with a preoperative diagnosis of IBS (p = 0.04), and with a longer than 5-year diverticular disease (p = 0.03). Cox regression analysis was not able to determine risks factors for recurrence (Table 4). Postoperative complications higher than Clavien-Dindo 3 rate did not affect the recurrence rate.
Discussion
Our study shows that recurrent diverticulitis is not a rare event since it was reported in almost 9% of the patients undergoing a colectomy. A preoperative diagnosis of IBS, diverticulitis involving del descending colon, and a long-lasting disease could be associated with recurrent diverticulitis.
Elective surgery is always carried out based on a shared decision between the surgeon and the patient, especially when treating a benign disease such as diverticulitis [10]. Consequently, a clinical recurrence could be considered as a treatment failure. The possibility that diverticulitis may recur must be carefully studied in order to be as comprehensive as possible at the time of explaining the outcomes of surgery to patients [11]. However, there is still few data about the incidence of recurrent diverticulitis. Indeed, literature reports the recurrence rate of patients operated for diverticulitis usually incorporating both urgent and elective procedures, thus becoming too heterogeneous [12]. Instead, there is a need to focus more on clinical outcomes following the elective procedures only. In this setting, the patient can be studied with more accuracy, and surgery can be planned accordingly.
A recent meta-analysis including 1062 patients reported the mean incidence of recurrence after surgery for diverticular disease of 5.8% (with a range between 3.5 and 8.7%) and identified IBS, uncomplicated recurrent diverticulitis, the anastomotic high, and age as associated factors for diverticulitis recurrence [4]. The data we report confirms that the presence of IBS and the chronicity of diverticular disease are associated with increased recurrent diverticulitis rates. Conversely, we did not find a significant association with patients’ age. However, it is possible that the sample size or the follow-up of this study was not sufficient to detect this association.
In patients with preoperative IBS, elective surgery has the role of preventing future complicated episodes. Similarly to Choi et al. [5], it is our practice to propose surgery in patients with IBS only after a CT confirmed diagnosis of acute diverticulitis. However, the possibility of recurrent diverticulitis and mostly the persistence of intestinal symptoms must be fully and clearly explained to the patient in the moment of the acquisition of the informed consent [13].
Recurrent diverticulitis occurred more frequently in patients suffering from an active disease for more than 5 years. It is conceivable that other colonic segments may become more susceptible to inflammation with the persistence of the inflammatory state correlated with diverticulitis [14]. Low-grade inflammation, sensory motor nerve damage, and dysbiosis in fact could be involved in colonic tissues as a result of continuous and chronic inflammatory stimulation [15, 16].
Surely, performing a colo-sigmoid anastomosis after sigmoidectomy means leaving the colo-rectal junction in place. A colonic resection not extended to the upper rectum is known to be associated with increased rates of recurrent diverticulitis on the sigmoid remnant [17]. Indeed, Thaler et al. reported four times higher risk of recurrent diverticulitis in patients with colo-sigmoid anastomosis [18]. All the procedures we report were performed with the excision of the colo-rectal junction as a mandatory surgical step.
Differently from other reported series, we found that patients in whom diverticular disease also involves the descending colon could have higher rates of recurrence. Indeed, when the descending colon was involved by diverticulitis, it has to be removed to avoid recurrence at that level [19]. An inaccurate preoperative diagnosis could be the cause of the relapse in these patients. Indeed, the presence of diverticulitis in colonic segments other than the sigmoid colon can essentially be documented by an abdominal CT scan performed during an acute attack. In case of an acute attack not investigated with a CT scan could cause an underestimation of the real extension of the disease [20].
The finding that only 8.6% of patients developed recurrent diverticular disease, none of which requiring reoperation is somehow comforting. Based on this, we believe that offering elective surgical resection to patients for active diverticular disease should be after all maintained. According to the same findings, extending the surgical resection to the descending colon, if not a site of severe diverticulosis, should also not be routinary performed.
As highlighted, the preoperative diagnostic analysis plays a central role in the correct surgical indication in patients suffering from diverticular disease. Therefore, an inaccurate preoperative work-up could be at the basis of the disease recurrence. However, the difficulty in finding associations strong enough to become risk factors suggests that, to a certain extent up to now, we do not have a definitive explanation for DD recurrence after elective surgery. Surely, the different clinical presentations for which patients are given the indication for elective surgery can generate a heterogeneity in the analyzed population such as to introduce factors related to the development of recurrences that, ultimately, we do not fully understand yet.
Conclusion
The rate of recurrent diverticulitis after elective sigmoid resection was 8.6% which is similar to previously reported studies. Our study showed that patients with a preoperative diagnosis of IBS, diverticulitis involving descending colon and a long-lasting disease is more likely to have recurrent diverticulitis. However, these variables could not be assumed as real risk factors in this study. Multicenter diverticular disease registries and longer follow-up may provide new evidences to guide patient selection for elective diverticular disease surgery and define who could benefit from surgery the most.
Data availability
All patients were enrolled in the Diverticular Disease Registry (DDR Trial) ClinicalTrials.gov (NCT04907383).
References
Hawkins AT, Wise PE, Chan T, Lee JT, Glyn T, Wood V, Eglinton T, Frizelle F, Khan A, Hall J, Ilyas MIM, Michailidou M, Nfonsam VN, Cowan ML, Williams J, Steele SR, Alavi K, Ellis CT, Collins D, Winter DC, Zaghiyan K, Gallo G, Carvello M, Spinelli A, Lightner AL (2020) Diverticulitis: an update from the age old paradigm. Curr Probl Surg 57(10):100862. https://doi.org/10.1016/j.cpsurg.2020.100862. Epub 2020 Jul 18. PMID: 33077029; PMCID: PMC7575828
Schultz JK, Azhar N, Binda GA, Barbara G, Biondo S, Boermeester MA, Chabok A, Consten ECJ, van Dijk ST, Johanssen A, Kruis W, Lambrichts D, Post S, Ris F, Rockall TA, Samuelsson A, Di Saverio S, Tartaglia D, Thorisson A, Winter DC, Bemelman W, Angenete E (2020) European Society of Coloproctology: guidelines for the management of diverticular disease of the colon. Colorectal Dis 22(Suppl 2):5–28. https://doi.org/10.1111/codi.15140 (Epub 2020 Jul 7 PMID: 32638537)
Cirocchi R, Sapienza P, Anania G, Binda GA, Avenia S, di Saverio S, Tebala GD, Zago M, Donini A, Mingoli A, Nascimbeni R (2021) State-of-the-art surgery for sigmoid diverticulitis. Langenbecks Arch Surg 2021 Sep 23. https://doi.org/10.1007/s00423-021-02288-5. Epub ahead of print. PMID: 34557938
Longchamp G, Abbassi Z, Meyer J, Toso C, Buchs NC, Ris F (2021) Surgical resection does not avoid the risk of diverticulitis recurrence-a systematic review of risk factors. Int J Colorectal Dis 36(2):227–237. https://doi.org/10.1007/s00384-020-03762-0. Epub 2020 Sep 28. PMID: 32989503; PMCID: PMC7801345
Choi KK, Martinolich J, Canete JJ, Valerian BT, Chismark DA, Ata A, Lee EC (2020) Elective laparoscopic sigmoid colectomy for diverticulitis-an updated look at recurrence after surgery. J Gastrointest Surg 24(2):388–395. https://doi.org/10.1007/s11605-018-04083-y (Epub 2019 Jan 22 PMID: 30671801)
Boostrom SY, Wolff BG, Cima RR, Merchea A, Dozois EJ, Larson DW (2012) Uncomplicated diverticulitis, more complicated than we thought. J Gastrointest Surg 16(9):1744–1749. https://doi.org/10.1007/s11605-012-1924-4 (Epub 2012 Jun 13 PMID: 22696233)
Maconi G (2017) Diagnosis of symptomatic uncomplicated diverticular disease and the role of Rifaximin in management. Acta Biomed 88(1):25–32. https://doi.org/10.23750/abm.v88i1.6360.PMID:28467330;PMCID:PMC6166204
Cremon C, Bellacosa L, Barbaro MR, Cogliandro RF, Stanghellini V, Barbara G (2017) Diagnostic challenges of symptomatic uncomplicated diverticular disease. Minerva Gastroenterol Dietol 63(2):119–129. https://doi.org/10.23736/S1121-421X.17.02370-4 (Epub 2017 Jan 12 PMID: 28079347)
Origi M, Achilli P, Calini G, Costanzi A, Monteleone M, Montroni I, Maggioni D, Cocozza E, Megna S, Totis M, Tamini N, Ziccarelli A, Filippone G, Ferrari G, Crippa J, Spinelli A, Mari GM (2021) AIMS Academy Clinical Research Network. The diverticular disease registry (DDR trial) by the advanced international mini-invasive surgery academy clinical research network: protocol for a multicenter, prospective observational study. Int J Surg Protoc 25(1):194–200. https://doi.org/10.29337/ijsp.157. PMID: 34541429; PMCID: PMC8415185
Collins D, Winter DC (2008) Elective resection for diverticular disease: an evidence-based review. World J Surg 32(11):2429–2433. https://doi.org/10.1007/s00268-008-9705-7 (PMID: 18712563)
Andeweg C, Peters J, Bleichrodt R, van Goor H (2008) Incidence and risk factors of recurrence after surgery for pathology-proven diverticular disease. World J Surg 32(7):1501–1506. https://doi.org/10.1007/s00268-008-9530-z.PMID:18330623;PMCID:PMC2480508
Mizrahi I, Al-Kurd A, Chapchay K, Ag-Rejuan Y, Simanovsky N, Eid A et al (2018) Long-term outcomes of sigmoid diverticulitis: a single-center experience. J Surg Res 221:8–14
Spiller R (2012) Is it diverticular disease or is it irritable bowel syndrome? Dig Dis 30(1):64–69. https://doi.org/10.1159/000335721 (Epub 2012 May 3 PMID: 22572688)
Strate LL, Modi R, Cohen E, Brennan MR, Spiegel MR (2012) Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol 107(10):1486–1493
Simpson J, Neal KR, Scholefield JH, Spiller RC (2003) Patterns of pain in diverticular disease and the influence of acute diverticulitis. Eur J Gastroenterol Hepatol 15(9):1005–1010. https://doi.org/10.1097/00042737-200309000-00011 (PMID: 12923374)
Tursi A, Elisei W, Picchio M, Giorgetti GM, Brandimarte G (2015) Moderate to severe and prolonged left lower-abdominal pain is the best symptom characterizing symptomatic uncomplicated diverticular disease of the colon: a comparison with fecal calprotectin in clinical setting. J Clin Gastroenterol 49(3):218–221. https://doi.org/10.1097/MCG.0000000000000094 (PMID: 24583746)
Ambrosetti P (2016) Acute left-sided colonic diverticulitis: clinical expressions, therapeutic insights, and role of computed tomography. Clin Exp Gastroenterol 18(9):249–257. https://doi.org/10.2147/CEG.S110428.PMID:27574459;PMCID:PMC4993273
Thaler K, Baig MK, Berho M, Weiss EG, Nogueras JJ, Arnaud JP, Wexner SD, Bergamaschi R (2003) Determinants of recurrence after sigmoid resection for uncomplicated diverticulitis. Dis Colon Rectum 46(3):385–388. https://doi.org/10.1007/s10350-004-6560-y (PMID: 12626916)
Ambrosetti P, Gervaz P (2014) Laparoscopic elective sigmoidectomy for diverticular disease: a plea for standardization of the procedure. Colorectal Dis 16(2):90–94. https://doi.org/10.1111/codi.12455 (PMID: 24128302)
Rausch VH, Weinrich JM, Schön G, Sabour L, Özden C, Kaul MG, Adam G, Bannas P, Henes FO (2021) Accuracy of preoperative CT staging of acute colonic diverticulitis using the classification of diverticular disease (CDD) - is there a beneficial impact of water enema and visceral obesity? Eur J Radiol 141:109813. https://doi.org/10.1016/j.ejrad.2021.109813 (Epub 2021 Jun 6 PMID: 34116453)
Author information
Authors and Affiliations
Contributions
Mari G. and Montroni I. conceived the study; Santambrogio G. wrote the manuscript; Costanzi A., Maggioni D., and Laporta A. collected the data; and Calini G. performed the statistical analysis. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of the medical center.
Consent to participate
All patients were enrolled in the Diverticular Disease Registry (DDR Trial) ClinicalTrials.gov (NCT04907383).
Consent for publication
All patients were enrolled in the Diverticular Disease Registry (DDR Trial) ClinicalTrials.gov (NCT04907383).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giulio, M., Gaia, S., Andrea, C. et al. Recurrent diverticulitis after elective surgery. Int J Colorectal Dis 37, 2149–2155 (2022). https://doi.org/10.1007/s00384-022-04248-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-022-04248-x