Abstract
Purpose
The standard treatment of stage II–III rectal cancer is preoperative chemoradiotherapy (CRT), followed by total mesorectal excision (TME). However, the rate of metastasis is still high following this treatment. Therefore, several adjuvant chemotherapy studies have been conducted on reducing subsequent metastases and increasing survival, although there are still no definite conclusions.
Methods
We searched for published prospective randomized controlled trials comparing adjuvant chemotherapy regimens following standard preoperative CRT and curative surgery in stage II–III rectal cancer. We systematically searched Medline, Embase, and the Cochrane Library for relevant trials done from January 2004 to January 2021. Review Manager (RevMan, version 5.3) was used to analyze the data.
Results
We initially searched 1955 studies. We screened and carefully selected four randomized controlled trials with 2897 patients. Compared to the 5-FU-based regimen group, the oxaliplatin-added regimen group attained a higher 3-year locoregional control rate (relative risk [RR] of 0.64, 95% confidence interval [CI], 0.48–0.86; p = 0.003) and 3-year distant metastasis control rate (RR of 0.82, 95% CI, 0.71–0.95; p = 0.007). The oxaliplatin-added regimen group had significantly increased 3-year disease-free survival with a hazard ratio (HR) of 0.85 (95% CI: 0.74–0.97, p = 0.020), but not overall survival (p = 0.740). Grade 3 or higher acute toxicity rates did not differ between the two groups (p = 0.190).
Conclusion
The addition of oxaliplatin to adjuvant therapy for stage II–III rectal cancer following preoperative CRT and TME may increase disease-free survival without significant increases in toxicity, but not overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard treatment for stage II–III rectal cancer is a multimodal approach, TME, concurrent preoperative chemoradiotherapy, and 5-fluorouracil (5-FU)-based postoperative chemotherapy [1]. With these multimodal treatments, the locoregional recurrence rate has dropped to around 10%. However, the future failure rate is still over 20% to 30% and affects patient survival [2,3,4,5]. Therefore, more intensified adjuvant chemotherapy regimens have been investigated to achieve better systemic disease control. The addition of oxaliplatin to 5-FU-based chemotherapy for colon cancer has been shown to improve survival. After the positive results of the MOSAIC trial [6] and the NSABP (National Surgical Adjuvant Breast and Bowel Project) C-07 trial [7], oxaliplatin-added regimens, such as FOLFOX, became a standard in high-risk stage-II and stage-III colon cancers [8].
These positive findings have prompted several studies to expand on them for stage II–III rectal cancer, based on these results [9, 10]. However, inconsistent results in these studies make it difficult to draw a definite conclusion. Therefore, the aim of this meta-analysis is to evaluate the efficacy of adding oxaliplatin to 5-FU-based chemotherapy for adjuvant treatment of stage II–III rectal cancer. We included only prospective randomized controlled trials.
Materials and methods
Literature search and selection criteria
We searched for published and unpublished prospective randomized controlled trials that compared adjuvant chemotherapy regimens following preoperative CRT and curative surgery for stage II–III rectal cancer. Patients aged 18 years and older were eligible for inclusion. All fluoropyrimidine (5-FU or capecitabine)-based or oxaliplatin-added chemotherapy regimens were accepted. Infusions of 5-FU/leucovorin and oral daily capecitabine were both accepted. Only studies in which total mesorectal excision following preoperative CRT had been performed were included. We excluded those studies from our analysis where total excision had been performed as an operation.
We searched Medline, Embase, and the Cochrane Library for relevant trials done from January 2004 to January 2021. We also searched abstracts from the major European or American international oncologic meetings: ASTRO, ASCO, ESTRO, and ESMO. Electronic database searches were conducted with MeSH terms (Rectal Neoplasms, Colorectal Neoplasms, Chemotherapy, and Radiotherapy) and free text terms (rectal cancer, adenocarcinoma, neoplasm, radiotherapy, chemotherapy, chemoradiation, radiochemotherapy, postoperative, and adjuvant). We restricted our searches to articles published in English or Korean. Two independent reviewers (Lee JH and Song JH) screened the searched articles’ titles and abstracts. Trials that seemed to meet the inclusion and exclusion criteria were selected for full-text review.
Outcome measures
We evaluated the following outcomes: 3-year overall survival (OS), disease-free survival (DFS), locoregional recurrence, distant metastasis, compliance to adjuvant chemotherapy, and toxicities. We defined OS as the time from curative surgery to death from any cause or to last follow-up. DFS was defined as time to any recurrence, malignancy, or death or to last follow-up from curative surgery. We analyzed the receipt of adjuvant chemotherapy following preoperative CRT. We compared grade 3 or higher toxicities from adjuvant chemotherapy for 5-FU-based or oxaliplatin-added chemotherapeutic regimens.
Statistical analyses
Two reviewers (Um JW and Kim SH) obtained the full text of relevant randomized controlled studies and assessed methodological quality according to the Cochrane Collaboration tool for assessing the risk of bias. Methodological details relevant for potential bias included random sequence generation, allocation concealment, blinding of participants, personal and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. Disagreements between the two reviewers were resolved by discussion and consensus. The data was extracted by one reviewer (Lee JH) on custom-designed forms and entered into a computer database for transfer and statistical analysis in the Review Manager software. The data extracted included first author, year of publication, source, sequence of adjuvant chemotherapy and surgery, clinical stage, number of patients included, and outcome parameters as listed above. Data accuracy was verified by the senior author (Kim SH). Differences between categorical outcome parameters were quantified using a hazard ratio and a corresponding 95% confidence interval (95% CI). Chi-squared and I-squared tests were used for testing heterogeneity between studies. If heterogeneity was not present (p > 0.10 and I2 < 50%), we adopted a fixed-effect model for data analysis. Otherwise, we employed a random-effect model. All statistical analyses were performed using Review Manager (RevMan, version 5.3) and R (version 3.1.0). A p-value < 0.05 was considered as being statistically significant.
Results
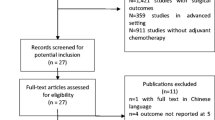
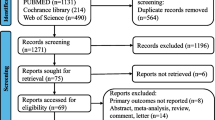
The search results are shown in Fig. 1. An initial literature search confirmed that 1955 studies were found. First, we excluded 727 duplicates, and then we excluded 1220 documents that did not meet the title review selection criteria. Only eight studies remained, and full-text articles were reviewed. Four documents were discarded because the patients were not randomized to fluorouracil-based or oxaliplatin-added adjuvant chemotherapy. At last, only four reports remained and are included in this meta-analysis.
The baseline characteristics of included studies are summarized in Table 1. The first study is the ADORE trial from South Korea [11, 12]. This is the sole result which randomized the patients to LF (leucovorin/5-FU) or FOLFOX chemotherapy following preoperative CRT and surgery. During preoperative CRT, oxaliplatin was not incorporated. The second study was the CAO/RAO/AIO-04 study from Germany. This is the largest trial, having more than 1200 patients [13]. This study compared 5-FU versus FOLFOX regimens during preoperative CRT and following surgery. The third one is the FOWARC study from China, the results of which were published twice (early result in 2016 / final result in 2019) [14, 15]. This was a three-arm study, which compared the LF versus FOLFOX regimens. Since the third arm group did not receive radiotherapy, the result from the third arm was excluded in our study. The last one is the PETACC-6 trial from Europe (EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD). This study compared capecitabine versus the CAPOX regimen used before surgery as well as after surgery [16]. The result was published in January 2021. All studies satisfied the inclusion criteria, and the total number of patients was more than 2800 included for this analysis.
The oncologic results of each study are summarized in Table 2. As shown in Fig. 2, the compliance was not different between the fluorouracil-based and oxaliplatin-added groups. The rate of patients who completed the planned chemotherapy regimen following surgery was 87.2% (1041 of 1194) in the fluorouracil-based group and 83.2% (902 of 1,084) in oxaliplatin-added group, with a relative risk ratio of 1.03 (95% CI: 0.93–1.13). The risk ratio (RR) was statistically significant only in the PETACC-6 study, in which significantly fewer patients (74% vs. 88%, p < 0.010) in the oxaliplatin-added group completed the planned adjuvant chemotherapy. However, in the three other included trials, there was no significant difference between the two groups.
The DFS and OS forest plot is shown in Fig. 3. The overall hazard ratio for DFS was 0.85 (95% CI: 0.74–0.97, p = 0.020), which shows a positive result in the oxaliplatin-added group. However, these results did not translate into OS benefits. The overall HR for OS was 1.02 (95% CI: 0.85–1.22, p = 0.850). The difference of DFS was affected by both in locoregional and distant metastasis control. The RR of 3-year loco-regional control rate was 0.64 (95% CI, 0.48–0.86; p = 0.003) which favors the oxaliplatin-added group. The 3-year distant metastasis control rate also favors the oxaliplatin-added group with an RR of 0.82 (95% CI, 0.71–0.95; p = 0.007).
The grade 3 or higher toxicity pooled analysis results are shown in Supplement Table. Although more toxicities were observed in the oxaliplatin-added group, the RR was 1.16 (95% CI, 0.78–1.72, p = 0.460) with no statistical significance (Fig. 4). In all trials, the most common toxicities were hematologic. However, the result was contradictory, and the detailed results of hematologic toxicities were not reported in the PETACC-6 trial. The frequently observed non-hematologic toxicities were nausea and vomiting, diarrhea, and sensory neuropathies. In all four studies, grade 3 or higher nausea and vomiting were more frequently observed in the oxaliplatin-added group. The overall RR was 2.66 (95% CI: 0.98–7.26, p = 0.060) with marginal significance (Supplement Figure). Diarrhea and sensory neuropathy were definitely higher in the oxaliplatin-added group. The RR was 1.79 (95% CI: 1.30–2.45, p < 0.001) for diarrhea, and 10.48 (95% CI: 6.52–16.84, p < 0.001) for grade 2 or higher sensory neuropathy.
Discussion
Since the publication of the German trial, the standard treatment of locally advanced rectal cancer (stage II or III) has been preoperative CRT and TME surgery, followed by fluorouracil-based adjuvant chemotherapy [1, 9]. This treatment strategy is widely accepted because of the possibility of sphincter saving, high locoregional control rate, and lower acute toxicities. However, survival is still not satisfactory because of high possibility of future recurrence. The subsequent 5-year metastatic rate was 36% in the preoperative CRT arm in German trial, as compared to a 6% locoregional recurrence rate [3].
Therefore, more intensified adjuvant chemotherapy regimens have been investigated to improve outcomes. In colon cancer, the benefits of adding oxaliplatin to a fluorouracil-based regimen have been proved. In the MOSAIC trial, they randomly assigned 2246 colon cancer patients at stage II–III to receive either the 5-FU plus leucovorin (LV5FU2) or the oxaliplatin-added group (FOLFOX4). The HR for DFS and OS was 0.80 (95% CI, 0.68–0.93, p = 0.003) and 0.84 (95% CI, 0.71–1.00, p = 0.046), which favors the oxaliplatin-added group [6, 17]. Similar results were shown in the NSABP C-07 trial. In this trial, which randomly assigned 2409 stage II–III colon cancer patients to either the 5-FU plus leucovorin (FULV) group or the oxaliplatin-added group (FLOX), the DFS improved with a HR of 0.82 (95% CI, 0.72–0.93) in the overall patient group. The OS improved in patients younger than age 70 (HR, 0.80; 95% CI, 0.68 to 0.95; p = 0.013) [7, 18].
With these positive colon cancer results, several trials were conducted to expand or these results in rectal cancer. Unlike colon cancer, in stage II–III rectal cancer, preoperative radiotherapy is usually incorporated in treatment modalities because of the high local recurrence rate in rectal cancer [19]. Therefore, the schedule for application chemotherapy is more complicated. Some trials added oxaliplatin only during preoperative CRT. Most of these trials failed to show improved survival because of high acute toxicities, although some studies showed a higher pathologic complete response (pCR) rate [20]. The ACCORD12 trial compared CAP45 (45 Gy RT with capecitabine) and CAPOX50 (50 Gy RT with capecitabine and oxaliplatin) [21]. The pCR rate was 13.9% versus 19.2%, with no statistical significance (p = 0.090). Local control, DFS, and OS were all similar. The STAR-01 trial also showed similar results, which compared infused 5-FU with 50.4 Gy RT (arm A) versus the same regimen plus weekly oxaliplatin (arm B) [22]. The pCR rate was 16% in both arms. The 5-year DFS was 66.3% vs. 69.2% (HR 0.89, 95% CI 0.60–1.15, p = 0.374). Grade 3 or higher toxicities were more frequent in arm B (24% vs. 8% of treated patients; p < 0.001). A meta-analysis result also showed adding-oxaliplatin in only a preoperative setting had no improvement [23]. The NCCN and ESMO guidelines also recommend not adding oxaliplatin in preoperative CRT [1, 24]. Therefore, we excluded studies which added oxaliplatin only in preoperative setting.
We performed a meta-analysis of trials which compares fluoropyrimidine (5-FU or capecitabine)-based versus oxaliplatin-added chemotherapy regimens in an adjuvant setting. We found four randomized controlled trials, in which the results differed among studies. These meta-analysis results showed adding oxaliplatin in adjuvant setting improved locoregional (HR 0.64, 95% CI 0.48–0.86) and future metastatic control rates (HR 0.82, 95% CI 0.71–0.95). These results lead to a better DFS with an HR of 0.85 (0.74–0.97) in the oxaliplatin-added arm. Overall, DFS benefits do not translate into OS benefits. The South Korean ADORE trial showed improved OS as well as DFS in the oxaliplatin-added group [11, 12]. This seems mainly to be because of the difference in inclusion criteria. Compared to the other three studies, which include clinical stage II or III rectal cancer patients, the ADORE trial only includes pathologically confirmed T3-4 or N + patients following preoperative CRT (ypT3-4 or ypN +). This led to a relatively poor treatment outcome (62.9% 3-year DFS) in the fluorouracil-based group. In our meta-analysis, the compliance rate was similar, and overall, grade 3 or higher acute complication rates did not differ between these groups. However, more grade 3 or worse diarrhea and grade 2 or worse neuropathy were found in the oxaliplatin-added group. Based on our meta-analysis results, it seems adding oxaliplatin in an adjuvant setting is an acceptable treatment option.
The limitation of our study is the small number of randomized trials included. Although the quality of included trials was not low, the large patient number of CAO/RAO/AIO-04 trials could make the result lean to one side. In addition, the reported toxicity outcomes were diverse among studies. So it is difficult to clearly analyze toxicity outcomes [26]. The PETACC-6 study did not report the hematologic toxicity rate [16]. Although it is generally known that adding oxaliplatin can increase diarrhea, stomatitis, neutropenia, or neuropathy [25], these seemed to be manageable in all included randomized studies.
Some meta-analyses comparing oncologic outcomes for 5-FU based- and oxaliplatin based-regimens in rectal cancer have been reported [27,28,29]. Although their results were similar to those of our study, the included trials were different to those of ours. Zheng et al. [27] and Yang et al. [28] included STAR-01, ACCORD-12, and NSABP-R04 trial which used oxaliplatin only in the preoperative setting, not in the adjuvant one. Zhao et al. [29] did not include the FOWARC, and the literature search was just limited by 2014. The final results of PETACC-6 included in our meta-analysis was published in 2021 [16]. Thus, more precise and updated data were analyzed comprehensively in our study.
Oxaliplatin-based postoperative adjuvant chemotherapy rather than fluorouracil-based chemotherapy in rectal cancer after preoperative CRT and curative surgery is significantly associated with increased disease-free survival with similar toxicity. Our results might be helpful to clinicians who need to decide on an adjuvant treatment regimen.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D (2018) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29(suppl 4):iv263
Riihimäki M, Hemminki A, Sundquist J, Hemminki K (2016) Patterns of metastasis in colon and rectal cancer. Sci Rep 6:29765
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933
Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT (2012) Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 13:579–588
Song JH, Jeong JU, Lee JH, Kim SH, Cho HM, Um JW (2017) Preoperative chemoradiotherapy versus postoperative chemoradiotherapy for stage II-III resectable rectal cancer: a meta-analysis of randomized controlled trials. Radiat Oncol J 35:198–207
André T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A (2015) Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC Study. J Clin Oncol 33:4176–4187
Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ (2011) Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 29:3768–3774
Meyers BM, Cosby R, Quereshy F, Jonker D (2017) Adjuvant chemotherapy for stage II and III colon cancer following complete resection: a cancer care ontario systematic review. Clin Oncol (R Coll Radiol) 29:459–465
Bregni G, Akin Telli T, Camera S, Deleporte A, Moretti L, Bali AM (2020) Adjuvant chemotherapy for rectal cancer: current evidence and recommendations for clinical practice. Cancer Treat Rev 83:101948
Glimelius B (2020) Adjuvant chemotherapy in rectal cancer: state of the art and future perspectives. Curr Opin Oncol 32:377–383
Hong YS, Kim SY, Lee JS, Nam BH, Kim KP, Kim JE (2019) Oxaliplatin-based adjuvant chemotherapy for rectal cancer after preoperative chemoradiotherapy (ADORE): long-term results of a randomized controlled trial. J Clin Oncol 37:3111–3123
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ, Park YS (2014) Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 15:1245–1253
Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D (2015) Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16:979–989
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L (2019) Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol 37:3223–3233
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L (2016) Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol 20(34):3300–3307
Schmoll HJ, Stein A, Van Cutsem E, Price T, Hofheinz RD, Nordlinger B (2021) Pre- and postoperative capecitabine without or with oxaliplatin in locally advanced rectal cancer: PETACC 6 Trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J Clin Oncol 39:17–29
André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27:3109–3116
Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G (2007) Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 25:2198–2204
Enríquez-Navascués JM, Borda N, Lizerazu A, Placer C, Elosegui JL, Ciria JP (2011) Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol 17:1674–1684
Wewala NT, Jameson MB (2017) The Role of Oxaliplatin in Chemoradiotherapy for Rectal Cancer. Asia Pac J Clin Oncol 13:341–342
Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne PL (2012) Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 30:4558–4565
Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G (2011) Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 29:2773–2780
Thavaneswaran S, Kok PS, Price T (2017) Evaluating the addition of oxaliplatin to single agent fluoropyrimidine in the treatment of locally advanced rectal cancer: a systematic review and meta-analysis. Expert Rev Anticancer Ther 17:965–979
NCCN guideline, Rectal Cancer (version 2.2021). Available from: http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed date 15 Sept 2021
Haller DG (2000) Safety of oxaliplatin in the treatment of colorectal cancer. Oncology (Williston Park) 14(12 Suppl 11):15–20
Bananzadeh A, Hafezi AA, Nguyen N, Omidvari S, Mosalaei A, Ahmadloo N, Ansari M, Mohammadianpanah M (2021) Efficacy and safety of sequential neoadjuvant chemotherapy and short-course radiation therapy followed by delayed surgery in locally advanced rectal cancer: a single-arm phase II clinical trial with subgroup analysis between the older and young patients. Radiat Oncol J 39:270–278
Zheng J, Feng X, Hu W, Wang J, Li Y (2017) Systematic review and meta-analysis of preoperative chemoradiotherapy with or without oxaliplatin in locally advanced rectal cancer. Medicine 96:e6487
Yang YJ, Cao L, Li ZW, Zhao L, Wu HF, Yue D, Yang JL, Zhou ZR, Liu SX (2016) Fluorouracil-based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: an updated systematic review and meta-analysis. Oncotarget 7:45513–45524
Zhao L, Liu R, Zhang Z, Li T, Li F, Liu H, Li G (2016) Oxaliplatin/fluorouracil-based adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery: a systematic review and meta-analysis of randomized controlled trials. Colorectal Dis 18:763–772
Acknowledgements
The statistical analyses performed in this article were advised by Catholic Medical Center Clinical Research Coordinating Center.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics approval
Approval of an Institute Review Board was waived because the patient data were collected from administrative data without identifiable personal information.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, J.H., Lee, J.H., Kim, S.H. et al. Oxaliplatin-based adjuvant chemotherapy rather than fluorouracil-based chemotherapy in rectal cancer is more efficient to decrease distant metastasis and increase survival after preoperative chemoradiotherapy and surgery: a meta-analysis. Int J Colorectal Dis 37, 649–656 (2022). https://doi.org/10.1007/s00384-022-04096-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-022-04096-9